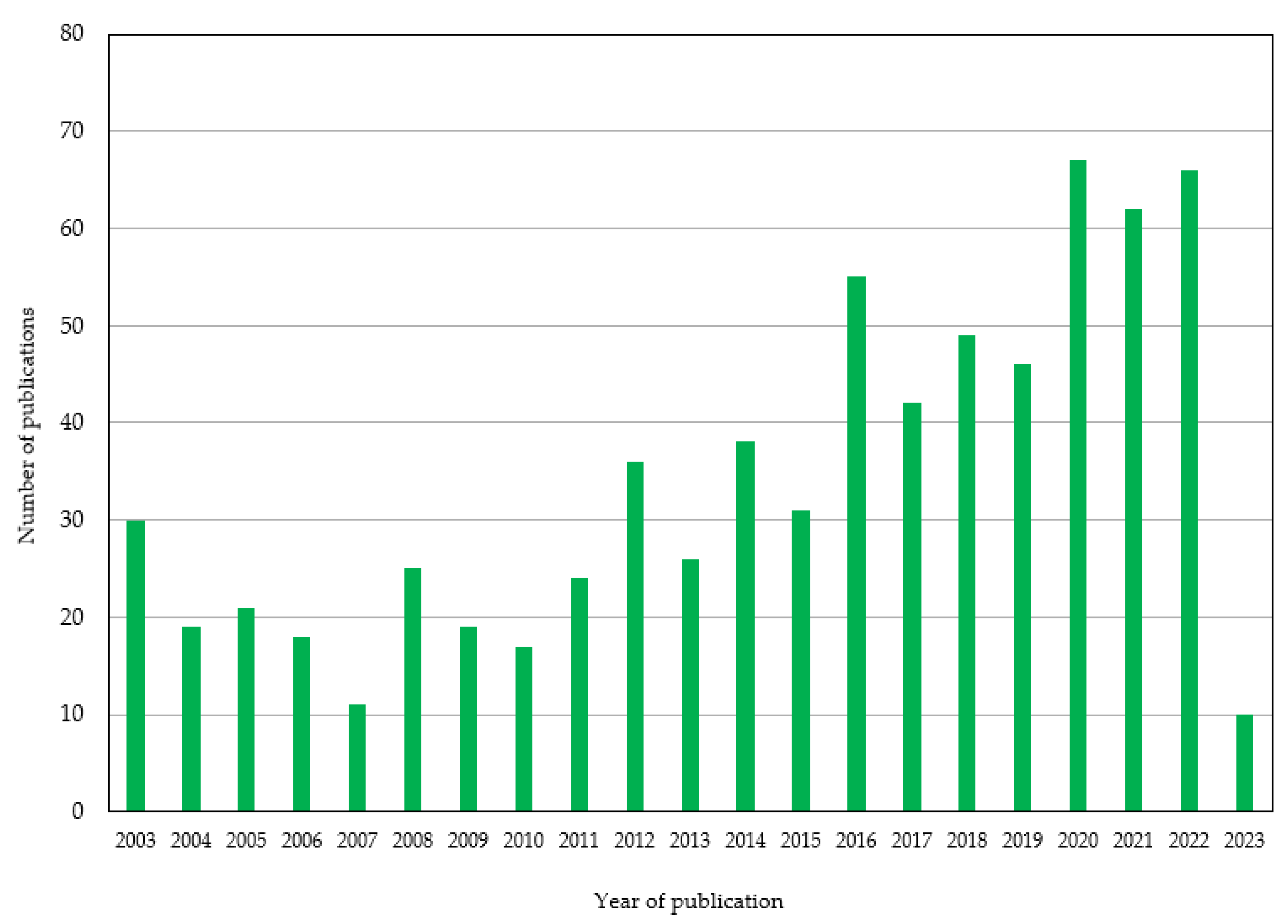
Submitted:
11 April 2023
Posted:
12 April 2023
You are already at the latest version
Abstract
Keywords:
1. Why Encapsulating Vitamins for Food and Cosmetics Application?
1.1. Hydrophobic Vitamins
1.2. Hydrophilic Vitamins
2. Formation and Classification of Liposomes
3. Methods for the Production of Vitamin-Loaded Liposomes
3.1. Conventional Methods
3.1.1. Thin-Film Hydration (TFH) Method (Bangham Method)
3.1.2. Reverse-Phase Evaporation (RPE) Method
3.1.3. Injection Methods
3.1.4. Detergent Removal (Depletion) Method
3.1.5. Double-Emulsion Method
3.1.6. Hydration of Proliposomes
3.2. Novel Methods
3.2.1. Heating Method
3.2.2. Membrane Contactor Method
3.2.3. Electroformation
3.2.4. Nanoprecipitation
3.2.5. Microfluidics Method
3.2.6. Dual Asymmetric Centrifugation (DAC)
3.2.7. Crossflow Filtration Detergent Depletion
3.2.8. Inkjet Method
3.2.9. Supercritical Technologies
4. Techniques for the Quantification of Vitamins into Liposomes
5. Applications of Vitamin-Loaded Liposomes
5.1. In Food
5.2. In Cosmetics
6. Discussion and Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tolonen, M. Vitamins. In: Vitamins and Minerals in Health and Nutrition; Woodhead Publishing, 1990, pp. 99–147. [CrossRef]
- Casas, C. Vitamins. In Analysis of Cosmetic Products, 1st ed.; Salvador, A., Chisvert, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 364–379. [Google Scholar] [CrossRef]
- Cilla, A.; Zanirato, V.; Rodriguez-Estrada, M.T.; Garcia-Llatas, G. Nutriential Hazards: Micronutrients: Vitamins and Minerals. In Encyclopedia of Food Safety. Motarjemi, Y., Ed.; Waltham: Academic Press, 2014, pp. 86–94. [CrossRef]
- Hon, S.L. Vitamin A. In: Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press, 2014, pp. 960–961. [CrossRef]
- Al Tanoury, Z.; Piskunov, A.; Rochette-Egly, C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J. Lipid Res. 2013, 54, 1761–1775. [Google Scholar] [CrossRef]
- Ross, A.C.; Stephensen, C.B. Vitamin A and retinoids in antiviral responses. FASEB J. 1996, 10, 979–985. [Google Scholar] [CrossRef]
- Rothman, K.J.; Moore, L.L.; Singer, M.R.; Nguyen, U.S.; Mannino, S.; Milunsky, A. Teratogenicity of high vitamin A intake. N. Engl. J. Med. 1995, 333, 1369–1373. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Caroli, A.; Poli, A.; Ricotta, D.; Banfi, G.; Cocchi, D. Invited review: Dairy intake and bone health: a viewpoint from the state of the art. J. Dairy Sci. 2011, 94, 5249–5262. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.A.; Oseliero-Filho, P.L.; Jange, C.G.; Sinigaglia-Coimbra, R.; Oliveira, C.L.P.; Pinho, S.C. Structural characterization of multilamellar liposomes coencapsulating curcumin and vitamin D3. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 549, 112–121. [Google Scholar] [CrossRef]
- Barkoukis, H. Nutrition recommendations in elderly and aging. Med. Clin. North Am. 2016, 100, 1237–1250. [Google Scholar] [CrossRef]
- Kira, M.; Kobayashi, T.; Yoshikawa, K. Vitamin D and the skin. J. Dermatol. 2003, 30, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Mitani, H.; Naru, E.; Yamashita, M.; Arakane, K.; Suzuki, T.; Imanari, T. Ergocalciferol promotes in vivo differentiation of keratinocytes and reduces photodamage caused by ultraviolet irradiation in hairless mice. Photodermatol. Photoimmunol. Photomed. 2004, 20, 215–223. [Google Scholar] [CrossRef]
- Jiang, Q. Metabolism of natural forms of vitamin E and biological actions of vitamin E metabolites. Free Radic. Biol. Med. 2022, 179, 375–387. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. The progress and application of vitamin E encapsulation – A review. Food Hydrocoll. 2021, 121, 106998. [Google Scholar] [CrossRef]
- Halder, M.; Petsophonsakul, P.; Akbulut, A.C.; Pavlic, A.; Bohan, F.; Anderson, E.; Maresz, K.; Kramann, R.; Schurgers, L. Vitamin K: Double bonds beyond coagulation insights into differences between vitamin K1 and K2 in health and disease. Int. J. Mol. Sci. 2019, 20, 896. [Google Scholar] [CrossRef]
- Weish, J.; Bak, M.J.; Narvaez, C.J. New insights into vitamin K biology with relevance to cancer. Trends Mol. Med. 2022, 28, 864–881. [Google Scholar] [CrossRef]
- Shah, N.S.; Lazarus, M.C.; Bugdodel, R.; Hsia, S.L.; He, J.; Duncan, R.; Baumann, L. The effects of topical vitamin K on bruising after laser treatment. J. Am. Acad. Dermatol. 2002, 47, 241–244. [Google Scholar] [CrossRef]
- Kamanna, V.S.; Kashyap, M.L. Mechanism of action of niacin. Am. J. Cardiol. 2008, 101, S20–S26. [Google Scholar] [CrossRef]
- Rémond, D.; Shakar, D.R.; Gille, D.; Pinto, P.; Kachal, J.; Peyron, M-A.; dos Santos, C.N.; Walther, B.; Bordoni, A.; Dupont, D.; Tomás-Cobos, L.; Vergères, G. Understanding the gastrointestinal tract of the elderly to develop dietary solutions that prevent malnutrition. Oncotarget. 2015, 6, 13858–13898. [Google Scholar] [CrossRef]
- Caritá, A.C.; Fonseca-Santos, B.; Schultz, J.D.; Michniak-Kohn, B.; Chorilli, M.; Leonardi, G.R. Vitamin C: One compound, several uses. Advances for delivery, efficiency and stability. Nanomed.: Nanotechnol. Biol. Med. 2020, 24, 102117. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Emami, S.; Azadmard-Damirchi, S.; Peighambardoust, S.H.; Valizadeh, H.; Hesari, J. Liposomes as carrier vehicles for functional compounds in food sector. J. Exp. Nanosci. 2016, 11, 737–759. [Google Scholar] [CrossRef]
- Shukla, S.; Haldorai, Y.; Hwang, S.K.; Bajpai, V.K.; Huh, Y.S.; Han, Y.-K. Current demands for food-approved liposome nanoparticles in food and safety sector. Front. Microbiol. 2017, 8, 2398. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Thompson, A.K.; Mozafari, M.R.; Singh, H. The properties of liposomes produced from milk fat globule membrane material using different techniques. Lait 2007, 87, 349–360. [Google Scholar] [CrossRef]
- Van Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed]
