After the 1930s, electricity replaced other forms of power and became the only power of mining excavators, and the steam shovel was replaced by the electric shovel. At this time, the shovel is mainly used for stripping mining, and the large mining shovel used for stripping is called stripping shovel. With the expansion of the scale of open-pit mining, the stripping shovel is developing towards large scale. In the 1970s, as surface mining technology improved, smaller pick-up shovels gradually replaced larger stripper shovels for easier matching with mining trucks. So far, this type of electric shovel is still the main equipment for open-pit mining mines.
Shovel is one of the most important equipment in open-pit mining technology. Lubrication of gear mechanism of shovel has always been a difficult problem. Improper selection of lubricant often causes serious pitting corrosion, abrasion, scratch, excessive wear and other phenomena, which makes the gear enter overhaul before its service lifetime and increase the maintenance cost. It is the bounden duty of SINOPEC to prolong the use of grease in mine shovel and reduce the surface pollution caused by exposed grease in open pit coal mine.
3.2.2.1. Research on Antioxidation of Electric Shovel Grease
According to
Table 7 and
Table 8 and
Figure 4, LDHs added to shovel grease can prolong the service lifetime of grease while the overall performance of grease is not affected.
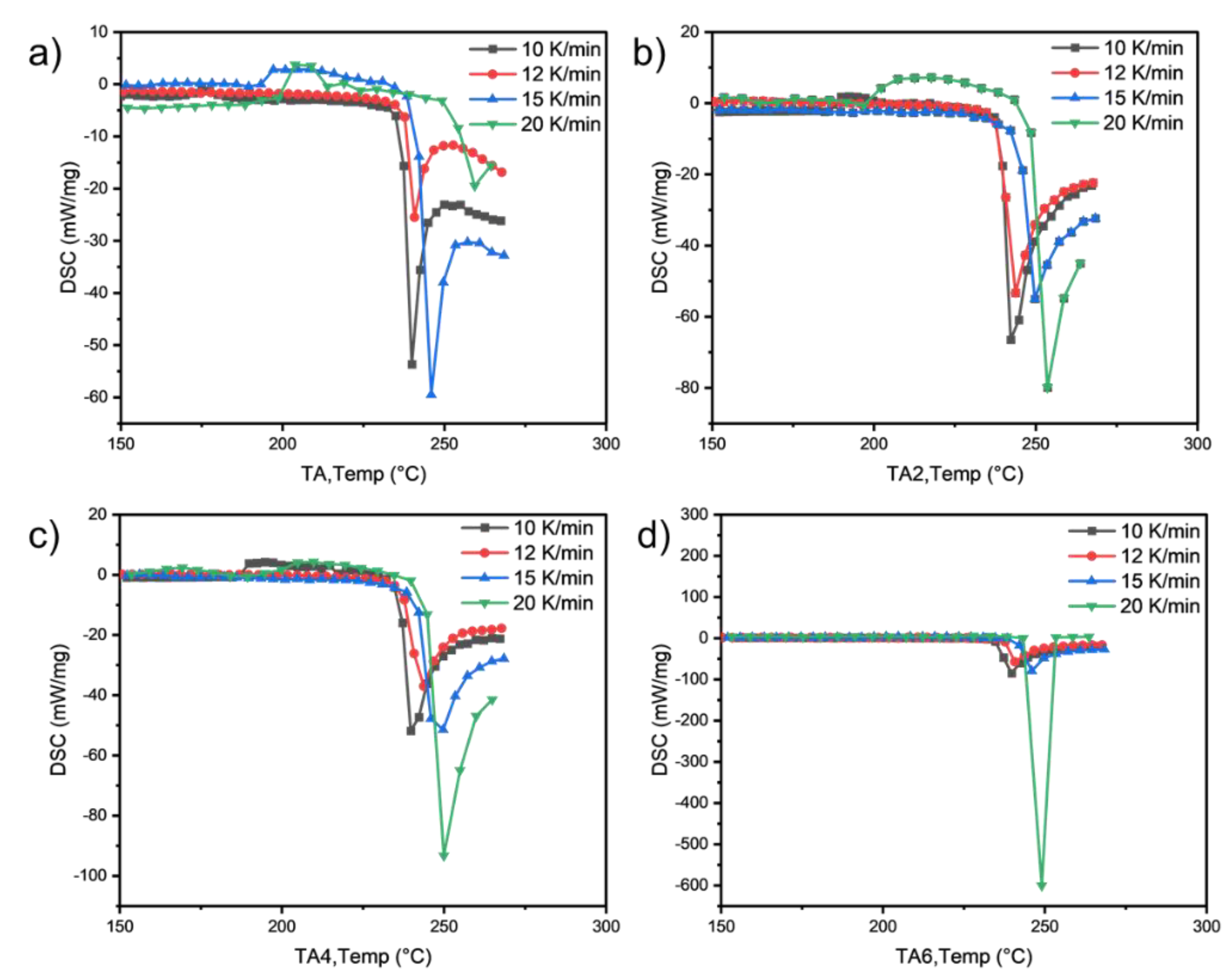
The main premise of DSC to study reaction kinetics is that the degree of reaction is proportional to the thermal effect released or absorbed by the reaction, that is, it is proportional to the area under the DSC curve. Can be expressed by the following formula:
where H - enthalpy, heat of reaction at temperature T; H
T - total enthalpy of the reaction. Thus, the basic kinetic equation of the reaction can be rewritten as,
On both sides of the exponential,
By comparing DSC scanning curves with the same change rates of the two reactions at different rates, the activation energy E can be obtained:
If the change rate α is the same at peak temperature, it is further expressed as:
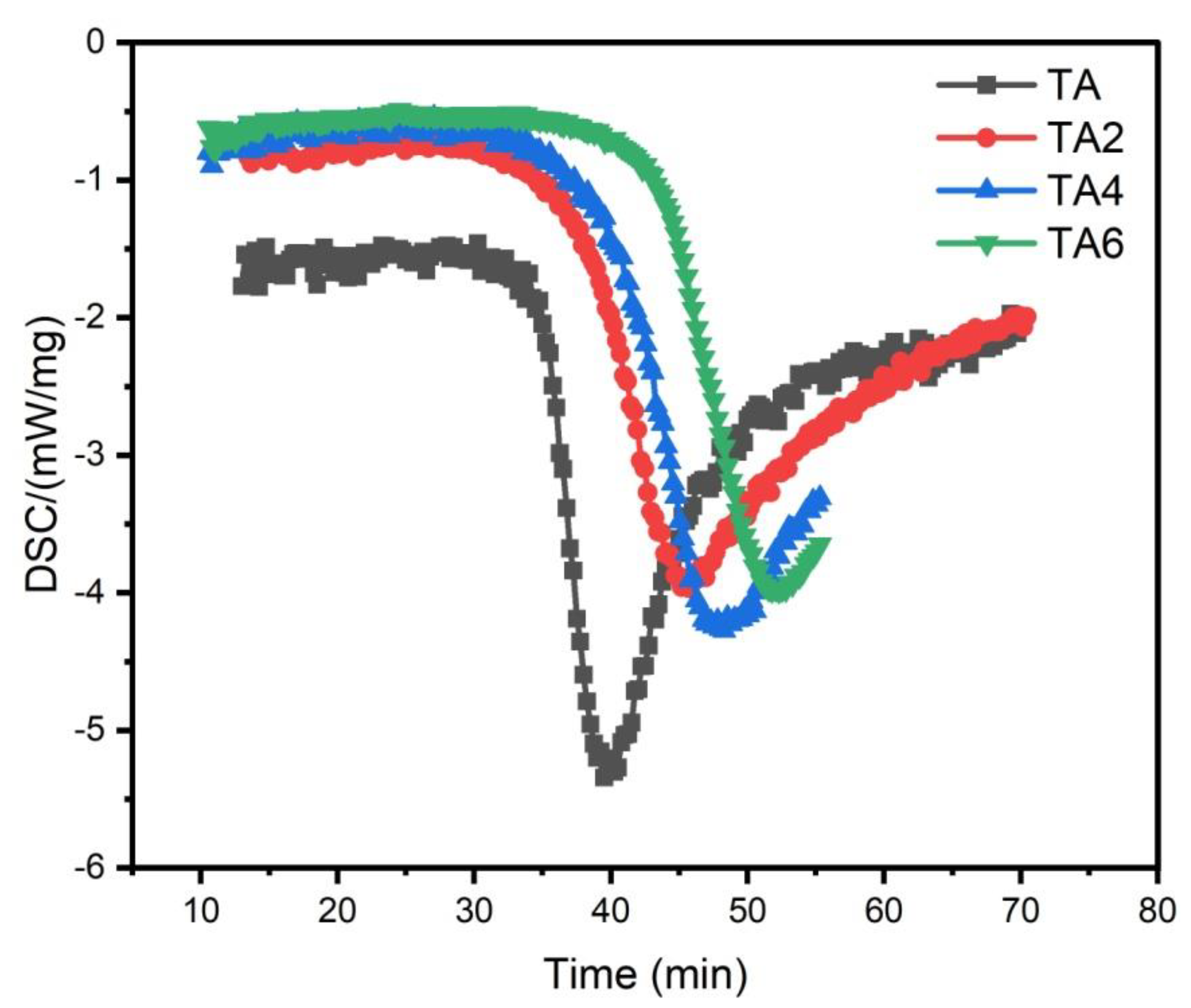
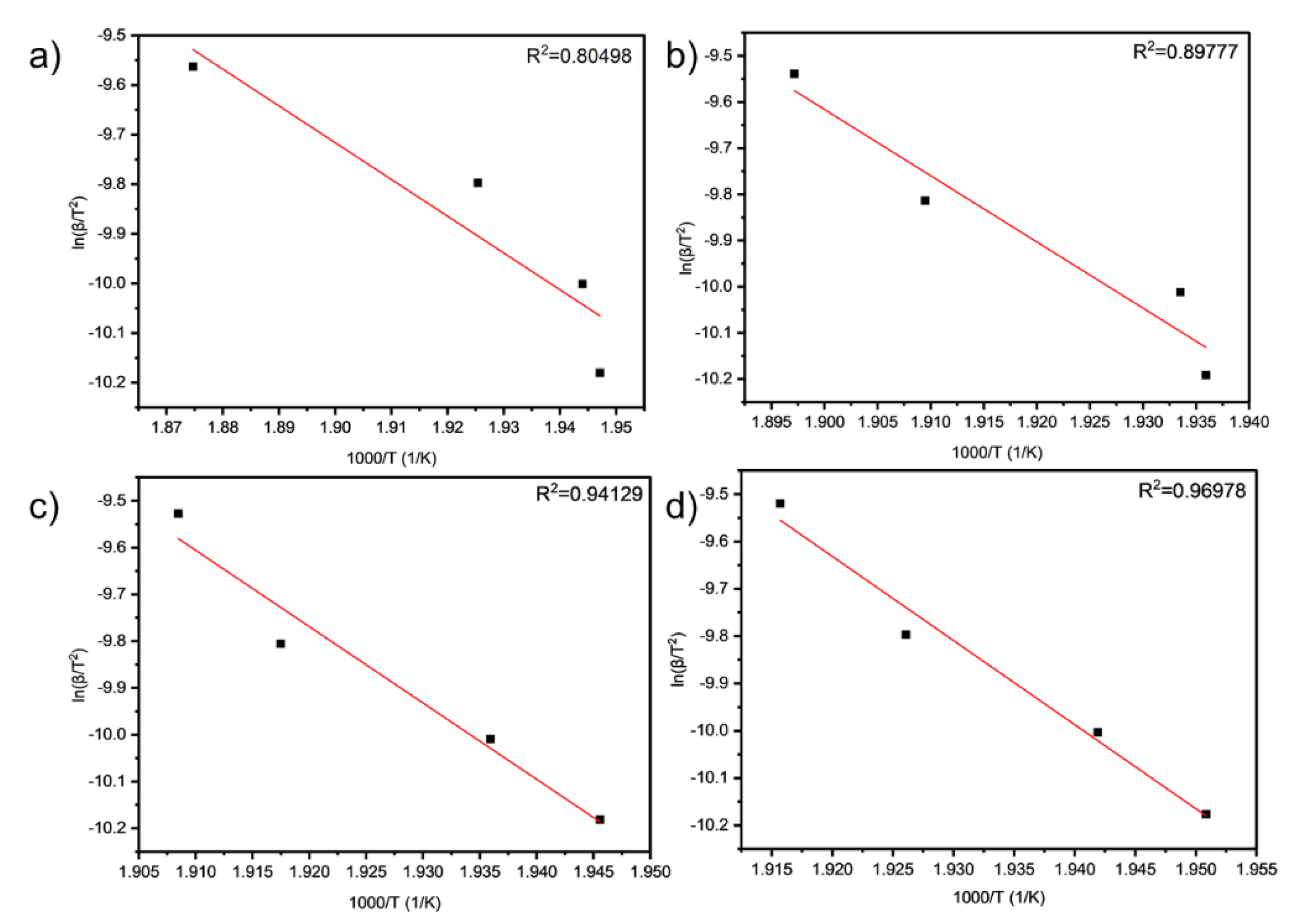
As can be seen from
Table 9 and
Table 10 and
Figure 5 and
Figure 6, the activation energies of the four samples all meet the increasing law of E
TA < E
TA2 < E
TA4 < E
TA6, indicating that with the increase of the amount of LDHs added, the larger the reaction activation energy of grease indicates the stronger its thermal anti-oxidation and decomposition ability.