- Priya, S.; Desai, V.M.; Singhvi, G. Surface modification of lipid-based nanocarriers: A potential approach to enhance targeted drug delivery. ACS Omega 2023, 8, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of liposomes preparation: Formation and control factors of versatile nanocarriers for biomedical and nanomedicine application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A review of liposomes as a drug delivery system: Current status of approved products, regulatory environments, and future perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Bi, H.; Xue, J.; Jiang, H.; Gao, S.; Yang, D.; Fang, Y.; Shi, K. Current developments in drug delivery with thermosensitive liposomes. Asian J. Pharm. 2019, 14, 365–379. [Google Scholar] [CrossRef]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Transferrin-functionalized liposomes loaded with vitamin VB12 for Alzheimer’s disease therapy. Int. J. Pharm. 2022, 626, 122167. [Google Scholar] [CrossRef]
- Campani, V.; Marchese, D.; Pitaro, M.T.; Pitaro, M.; Grieco, P.; de Rosa, G. Development of a liposome-based formulation for vitamin K1 nebulization on the skin. Int. J. Nanomedicine 2014, 9, 1823–1832. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, L.; Song, L.; Chen, S.; Gao, Y. α-Tocopherol liposome loaded chitosan hydrogel to suppress oxidative stress injury in cardiomyocytes. Int. J. Biol. Macromol. 2019, 125, 1192–1202. [Google Scholar] [CrossRef] [PubMed]
- Szoka Jr., F.; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. 1978, 75, 4194–4198. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154-155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Bigazzi, W.; Penoy, N.; Evrard, B.; Piel, G. Supercritical fluid methods: An alternative to conventional methods to prepare liposomes. Chem. Eng. J. 2020, 383, 123106. [Google Scholar] [CrossRef]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef]
- Favarin, F.R.; Gündel, S.S.; Ledur, C.M.; Roggia, I.; Fagan, S.B.; Gündel, A.; Fogaça, A.O.; Ourique, A.F. Vitamin C as a shelf-life extender in liposomes. Braz. J. Pharm. Sci. 2022, 58, e20492. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Limayem-Blouza, I.; Charcosset, C.; Fessi, H. Preparation, characterization and applications of liposomes: State of the art. J. Colloid Sci. Biotechnol. 2012, 1, 147–168. [Google Scholar] [CrossRef]
- Batzri, S.; Korn, E.D. Single bilayer liposomes prepared without sonication. Biochim. Biophys. Acta 1973, 16, 1015–1019. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Rizvi, S.S.H. Liposomal microencapsulation using the conventional methods and novel supercritical fluid processes. Trends Food Sci. Technol. 2016, 55, 61–71. [Google Scholar] [CrossRef]
- Woodle, M.C.; Papahadjopoulos, D. Liposome preparation and size characterization. Meth. Enzymol. 1989, 171, 193–217. [Google Scholar] [CrossRef]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J Control Release 2019, 300, 114–140. [Google Scholar] [CrossRef] [PubMed]
- Charcosset, C.; Juban, A.; Valour, J.-P.; Urbaniak, S.; Fessi, H. Preparation of liposomes at large scale using the ethanol injection method: Effect of scale-up and injection devices. Chem. Eng. Res. Des. 2015, 94, 508–515. [Google Scholar] [CrossRef]
- Deamer, D.W.; Bangham, A.D. Large volume liposomes by an ether vaporization method. Biochim. Biophys. Acta 1973, 443, 629. [Google Scholar] [CrossRef]
- Farag, M.A.; Elimam, D.M.; Afifi, S.M. Outgoing and potential trends of the omega-3 rich linseed oil quality characteristics and rancidity management: A comprehensive review for maximizing its food and nutraceutical Applications. Trends Food Sci. Technol. 2021, 114, 292–309. [Google Scholar] [CrossRef]
- Maherani., B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci 2011, 7, 136–452. [Google Scholar] [CrossRef]
- Cardoza, J.D.; Kleinfeld, A.M.; Stallcup, K.C.; Merscher, M.F. Hairpin configuration of H-2Kk in liposomes formed by detergent dialysis. Biochemistry 1984, 23, 4401–4409. [Google Scholar] [CrossRef]
- Zumbuehl, O.; Weder, H.G. Liposomes of controllable size in the range of 40 to 180 nm by defined dialysis of lipid/detergent mixed micelles. Biochim. Biophys. Acta Biomembr. 1981, 640, 252–262. [Google Scholar] [CrossRef]
- Lasch, J.; Weissig, V.; Brandl, M. Preparation of liposomes. In Liposomes – a practical approach, 2nd ed.; Torchilin, V.P., Weissig, V., Eds.; Oxford University Press: Oxford, UK, 2003. [Google Scholar]
- Mastrogiacomo, D.; Lenucci, M.S.; Bonfrate, V.; di Carolo, M.; Piro, G.; Valli, L.; Rescio, L.; Milano, F.; Comparelli, R.; de Leo, V.; Fiotta, L. Lipid/detergent mixed micelles as a tool for transferring antioxidant power from hydrophobic natural extracts into bio-deliverable liposome carriers: the case of lycopene rich oleoresins. RSC Adv. 2015, 5, 3081–3093. [Google Scholar] [CrossRef]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef]
- Meure, L.A.; Foster, N.R.; Dehghani, F. Conventional and dense gas techniques for the production of liposomes: A review. AAPS PharmSciTech 2008, 9, 798–809. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, T.; Horikoshi, K.; Suzuki, A.; Neves, M.A.; Kobayashi, I.; Uemura, K.; Nakajima, M.; Kanazawa, A.; Ichikawa, S. Efficient encapsulation of a water-soluble molecule into lipid vesicles using W/O/W multiple emulsions via solvent evaporation. J. Am. Oil Chem. Soc. 2016, 93, 421–430. [Google Scholar] [CrossRef]
- Li, T.; Yang, S.; Liu, W.; Liu, C.; Liu, W.; Zheng, H.; Zhou, W.; Tong, G. Preparation and characterization of nanoscale complex liposomes containing medium-chain fatty acids and vitamin C. Int. J. Food Prop. 2015, 18, 113–124. [Google Scholar] [CrossRef]
- Yang, S.; Liu, C.; Liu, W.; Yu, H.; Zheng, H.; Zhou, W.; Hu, Y. Preparation and characterization of nanoliposomes entrapping medium-chain fatty acids and vitamin C by lyophilization. Int. J. Mol. Sci. 2013, 14, 19763–19773. [Google Scholar] [CrossRef]
- Pattnaik, M.; Mishra, H.N. Effect of ultrasonication and wall materials on the stability, rheology, and encapsulation efficiency of vitamins in a lipid-based double emulsion template. J. Food Process Eng. 2022, e14201. [Google Scholar] [CrossRef]
- Payne, N.I.; Timmins, P.; Ambrose, C.V.; Ward, M.D.; Ridgway, F. Proliposomes: a novel solution to an old problem. J. Pharm. Sci. 1986, 75, 325–329. [Google Scholar] [CrossRef]
- Xu, H.; He, L.; Nie, S.; Guan, J.; Zhang, X.; Yang, X.; Pan, W. Optimized preparation of vinpocetine proliposomes by a novel method and in vivo evaluation of its pharmacokinetics in New Zealand rabbits. J. Control. Release. 2009, 140, 61–68. [Google Scholar] [CrossRef]
- Dhakal, S.P.; He, J. Microencapsulation of vitamins in food applications to prevent losses in processing and storage: A review. Food. Res. Int. 2020, 137, 109326. [Google Scholar] [CrossRef]