3.2.2.2. Analysis of Rheological Properties of Electric Shovel Grease
Grease is a viscoelastic non-Newtonian fluid. The elastic part of viscoelasticity is represented by the energy storage modulus G', which indicates that the stress energy can be temporarily stored and recovered during the experiment. The viscous part is represented by the loss modulus G″, which indicates that the grease loses energy during the initial flow, which is converted into shear heat, and the loss is irreversible. When rheological experiments are performed, the shear stress increases, and the point where the energy storage modulus G' begins to decrease is usually defined as the end of the linear viscoelastic zone (LVE), called the yield point, and the shear stress at this point is also called the yield stress τy. The maximum elastic deformation that the grease can withstand can be determined by this point. Shear stress and deformation continue to increase, G' line and G″line intersection, the intersection point is called the flow point (Flow point), the shear stress at this point is called the flow stress τf, at this time the grease thickener structure is more damaged, the grease began to flow.
The oscillatory rheological experiments were performed on four grease samples using the method.
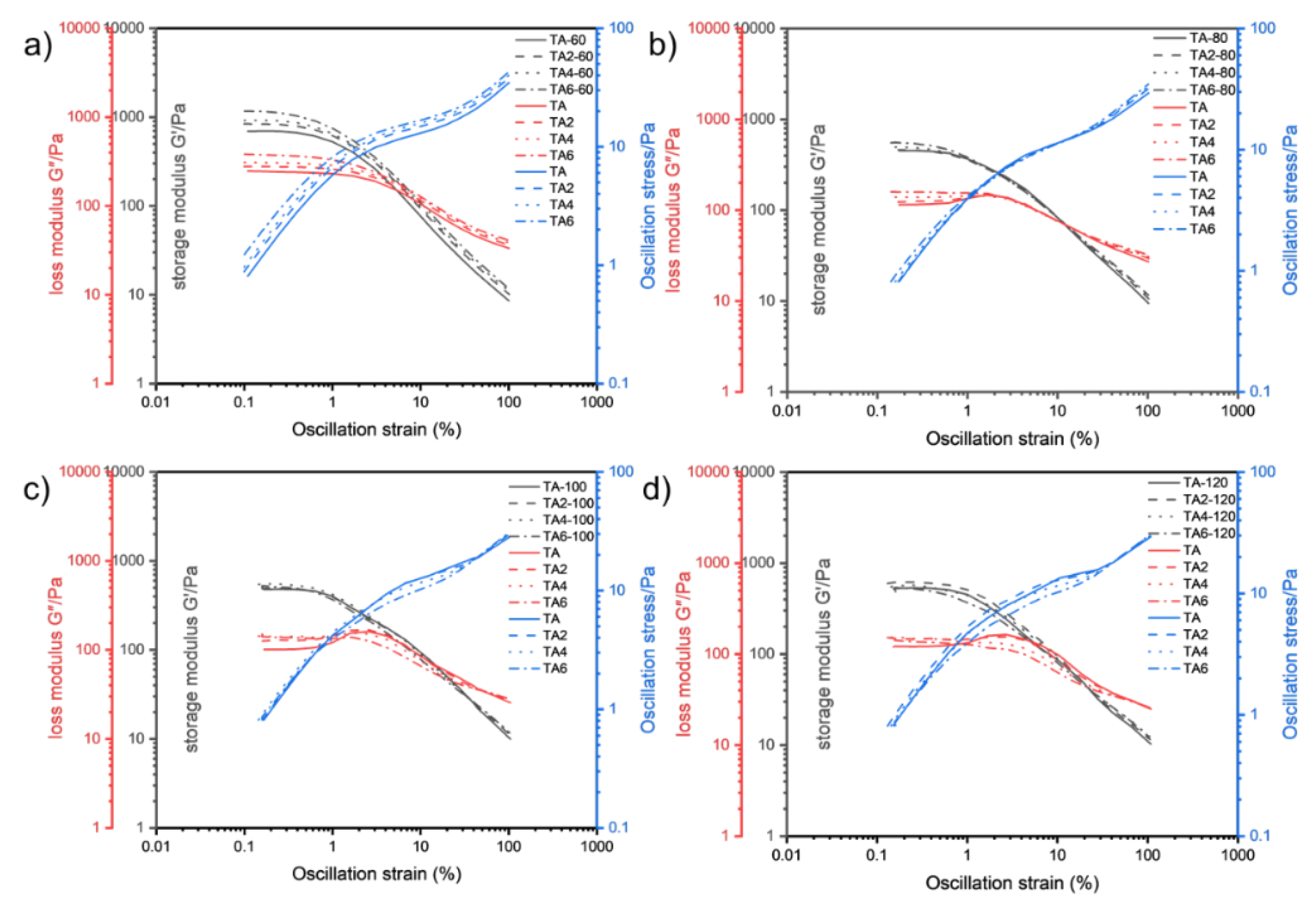
Figure 7 shows the relationship between the storage modulus G' and loss modulus G″with the variation of shear deformation γ at different temperatures measured by changing the experimental temperatures (60, 80, 100 and 120 °C)
It can be seen from
Figure 7 that the overall pattern of energy storage modulus G' of the four greases entering the rheological transition zone at 60, 80, 100 and 120 °C is gradually decreasing with the increase of temperature. From the previous principle analysis, it is known that the larger the G' is, the better the structure of the grease is maintained before it is in gel state, and the lubricating performance of the grease inside the electric shovel gear is often carried out within a few seconds after the start of operation. Therefore, it is beneficial to improve the lubrication of gears by appropriately improving the energy storage modulus and fluidity performance of grease. At 60 °C, as the proportion of LDHs increases, the energy storage modulus of TA-6 grease sample is the largest, and TA-2 is the second at 120 °C, as the proportion of LDHs increases, the energy storage modulus of TA-6 grease sample is the largest, and TA-4 is the second; at 100 °C, the energy storage modulus of TA-4 grease sample is the largest, and TA-2 is the second; at 120 °C, the energy storage modulus of TA-2 grease sample is the largest, TA is the second largest and TA-6 is the smallest; it means that at low temperature, the energy storage modulus of grease increases simultaneously with the increase of LDHs ratio, and with the increase of temperature, the larger the LDHs ratio is, the larger the system elastic deformation damage is, so it is necessary to consider the effect of suitable ratio of LDHs on energy storage modulus. Combined with the flow transition index and energy storage modulus in different linear viscoelastic intervals, it can be seen that adding two percent of LDHs is the best for the system elastic deformation and energy storage modulus. It indicates that the internal structure of sample TA-2 is more stable under shear, and the fiber structure is better maintained, which is better for gear support and lubrication, and is conducive to reducing gear wear.
The values of energy storage modulus and loss modulus, shear stress and corresponding strain values of the four greases at yield point and flow point at the two sets of experimental temperatures can be calculated and the numerical results are listed in
Table 11.
Table 11 shows that: at 60 °C, the yield point energy storage modulus G' is ranked as G'(TA-6) > G'(TA-2) > G'(TA-4) > G'(TA); at 80 °C, G'(TA-6) > G'(TA-4) > G'(TA) > G'(TA-2); at 100 °C, G'(TA-4) > G'(TA-2) > G'(TA) > G'(TA6); at 120 °C, G'(TA-2) > G'(TA) > G'(TA4) > G'(TA6);
At the flow point, the strain amplitude γ is ordered as follows: 60 °C, γ(TA-6) > γ(TA-4) > γ(TA-2) > γ(TA); 80 °C, γ(TA-4) > γ(TA-2) > γ(TA-6) > γ(TA); 100 °C, γ(TA-4) > γ(TA-6) > γ(TA-2) > γ(TA); 120 °C, γ(TA-2) > γ(TA-6) > γ(TA-4) > γ(TA);. The energy storage modulus G' is ordered as follows: 60 °C, G' (TA-2) > G' (TA-4) > G' (TA) > G' (TA-6); 80 °C, G' (TA) > G' (TA-6) > G' (TA-2) > G' (TA-4); 100°C, G' (TA) >G' (TA-2) > G' (TA-4) > G' (TA-6); 120 °C, G' (TA) > G' (TA-4) > G' (TA-2) > G' (TA-6). The shear stress τf is sorted as follows: 60 °C, τf (TA-6) > τf (TA-4) > τf (TA-2) > τf (TA); 80 °C, τf (TA-4) > τf (TA-2) > τf (TA-6) > τf (TA); 100 °C, τf (TA-4) > τf (TA-2) > τf (TA-6) > τf (TA); 120 °C, τf (TA-2) > τf (TA-4) > τf (TA-6) > τf (TA);.
At the flow point, the flow transition index τf/τy is ordered as follows: τf/τy(TA-2) > τf /τy(TA) > τf /τy(TA-4) > τf/τy (TA-6) for 60 °C; τf/τy(TA-2) > τf /τy(TA-4) > τf /τy(TA-6) > τf/τy (TA) for 80 °C; τf/τy (TA-2) > τf /τy(TA-6) > τf/τy(TA-4) > τf/τy(TA) for 100 °C; τf/τy(TA-2) > τf /τy(TA-6) > τf /τy(TA-4) > τf/τy (TA) for 120 °C.
The maximum value of τf/τy for sample TA-2 at the test temperature indicates that at this temperature, TA-2 grease-like soap fibers have the best structural stability and the soap fibers are less likely to fracture or break. Comparing the storage modulus and flow transition index at different temperatures, it can be seen that the addition of the appropriate amount of LDHs for the system oxidation resistance and viscoelasticity requires close attention. For the electric shovel grease system adding 2 % of LDHs can achieve the best oxidation resistance and rheological properties.