- Lee, S.J.; Wong, M. Nano- and microencapsulation of phytochemicals. In Nano- and Microencapsulation for Foods; Kwak, H-S., Ed.; John Wiley & Sons, Ltd, 2014, pp. 117–165. [CrossRef]
- Yu, J.Y.; Chuesiang, P.; Shin, G.H.; Park, H.J. Post-processing techniques for the improvement of liposome stability. Pharmaceutics 2021, 13, 1023. [Google Scholar] [CrossRef]
- Has, C.; Sunthar, P. A comprehensive review on recent preparation techniques of liposomes. J. Liposome Res. 2020, 30, 336–365. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Carlan, I.C.; Estevinho, B.N.; Rocha, F. Production of vitamin B1 microparticles by a spray drying process using different biopolymers as wall materials. Can. J. Chem. Eng. 2020, 98, 1682–1695. [Google Scholar] [CrossRef]
- Estevinho, B.N.; Mota, R.; Leite, J.P.; Tamagnini, P.; Gales, L.; Rocha, F. Application of a cyanobacterial extracellular polymeric substance in the microencapsulation of vitamin B12. Powder Technol. 2019, 343, 644–651. [Google Scholar] [CrossRef]
- Toniazzo, T.; Berbel, I.F.; Cho, S.; Fávaro-Trindade, C.S.; Moraes, I.C.F.; Pinho, S.C. β-carotene-loaded liposome dispersions stabilized with xanthan and guar gums: Physico-chemical stability and feasibility of application in yogurt. LWT 2014, 59, 1265–1273. [Google Scholar] [CrossRef]
- Chaves, M.A.; Pinho, S.C. Unpurified soybean lecithins impact on the chemistry of proliposomes and liposome dispersions encapsulating vitamin D3. Food Biosci. 2020, 37, 100700. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Reed, C.J.; Rostron, C.; Kocum, C.; Piskin, E. Constructing of stable anionic liposome-plasmid particles using the heating method: A preliminary investigation. Cell. Mol. Biol. Lett. 2002, 7, 923–928. [Google Scholar]
- Mozafari, M.R. Liposomes: Na overview of manufacturing techniques. Cell. Mol. Biol. Lett. 2005, 10, 711–719. [Google Scholar]
- Charcosset, C.; Limayem, I.; Fessi, H. The membrane emulsification process – a review. J. Chem. Technol. Biotechnol. 2004, 79, 209–218. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Charcosset, C.; Fessi, H. A new method for liposome preparation using a membrane contactor. J. Liposome Res. 2011, 21, 213–220. [Google Scholar] [CrossRef]
- Laouini, A.; Jaafar-Maalej, C.; Sfar, S.; Charcosset, C.; Fessi, H. Liposome preparation using a hollow fiber membrane contactor—Application to spironolactone encapsulation. Int. J. Pharm. 2011, 415, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Laouini, A.; Charcosset, C.; Fessi, H.; Holdich, R.G.; Vladisavljević, G.T. Preparation of liposomes: a novel application of microengineered membranes - investigation of the process parameters and application to the encapsulation of vitamin E. RSC Adv. 2013, 3, 4985–4994. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Angelova, M.I.; Soléau, S.; Méléard, Ph.; Faucon, F.; Bothorel, P. Preparation of giant vesicles by external AC electric fields. Kinetics and applications. In Trends in Colloid and Interface Science VI; Progress in Colloid & Polymer Science, volume 89; Lösche, M., Möhwald, H., Helm, C., Eds.; Steinkopff: Darmstadt, Germany, 1992. [Google Scholar] [CrossRef]
- DiPasquale, M.; Nguyen, M.H.L.; Rickeard, B.W.; Cesca, N.; Tannous, C.; Castillo, S.R.; Katsaras, J.; Kelley, E.G.; Heberle, F.A.; Masquardt, D. The antioxidant vitamin E as a membrane raft modulator: Tocopherols do not abolish lipid domains. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183189. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.L.; Monaco, I.; Kostevšek, N.; Franchini, M.C.; Al-Jamal, W.T. Nanoprecipitation preparation of low temperature-sensitive magnetoliposomes. Colloids Surf. B. 2021, 198, 111453. [Google Scholar] [CrossRef] [PubMed]
- Jash, A.; Rizvi, S.S.H. Heat-stable liposomes from milk fat globule membrane phospholipids for pH-triggered delivery of hydrophilic and lipophilic bioactives. Innov. Food Sci. Emerg. Technol. 2022, 79, 103030. [Google Scholar] [CrossRef]
- Ottino, J.M.; Wiggins, S. Introduction: Mixing in microfluidics. Phil. Trans. R. Soc. Lond. A 2004, 362, 923–935. [Google Scholar] [CrossRef]
- Carugo, D.; Bottaro, E.; Owen, J.; Stride, E.; Nastruzzi, C. Liposome production by microfluidics: potential and limiting factors. Sci. Rep. 2016, 6, 25876. [Google Scholar] [CrossRef]
- Dalmoro, A.; Bochicchio, S.; Lamberti, G.; Bertoncin, P.; Janssens, B.; Barba, A.A. Micronutrients encapsulation in enhanced nanoliposomal carriers by a novel preparative technology. RSC Adv. 2019, 9, 19800–19812. [Google Scholar] [CrossRef]
- Ingebrigtsen, S.G.; Škalko-Basnet, N.; Holsæter, A.M. Development and optimization of a new processing approach for manufacturing topical liposomes-in-hydrogel drug formulations by dual asymmetric centrifugation. Drug Dev. Ind. Pharm. 2016, 42, 1375–1383. [Google Scholar] [CrossRef]
- Massing, U.; Cicko, S.; Ziroli, V. Dual asymmetric centrifugation (DAC)—A new technique for liposome preparation. J. Control. Release 2008, 125, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Li, X.; Zhang, T.; Song, Y.; She, Z.; Li, J.; Deng, Y. Progress involving new techniques for liposome preparation. Asian J. Pharm. 2014, 9, 176–182. [Google Scholar] [CrossRef]
- Peschka, R.; Dennehy, C.; Szoka, F.C., Jr. A simple in vitro model to study the release kinetics of liposome encapsulated material. J. Control. Release 1998, 56, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, B.; Gupta, R.; Gulati, M.; Singh, S.K.; Khursheed, R.; Gupta, M. The Why, Where, Who, How, and What of the vesicular delivery systems. Adv. Colloid Interface Sci. 2019, 271, 101985. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, S.; Lipprandt, U.; Rumplecker, A.; Borchert, U.; Rank, A.; Schubert, R.; Förster, S. Direct preparation and loading of lipid and polymer vesicles using inkjets. Small 2005, 1, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Bnyan, R.; Cesarini, L.; Khan, I.; Roberts, M.; Ehtezazi, T. The effect of ethanol evaporation on the properties of inkjet produced liposomes. DARU J. Pharm. 2020, 28, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.; Monou, P.K.; Andriotis, E.; Mitsouli, E.; Moutafidou, N.; Markopoulou, C.; Bouropoulos, N.; Fatouros, D. Development and characterization of inkjet printed edible films for buccal delivery of b-complex vitamins. Pharmaceutics 2020, 13, 203. [Google Scholar] [CrossRef]
- Merlo-Mas, J.; Tomsen-Melero, J.; Corchero, J-L.; González-Mira, E.; Font, A.; Pedersen, J.N.; García-Aranda, N.; Cristóbal-Lecina, E.; Alcaina-Hernando, M.; Mendoza, R.; Garcia-Fruitós, E.; Lizarraga, T.; Resch, S.; Schimpel, C.; Falk, A.; Pulido, D.; Royo, M.; Schwartz Jr, S.; Abasolo, I.; Pedersen. J.S.; Danino, D.; Soldevila, A.; Veciana, J.; Sala, S.; Ventosa, N.; Córdoba, A. Application of Quality by Design to the robust preparation of a liposomal GLA formulation by DELOS-susp method. J. Supercrit. Fluids 2021, 173, 105204. [CrossRef]
- Cano-Sarabia, M.; Ventosa, N.; Sala, S.; Patiño, C.; Arranz, R.; Veciana, J. Preparation of uniform rich cholesterol unilamellar nanovesicles using CO2-expanded solvents. Langmuir 2008, 24, 2433–2437. [Google Scholar] [CrossRef]
- Leitgeb, M.; Knez, Ž.; Primožič, M. Sustainable technologies for liposome preparation. J, Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F. Preparation of liposomes using a modified supercritical process via depressurization of liquid phase. J. Supercrit. Fluids 2015, 100, 110–120. [Google Scholar] [CrossRef]
- Elizondo, E.; Moreno, E.; Cabrera, I.; Córdoba, A.; Sala, S.; Veciana, J.; Ventosa, N. Liposomes and other vesicular systems: Structural characteristics, methods of preparation, and use in nanomedicine. Prog. Mol. Biol. Transl. Sci. 2011, 104, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Meure, L.A.; Knott, R.; Foster, N.R.; Dehgani, F. The depressurization of an expanded solution into aqueous media for the bulk production of liposomes. Langmuir 2009, 25, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Beh, C.C.; Mammucari, R.; Foster, N.R. Process intensification: Nano-carrier formation by a continuous dense gas process. Chem. Eng. J. 2015, 266, 320–328. [Google Scholar] [CrossRef]
- Beh, C.C.; Wong, M.G.; Olet, V.; Foster, N. Development of a novel continuous dense gas process for the production of residual solvent-free self-assembled nano-carriers. Chem. Eng. Process. 2019, 143, 107589. [Google Scholar] [CrossRef]
- Karn, P.R.; Cho, W.; Hwang, S.-J. Liposomal drug products and recent advances in the synthesis of supercritical fluid-mediated liposomes. Nanomedicine 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Frederiksen, L.; Anton, K.; van Hoogevest, P.; Keller, H.R.; Leuenberger, H. Preparation of liposomes encapsulating water-soluble compounds using supercritical carbon dioxide. J. Pharm. Sci. 2000, 86, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Liu, B.; Zheng, Z.K.; You, X.K.; Pu, Y.T.; Li, Q. Preparation of liposome particle of atractylone by supercritical carbon dioxide process. Adv. Mater. Res. 2010, 92, 177–182. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Liposomes: From Bangham to supercritical fluids. Processes 2020, 8, 1022. [Google Scholar] [CrossRef]
- Tsai, W.-C.; Rizvi, S.S.H. Simultaneous microencapsulation of hydrophilic and lipophilic bioactives in liposomes produced by an ecofriendly supercritical fluid process. Food Res. Int. 2017, 99, 256–262. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Han, S.; Zha, X.; Xia, J. Preparation of vitamin C liposomes by rapid expansion of supercritical solution process: Experiments and optimization. J. Drug Deliv. Sci. Technol. 51, 1–6. [CrossRef]
- Jiao, Z.; Han, S.; Wang, W.; Song, J.; Cheng, J. Preparation and optimization of Vitamin E acetate liposomes using a modified RESS process combined with response surface methodology. Part. Sci. Technol. 38, 863–875. [CrossRef]
- Jash, A.; Ubeyitogullari, A.; Rizvi, S.S.H. Liposomes for oral delivery of protein and peptide-based therapeutics: challenges, formulation strategies, and advances. J. Mater. Chem. B, 2021, 9, 4773–4792. [CrossRef]
- Sharifi, F.; Jash, A.; Abbaspourrad, A.; Rizvi, S.S.H. Generation of ironized and multivitamin-loaded liposomes using venturi-based rapid expansion of a supercritical solution (Vent-RESS). Green Chem. 2020, 22, 1618–1629. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.; Ruan, N.; Jiao, Z. Preparation of liposomes composed of supercritical carbon dioxide-philic phospholipids using the rapid expansion of supercritical solution process. J. Drug Deliv. Sci. Technol. 2021, 64, 102568. [Google Scholar] [CrossRef]
- Otake, K.; Imura, T.; Sakai, H.; Abe, M. Development of a new preparation method of liposomes using supercritical carbon dioxide. Langmuir 2001, 17, 3898–3901. [Google Scholar] [CrossRef]
- Imura, T.; Otake, K.; Hashimoto, S.; Gotoh, T.; Yuasa, M.; Yokoyama, S.; Sakai, H.; Rathman, J.F.; Abe, M. Preparation and physicochemical properties of various soybean lecithin liposomes using supercritical reverse phase evaporation method. Colloids Surf. B 2003, 27, 133–140. [Google Scholar] [CrossRef]
- Otake, K.; Shimomura, T.; Goto, T.; Imura, T.; Furuya, T.; Yoda, S.; Takebayashi, Y.; Sakai, H.; Abe, M. Preparation of liposomes using an improved supercritical reverse phase evaporation method. Langmuir 2006, 22, 2543–2550. [Google Scholar] [CrossRef]
- Zhao, L.; Temelli, F. Preparation of anthocyanin-loaded liposomes using an improved supercritical carbon dioxide method. Innov. Food Sci. Emerg. Technol. 2017, 39, 119–128. [Google Scholar] [CrossRef]
- Lesoin, L.; Boutin, O.; Crampon, C.; Badens, E. CO2/water/surfactant ternary systems and liposome formation using supercritical CO2: A review. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 377, 1–14. [Google Scholar] [CrossRef]
- Xia, F.; Jin, H.; Zhao, Y.; Guo, X. Supercritical antisolvent-based technology for preparation of vitamin D3 proliposome and its characteristics. Chin. J. Chem. Eng. 2011, 19, 1039–1046. [Google Scholar] [CrossRef]
- Espírito-Santo, I.; Campardelli, R.; Albuquerque, E.C.; de Melo, S.V.; della Porta, G.; Reverchon, E. Liposomes preparation using a supercritical fluid assisted continuous process. Chem. Eng. J. 2014, 249, 153–159. [Google Scholar] [CrossRef]
- Campardelli, R.; Espírito-Santo, I.; Albuquerque, E.C.; de Melo, S.V.; della Porta, G.; Reverchon, E. Efficient encapsulation of proteins in submicro liposomes using a supercritical fluid assisted continuous process. J. Supercrit. Fluids 2016, 107, 163–169. [Google Scholar] [CrossRef]