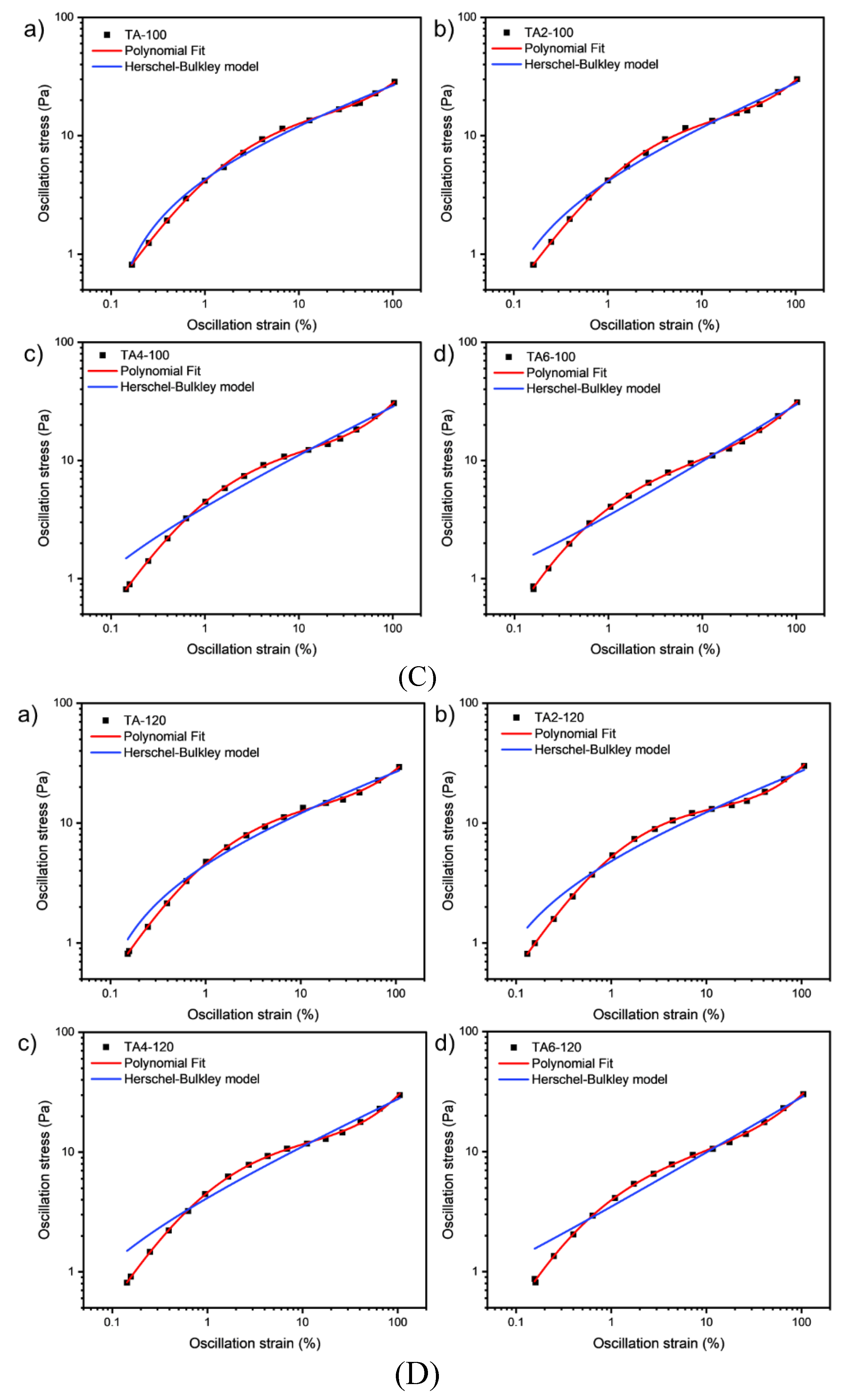
Since grease is a non-Newtonian fluid, curve fitting in this paper refers to the streamlining of flow curves of varying graphical complexity into an equation with two, three or four coefficients. Curve fitting has the following advantages: in quality control, it is easier to mathematically set the tolerance range with the help of standard regression coefficients, and it is more difficult to visually compare the shape difference between the unique flow curve formed by the measured test point and the standard curve. After completing the procedural processing of the experimental data, the second step is obviously to continue the automatic evaluation of the samples for compliance with the technical specifications. Regression calculations help to solve this problem.