- Espírito-Santo, I.; Campardelli, R.; Albuquerque, E.C.; de Melo, S.A.B.V.; Reverchon, E.; della Porta, G. Liposomes size engineering by combination of ethanol injection and supercritical processing. J. Pharm. Sci. 2015, 104, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P.; Campardelli, R.; Scognamiglio, M.; Reverchon, E. Control of liposomes diameter at micrometric and nanometric level using a supercritical assisted technique. J. CO2 Util. 2019, 32, 119–127. [Google Scholar] [CrossRef]
- Campardelli, R.; Trucillo, P.; Reverchon, E. A supercritical fluid-based process for the production of fluorescein-loaded liposomes. Ind. Eng. Chem. Res. 2016, 55, 5359–5365. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Supercritical CO2 assisted liposomes formation: Optimization of the lipidic layer for an efficient hydrophilic drug loading. J. CO2 Util. 2017, 18, 181–188. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. Production of liposomes loaded with antioxidants using a supercritical CO2 assisted process. Powder Technol. 2018, 323, 155–162. [Google Scholar] [CrossRef]
- Trucillo, P.; Campardelli, R.; Reverchon, E. A versatile supercritical assisted process for the one-shot production of liposomes. J. Supercrit. Fluids 2019, 146, 136–143. [Google Scholar] [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Supercritical CO2 assisted process for the production of mixed phospholipid nanoliposomes: Unloaded and vitamin D3-loaded vesicles. J. Food. Eng. 316, 110851. [CrossRef]
- Chaves, M.A.; Baldino, L.; Pinho, S.C.; Reverchon, E. Co-encapsulation of curcumin and vitamin D3 in mixed phospholipid nanoliposomes using a continuous supercritical CO2 assisted process. J. Taiwan Inst. Chem. Eng. 132, 104120. [CrossRef]
- Bleich, J.; Müller, B.W. Production of drug loaded microparticles by the use of supercritical gases with the Aerosol Solvent Extraction System (ASES) process. J. Microencapsul. 1996, 13, 131–139. [Google Scholar] [CrossRef]
- Kunastitchai, S.; Pichert, L.; Sarisuta, N.; Müller, B.W. Application of aerosol solvent extraction system (ASES) process for preparation of liposomes in a dry and reconstitutable form. Int. J. Pharm. 2006, 316, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Kunastitchai, S.; Sarisuta, N.; Panyarachun, B.; Müller, B.W. Physical and chemical stability of miconazole liposomes prepared by Supercritical Aerosol Solvent Extraction System (ASES) process. Pharm. Dev. 2007, 12, 361–370. [Google Scholar] [CrossRef] [PubMed]
- De Paz, E.; Martín, Á.; Cocero, M.J. Formulation of β-carotene with soybean lecithin by PGSS (Particles from Gas Saturated Solutions)-drying. J. Supercrit. Fluids 2012, 72, 125–133. [Google Scholar] [CrossRef]
- Varona, S.; Martín, Á.; Cocero, M.J. Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. Ind. Eng. Chem. Res. 2011, 50, 2088–2097. [Google Scholar] [CrossRef]
- Rovoli, M.; Pappas, I.; Lalas, S.; Gortzi, O.; Kontopidis, G. In vitro and in vivo assessment of vitamin A encapsulation in a liposome–protein delivery system. J. Liposome. Res. 2019, 29, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-U.; Park, H.-W.; Lee, S.-C. Comparing the stability of retinol in liposomes with cholesterol, β-sitosterol, and stigmasterol. Food Sci. Biotechnol. 2021, 30, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Pezeshky, A.; Ghanbarzadeh, B.; Hamishehkar, H.; Moghadam, M.; Babazadeh, A. Vitamin A palmitate-bearing nanoliposomes: Preparation and characterization. Food Biosci. 2016, 13, 49–55. [Google Scholar] [CrossRef]
- Fan, C.; Feng, T.; Wang, X.; Xia, S.; Swing, C.J. Liposomes for encapsulation of liposoluble vitamins (A, D, E and K): Comparation of loading ability, storage stability and bilayer dynamics. Food Res. Int. 2023, 163, 112264. [Google Scholar] [CrossRef]
- Cansell, M.; Moussaoui, N.; Lefrançois, C. Stability of marine lipid based-liposomes under acid conditions. Influence of xanthan gum. J. Liposome Res. 2001, 11, 229–242. [Google Scholar] [CrossRef]
- Fathima, S.J.; Fathima, I.; Abhishek, V.; Khanum, F. Phosphatidylcholine, an edible carrier for nanoencapsulation of unstable thiamine. Food Chem. 2016, 197, 562–570. [Google Scholar] [CrossRef]
- Ahmad, I.; Arsalan, A.; Ali, S.A.; Sheraz, M.A.; Ahmed, S.; Anwar, Z.; Munir, I.; Shah, M.R. Formulation and stabilization of riboflavin in liposomal preparations. J. Photochem. Photobiol. B Biol. 2015, 153, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Ota, A.; Istenič, K.; Skrt, M.; Šegatin, N.; Žnidaršič, N.; Kogej, K.; Ulrih, N.P. Encapsulation of pantothenic acid into liposomes and into alginate or alginate–pectin microparticles loaded with liposomes. J. Food Eng. 2018, 229, 21–31. [Google Scholar] [CrossRef]
- Jiao, Z.; Wang, X.; Yin, Y.; Xia, J.; Mei, Y. Preparation and evaluation of a chitosan-coated antioxidant liposome containing vitamin C and folic acid. J. Microencapsul. 2018, 35, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Marchianò, V.; Matos, M.; Serrano, E.; Álvarez, J.R.; Marcet, I.; Blanco-López, M.C.; Gutiérrez, G. Lyophilised nanovesicles loaded with vitamin B12. J. Mol. Liq. 2022, 365, 120129. [Google Scholar] [CrossRef]
- Bochicchio, S.; Barba, A.A.; Grassi, G.; Lamberti, G. Vitamin delivery: Carriers based on nanoliposomes produced via ultrasonic irradiation. LWT 2016, 69, 9–16. [Google Scholar] [CrossRef]
- Liu, X.; Wang, P.; Zou, Y.-X.; Luo, Z.-G.; Tamer, T.M. Co-encapsulation of vitamin C and β-Carotene in liposomes: Storage stability, antioxidant activity, and in vitro gastrointestinal digestion. Food Res. Int. 2020, 136, 109587. [Google Scholar] [CrossRef] [PubMed]
- Łukawski, M.; Dałek, P.; Borowik, T.; Foryś, A.; Langner, M.; Witkiewicz, W.; Przybyło, M. New oral liposomal vitamin C formulation: properties and bioavailability. J. Liposome Res. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Huang, Z.; Brennan, C.S.; Zhao, H.; Liu, J.; Guan, W.; Mohan, M.S.; Stipkovits, L.; Zheng, H.; Kulasiri, D. Fabrication and assessment of milk phospholipid-complexed antioxidant phytosomes with vitamin C and E: A comparison with liposomes. Food Chem. 2020, 324, 126837. [Google Scholar] [CrossRef]
- Kunthia, A.; Kumar, R.; Premjit, Y.; Mitra, J. Release behavior of vitamin C nanoliposomes from starch–vitamin C active packaging films. J. Food Process Eng. 2022, 45, e14075. [Google Scholar] [CrossRef]
- Farhang, B.; Kakuda, Y.; Corredig, M. Encapsulation of ascorbic acid in liposomes prepared with milk fat globule membrane-derived phospholipids. Dairy Sci. Technol. 2012, 92, 353–366. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Alonso, S.V.; Chiaramoni, N.S. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Liu, H.; Meng, X.; Li, L.; Hu, X.; Fang, Y.; Xia, Y. Synergistic effect on antioxidant activity of vitamin C provided with acidic vesiculation of hybrid fatty acids. J. Funct. Foods 2021, 85, 104647. [Google Scholar] [CrossRef]
- Banville, C.; Vuillemard, J.C.; Lacroix, C. Comparison of different methods for fortifying Cheddar cheese with vitamin D. Int. Dairy J. 2000, 10, 375–382. [Google Scholar] [CrossRef]
- Dałek, P.; Drabik, D.; Wołczańska, H.; Foryś, A.; Jagas, M.; Jędruchniewicz, N.; Przybyło, M.; Witkiewicz, W.; Langner, M. Bioavailability by design — Vitamin D3 liposomal delivery vehicles. Nanomed.: Nanotechnol. Biol. Med. 2022, 43, 102552. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhang, J.; Jin, R.; Cheng, R.; Wang, X.; Yuan, C.; Gan, C. Physicochemical properties, antioxidant activities and in vitro sustained release behaviour of co-encapsulated liposomes as vehicle for vitamin E and β-carotene. J. Sci. Food Agric. 2022, 102, 5759–5767. [Google Scholar] [CrossRef]
- Jiao, Z.; Han, S.; Wang, W.; Song, J.; Cheng, J. Preparation and optimization of Vitamin E acetate liposomes using a modified RESS process combined with response surface methodology. Part. Sci. Technol. 2020, 38, 863–875. [Google Scholar] [CrossRef]
- Amiri, S.; Ghanbarzadeh, B.; Hamishehkar, H.; Hosein, M.; Babazadeh, A.; Adun, P. Vitamin E loaded nanoliposomes: effects of gammaoryzanol, polyethylene glycol and lauric acid on physicochemical properties. Colloids Interface Sci. Commun. 2018, 26, 1–6. [Google Scholar] [CrossRef]
- Ma, Q.H.; Kuang, Y.Z.; Hao, X.Z.; Gu, N. Preparation and characterization of tea polyphenols and vitamin E loaded nanoscale complex liposome. J. Nanosci. Nanotechnol. 2009, 9, 1379–1383. [Google Scholar] [CrossRef]
- Souri, J.; Almasi, H.; Hamishehkar, H.; Amjadi, S. Sodium caseinate-coated and β-cyclodextrin/vitamin E inclusion complex-loaded nanoliposomes: A novel stabilized nanocarrier. LWT 2021, 151, 112174. [Google Scholar] [CrossRef]
- Xia, S.; Tan, C.; Xue, J.; Lou, X.; Zhang, X.; Feng, B. Chitosan/tripolyphosphate-nanoliposomes core-shell nanocomplexes as vitamin E carriers: shelf-life and thermal properties. Int. J. Food Sci. 2013, 49, 1367–1374. [Google Scholar] [CrossRef]
- Samadi, N.; Azar, P.A.; Husain, S.W.; Maibach, H.I.; Nafisi, S. Experimental design in formulation optimization of vitamin K1 oxide-loaded nanoliposomes for skin delivery. Int. J. Pharm. 2020, 579, 119136. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-H. Y.; Baianu, I.C. Novel liposome microencapsulation techniques for food applications. Trends Food Sci. Technol. 1991, 2, 55–61. [Google Scholar] [CrossRef]
- Marsanasco, M.; Márquez, A.L.; Wagner, J.R.; Chiaramoni, N.S.; Alonso, S.V. Bioactive compounds as functional food ingredients: characterization in model system and sensory evaluation in chocolate milk. J. Food Eng. 2015, 166, 55–63. [Google Scholar] [CrossRef]
- Wechtersbach, L.; Ulrih, N.P.; Cigić, B. Liposomal stabilization of ascorbic acid in model systems and in food matrices. LWT 2012, 45, 43–49. [Google Scholar] [CrossRef]
- Mohammadi, M.; Ghanbarzadeh, B.; Hamishehkar, H. Formulation of nanoliposomal vitamin D3 for potential application in beverage fortification. Adv. Pharm. Bull. 2014, 4, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Didar, Z. Enrichment of dark chocolate with vitamin D3 (free or liposome) and assessment quality parameters. J. Food Sci. Technol. 58, 3065–3072. [CrossRef] [PubMed]
- Didar, Z. Inclusion of vitamin D3 (free or liposome) into white chocolate and an investigation of its stability during storage. J. Food Process. Preserv. 45, e15231. [CrossRef]
- Chaves, M.A.; Franckin, V.; Sinigaglia-Coimbra, R.; Pinho, S.C. Nanoliposomes coencapsulating curcumin and vitamin D3 produced by hydration of proliposomes: Effects of the phospholipid composition in the physicochemical characteristics of vesicles and after incorporation in yoghurts. Int. J. Dairy Technol. 2021, 74, 107–117. [Google Scholar] [CrossRef]
- Liu, W.; Tian, M.; Kong, Y.; Lu, J.; Li, N.; Han, J. Multilayered vitamin C nanoliposomes by self-assembly of alginate and chitosan: Long-term stability and feasibility application in mandarin juice. LWT 2017, 75, 608–615. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Malheiros, P.S.; Sant’Anna, V.; Barbosa, M.S.; Brandelli, A.; Franco, B.D.G.M. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Polo, J.; Silva-Weiss, A.; Zamorano, M.; Osorio, F.A. Humectability and physical properties of hydroxypropyl methylcellulose coatings with liposome-cellulose nanofibers: Food application. Carbohydr. Polym. 2020, 231, 115702. [Google Scholar] [CrossRef] [PubMed]
- Toniazzo, T.; Galeskas, H.; Dacanal, G.C.; Pinho, S.C. Production of cornstarch granules enriched with quercetin liposomes by aggregation of particulate binary mixtures using high shear process. J. Food Sci. 2017, 82, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the stability of betanin by liposomal nanocarriers: Its application in gummy candy as a food model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Tao, J.; Liu, X.; Wen, Z. Baicalin-liposomes loaded polyvinyl alcohol-chitosan electrospinning nanofibrous films: Characterization, antibacterial properties and preservation effects on mushrooms. Food Chem. 2022, 371, 131372. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Bai, M.; Chen, X.; Hu, W.; Cui, H.; Lin, L. Controlled release and antibacterial activity of nanofibers loaded with basil essential oil-encapsulated cationic liposomes against Listeria monocytogenes. Food Biosci. 2022, 46, 101578. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Thys, R.C.S.; Brandelli, A. Antifungal properties of phosphatidylcholine-oleic acid liposomes encapsulating garlic against environmental fungal in wheat bread. Int. J. Food. Microbiol. 2019, 293, 72–78. [Google Scholar] [CrossRef]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef]
- US Food and Drug Administration (FDA), Cosmeceutical, https://www.fda.gov/cosmetics/labeling/claims/ucm127064.htm, 2018.