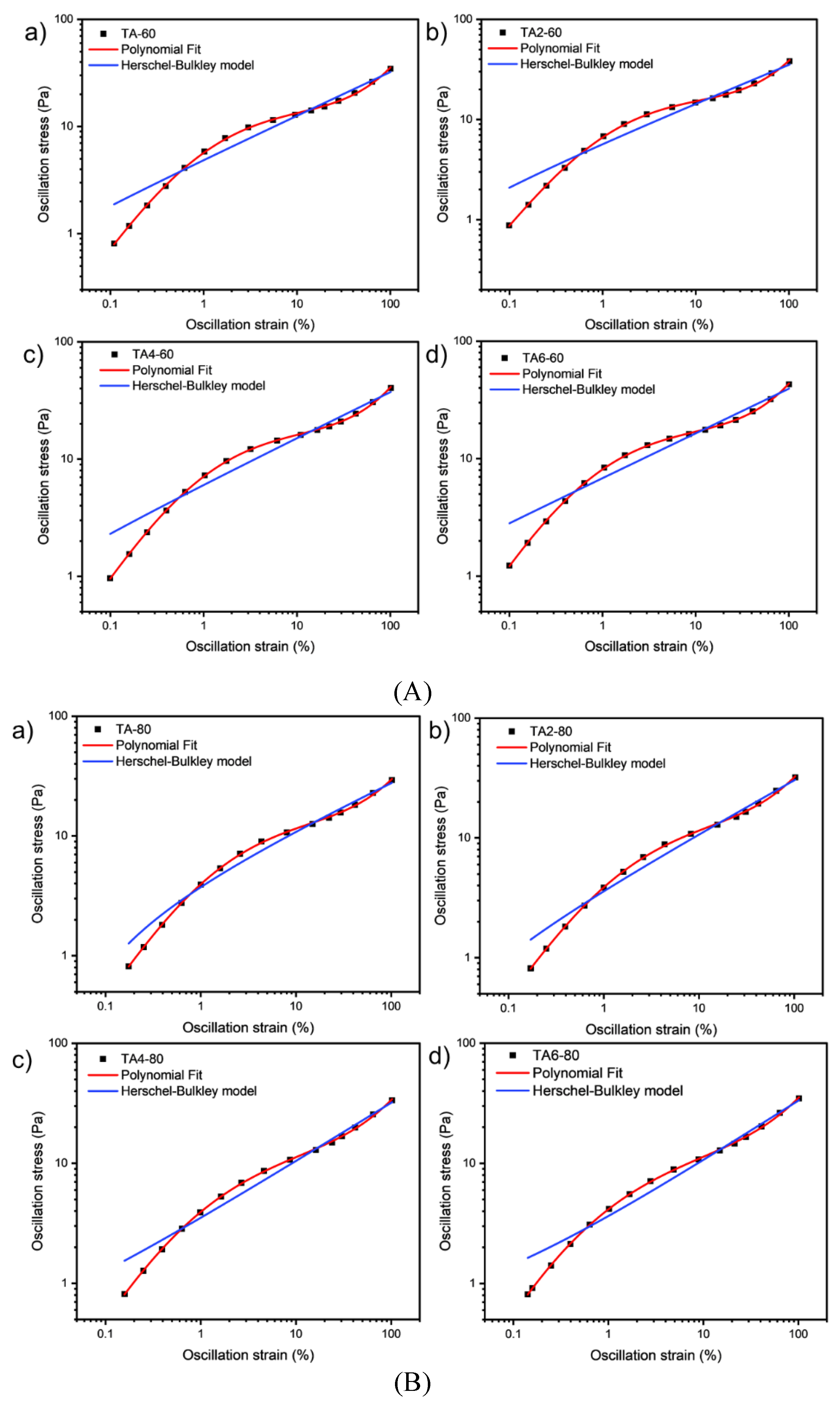
These data were not fitted to the Ostwald-de-Waele model (Equation (1)) because of R
2. These presented a value of n not close to 1, as was expected for not Newtonian fluids. The existence of a yield stress value can be recognized in
Figure 8, and the data were not satisfactorily fitted to a Herschel-Bulkley model (Equation (2)) and the results are collected in
Table 12.The data were satisfactorily fitted to a Polynomial model (Equation (3),R
2>0.999) and the results are collected in
Table 12.
3.2.2.3. Electric Shovel Grease Viscosity and Temperature Analysis
The viscosity-temperature property of grease is the performance of viscosity changed by the temperature. Viscosity usually decreases with the increase of temperature. The change in viscosity of a grease is highly correlated with temperature, and there is a pattern, and the viscosity reflects the rheology of the fluid. The most commonly used equations for calculating viscosity are derived from the corresponding state or statistical principles, but this method has some limitations.