- Dubey, S.K.; Dey, A.; Singhvi, G.; Pandey, M.M.; Singh, V.; Kesharwani, *!!! REPLACE !!!*. Emerging trends of nanotechnology in advanced cosmetics. Colloids Surf. B 2022, 214, 112440. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of phenolic compounds with liposomal improvement in the cosmetic industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems – the current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dreier, J.; Sørensen, J.A.; Brewer, J.R. Superresolution and fluorescence dynamics evidence reveal that intact liposomes do not cross the human skin barrier. PLoS One 2016, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Touti, R.; Noun, M.; Guimberteau, F.; Lecomte, S.; Faure, C. What is the fate of multilamellar liposomes of controlled size, charge and elasticity in artificial and animal skin? Eur. J. Pharm. Biopharm. 2020, 151, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.U.; Lee, S.W.; Pangeni, R.; Byun, Y.; Yoon, I.S.; Park, J.W. Preparation and in vivo evaluation of cationic elastic liposomes comprising highly skin-permeable growth factors combined with hyaluronic acid for enhanced diabetic wound-healing therapy. Acta Biomater. 2017, 57, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Manconi, M.; Fadda, A.M.; Lai, F.; Lampis, S.; Diez0Sales, O.; Sinico, C. Nanocarriers for antioxidant resveratrol: Formulation approach, vesicle self-assembly and stability evaluation. Colloids Surf. B 2013, 111, 327–332. [Google Scholar] [CrossRef]
- Nguyen, S.; Alund, S.J.; Hiorth, M.; Kjøniksen, A.-L.; Smistad, G. Studies on pectin coating of liposomes for drug delivery. Colloids Surf. B 2011, 88, 664–673. [Google Scholar] [CrossRef]
- Nadaf, S.J.; Killedar, S.G. Ultradeformable liposomal nanostructures: Role in transdermal delivery of therapeutics. In Nanoscale Processing; Thomas, S., Balakrishnan, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 492–516. [Google Scholar] [CrossRef]
- Ahmadi Ashtiani, H. R.; Bishe, P.; Lashgari, N.; Nilforoushzadeh, M. A.; Zare, S. Liposomes in Cosmetics. J. Skin Stem. Cell. 2016, 3, e65815. [Google Scholar] [CrossRef]
- Shaw, T.K.; Paul, P.; Chatterjee, B. Research-based findings on scope of liposome-based cosmeceuticals: an updated review. Futur. J. Pharm. Sci. 2022, 8, 46. [Google Scholar] [CrossRef]
| Vitamin | Chemical structure | Way of obtainment | Sources |
|---|---|---|---|
| A (retinol) |  |
Intake of dietary sources or vitamin supplements | Fish, meat, eggs, whole milk, carrots, spinach, and mango |
| D3 (cholecalciferol) | 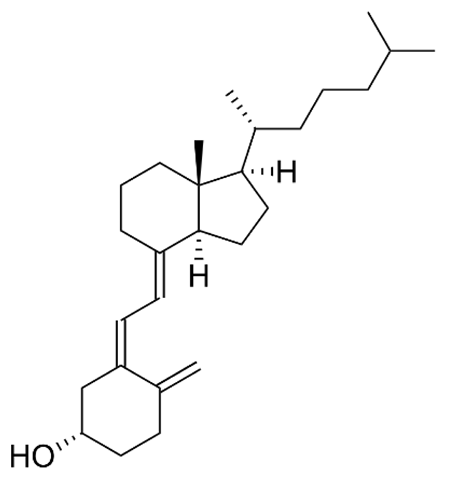 |
Synthesized by the human epidermis or consumed in the form of supplements or fortified foods | Fish, as salmon and sardines, butter and eggs |
| E (α-tocopherol) | 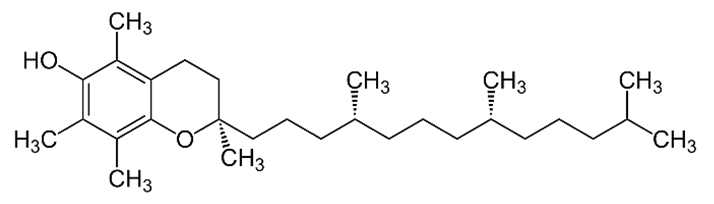 |
Intake of dietary sources or vitamin supplements | Vegetable oils of peanut, soya, palm, corn, safflower, sunflower, and wheat germ |
| K1 (phylloquinone) | 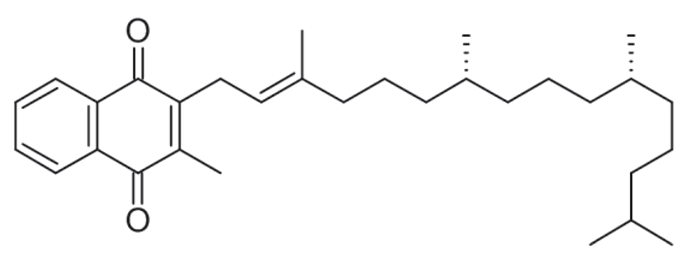 |
Plants | Green leafy vegetables, liver, lean meat, cow's milk, egg yolks, and whole wheat products |
| K2 (menaquinone) | 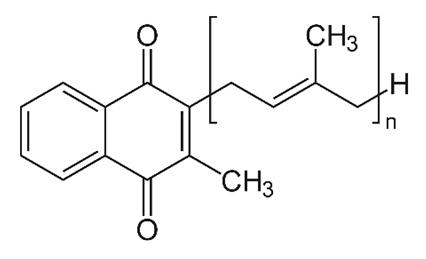 |
Synthesized by bacteria in the human and animal intestines | - |
| K3 (menadione) |  |
Converted to K2 in the intestinal tract | - |
| B1 (thiamine) |  |
Intake of dietary sources or vitamin supplements | Brewer's dried yeast and pork, lamb and beef, poultry, whole grains, nuts, vegetables, and legumes |
| B2 (riboflavin) | 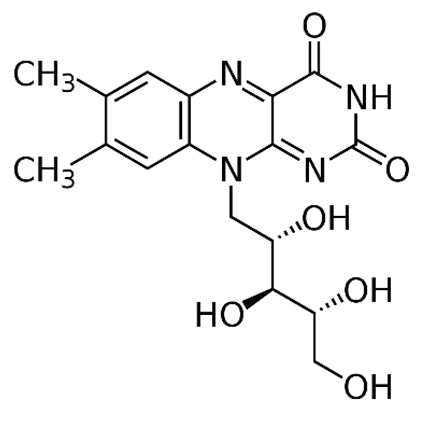 |
Plants and animal cells | Milk and milk products, meat, eggs, and leafy green vegetables |
| B3 (niacin) | 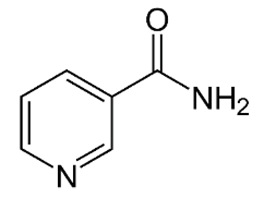 |
Intake of dietary sources or vitamin supplements | Yeast, liver, poultry, lean meats, nuts, and legumes |
| B5 (pantothenic acid) | 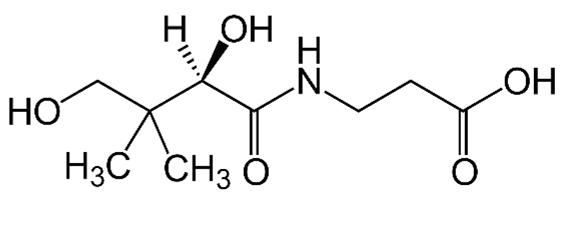 |
Intake of dietary sources or cosmetic products | Organ of animals, eggs, milk, vegetables, legumes, and whole-grain cereals |
| B12 (cobalamin) | 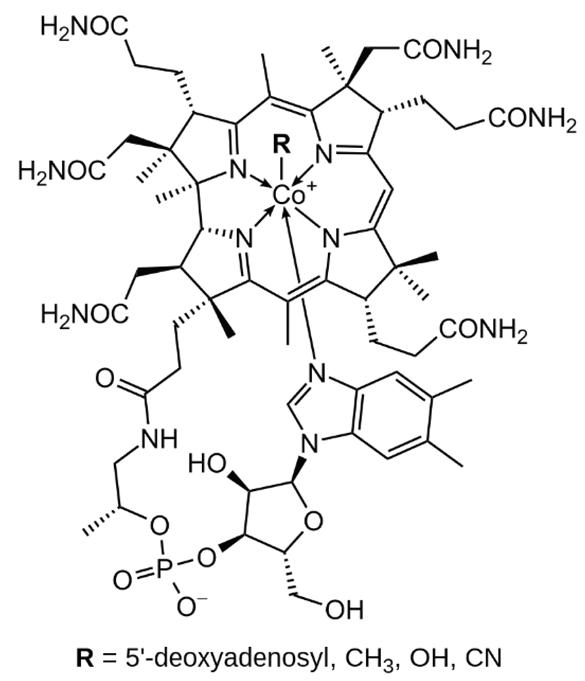 |
Intake of dietary sources or vitamin supplements | Milk, and dairy products, eggs, salmon |
| C (ascorbic acid) | 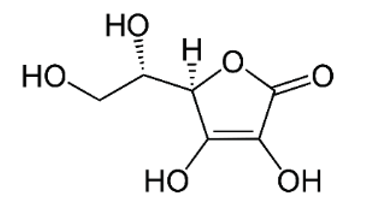 |
Intake of dietary sources or vitamin supplements | Citric fruits, currants, peppers, parsley, cauliflower, potatoes, sweet potatoes, broccoli, brussels sprouts, strawberries, guava, and mango |
| Type of liposome | Abbreviation | Characteristic | Diameter | Schematic representation |
|---|---|---|---|---|
| Small unilamellar vesicles | SUV | Small unilamellar vesicles | 20 to 200 nm | 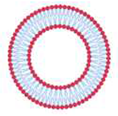 |
| Large unilamellar vesicles | LUV | Unilamellar liposomes with average diameters higher than SUV | Above 200 nm |  |
| Giant unilamellar vesicles | GUV | Unilamellar liposomes with average diameters higher than LUV | Higher than 1 µm | 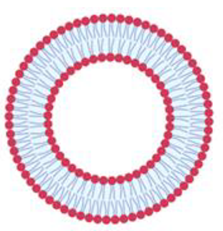 |
| Multilamellar vesicles | MLV | Several concentrically arranged vesicles | 0.5 to 5 µm | 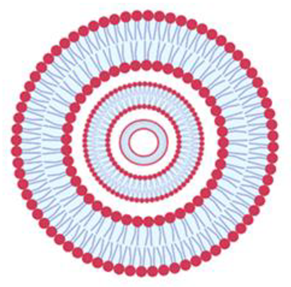 |
| Multivesicular vesicles | MVV | Smaller vesicles within a vesicle of large size | Higher than 1 µm | 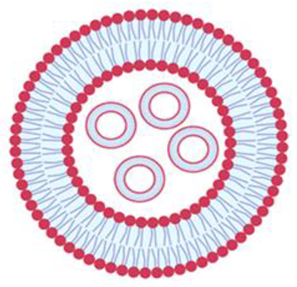 |
| Vitamin | Liposome type/size | Chemical used to demulsify/precipitate the liposomes | Method for vitamin quantification | EE% | Reference |
|---|---|---|---|---|---|
| Vitamin A | SUV | Methanol | HPLC | 50.6-56.2% | [134] |
| Vitamin A | MLV | Chloroform /Methanol 2:1 v/v | UV-Vis spectrophotometry | 99% | [135] |
| Vitamin A | MLV/SUV | Chloroform | HPLC | 15.8% | [136] |
| Vitamins A, D, E, and K | n.s. | Tween 80 | HPLC | 20-100% | [137] |
| Vitamin B1 | MLV | n-octyl β-D-glucopyranoside | Optical density | 31.2% | [138] |
| Vitamin B1 | n.s. | PBS | HPLC | 97% | [139] |
| Vitamin B2 | n.s. | Triton X-100 | Indirect method (photolysis) | 42.3% | [140] |
| Vitamin B5 | n.s. | Chloroform/Methanol | Direct method (mass weight) | 75% | [141] |
| Vitamin B9 | n.s. | Ethanol | Ultrafiltration in centrifuge tube followed by HPLC | 87.4% | [142] |
| Vitamin B12 | MLV | Methanol/Water 1:10 v/v | HPLC | 70% | [143] |
| Vitamin B12 | LUV | - | Centrifugation followed by UV-Vis spectroscopy | 27% | [33] |
| Vitamins B12, D2 and E | SUV | Ethanol | UV-Vis spectrophotometry | 56-76% | [144] |
| Vitamin C | n.s. | Ethanol | Dialysis followed by UV-vis spectrophotometry | 77.9% | [145] |
| Vitamin C | n.s. | Methanol, chloroform, and ammonium buffer | HPLC | 99.2% | [146] |
| Vitamin C | n.s. | Ethanol | HPLC | 75.4% | [108] |
| Vitamins C and E | n.s. | Triton X-100 / DMSO | UV-Vis spectrophotometry | 93-95% | [83] |
| Vitamins C and E | n.s. | Assay kit BC1235 | Indirect method | 98.5% | [147] |
| Vitamin C | SUV/LUV | Meta-phosphoric acid | Titration with indophenol solution | 94.2% | [148] |
| Vitamin C | SUV/MLV | - | Gel permeation chromatography | 5-16% | [149] |
| Vitamins C and E | MLV/MVV | Protamine sulfate solution followed by Triton X-100 | Protamine aggregation method followed by UV-Vis spectrophotometry | 12-88% | [111] |
| Vitamins C and E | MLV | Acetic acid and protamine solution | UV-Vis spectrophotometry | 38% | [150] |
| Vitamin C | n.s. | - | Dialysis followed by UV-Vis spectrophotometry | 100% | [151] |
| Vitamin D | MLV | Triton X-100 | Ultracentrifugation followed by HPLC | 61.5% | [152] |
| Vitamin D3 | n.s. | Methanol | Ultrafiltration in centrifuge tube followed by HPLC | 74% | [127] |
| Vitamin D3 | SUV/LUV | Methanol | HPLC | 90.2% | [72] |
| Vitamin D3 | SUV/MLV | Water | Ultrafiltration in centrifuge tube followed by RP-HPLC | 100% | [153] |
| Vitamins D3 and K2 | SUV/LUV | Ethanol | UV-Vis spectrophotometry | 98% | [86] |
| Vitamin E | n.s. | Methanol | Dialysis followed by HPLC | 92.5% | [154] |
| Vitamin E | n.s. | Ethanol | UV-Vis spectrophotometry | 98.6% | [155] |
| Vitamin E | n.s. | Chloroform/Methanol 2:1 v/v | UV-Vis spectrophotometry | 78% | [156] |
| Vitamin E | n.s. | Water | Centrifugation followed by HPLC | 94% | [157] |
| Vitamin E | n.s. | - | Centrifugation followed by UV-Vis spectrophotometry | 83.8% | [158] |
| Vitamin E | n.s. | Chloride acid, Tween 80:ethanol and n-pentane | UV-Vis spectrophotometry | 97% | [159] |
| Vitamin K1 | n.s. | - | Ultracentrifugation followed by HPLC | 79.2% | [160] |
| Vitamin K1 | n.s. | Methanol | HPLC | 3.4-154 µg/mg | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