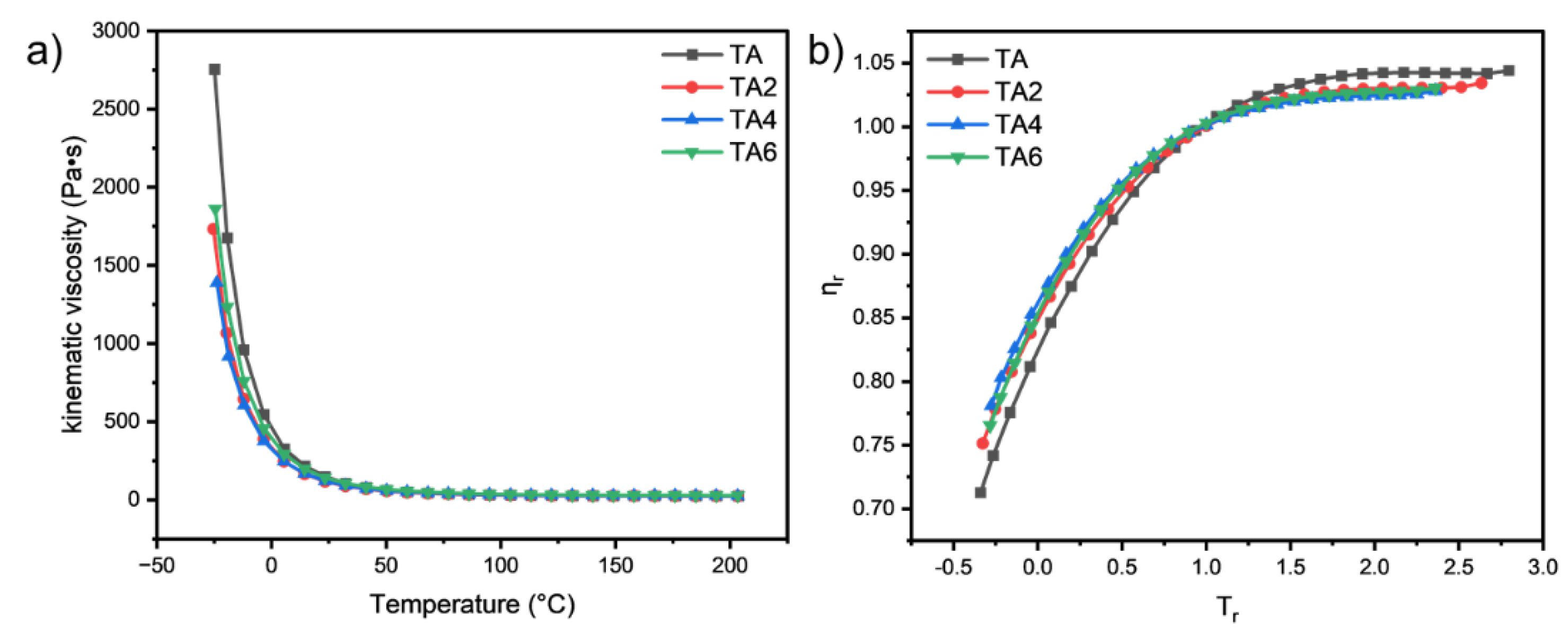
In this paper, the rheological properties of mine grease with different LDHs additions were investigated using a DISCOVERY HR-3 rotational rheometer. It can be found by
Figure 9 that the viscosity changes with temperature in an approximate exponential manner, and the kinematic viscosity of the four types of grease increases sharply when cooling from 200 ℃ to -30 ℃, but the curve is relatively flat and the decline becomes smaller from 200 ℃ to 5 ℃. In the low temperature working interval (-30 ℃ to -5 ℃), the grease samples with different proportions of solid LDHs added are better than mine grease low temperature, indicating that the addition of solid LDH can change the low temperature performance of mine grease. In the high temperature working interval (40 ℃ ~ 200 ℃), the four grease samples power viscosity is comparable, indicating that after adding nano-solid LDHs, the grease in the high temperature working interval viscosity decline trend and adding three proportions of LDHs viscosity decline trend the same, especially adding 6% of LDHs viscosity slightly higher.
Further information was obtained when the viscosity data were fitted to the temperature Power Law model (Equation (4)).
In this model,
represents the asymptote value,
is a threshold temperature at which the sample becomes fragile and
is the parameter that modulates the effect of the temperature on the viscosity. This model allowed us to construct a master curve using the relative viscosity,
, and relative temperature, Tr, described by Equations (5) and (6).
The master curves are shown in
Figure 9b and
Table 13. This kind of representation has been satisfactorily used in lubricant. It is clearly seen that the addition of LDH doesn’t cause a deviation in the model. LDH reduce the effect of temperature on the viscosity of the lubricant. In the case of LDH, we found a good agreement with the model as expected.