Submitted:
30 March 2023
Posted:
31 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
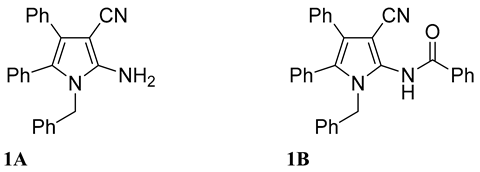
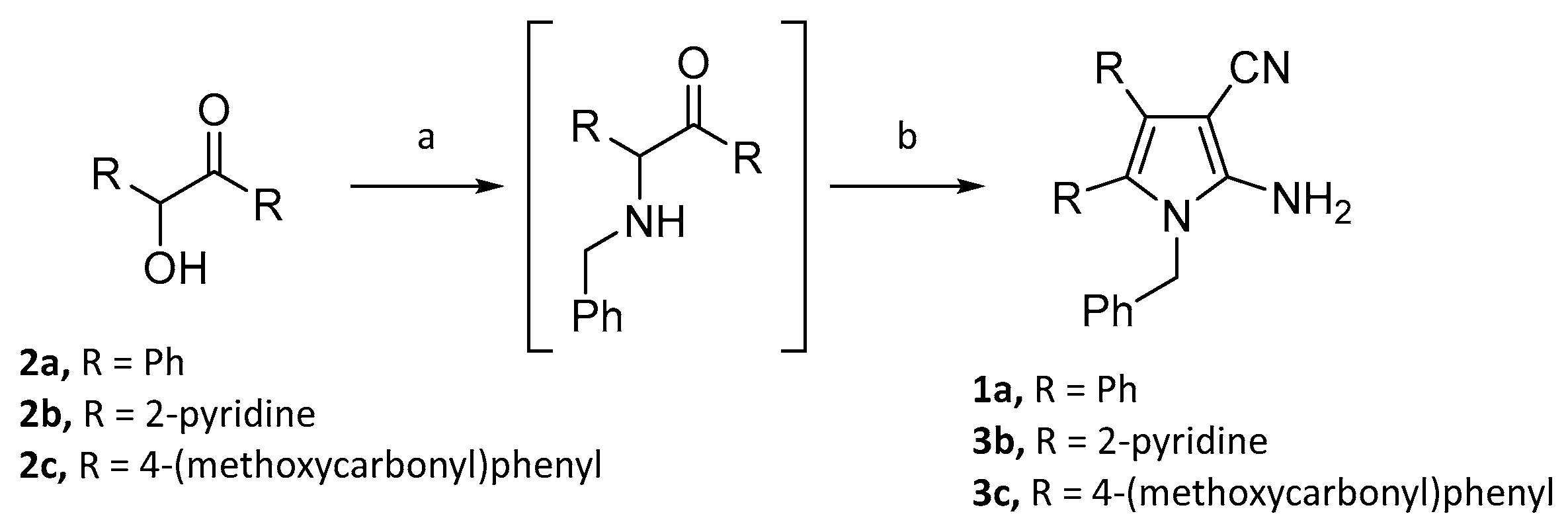
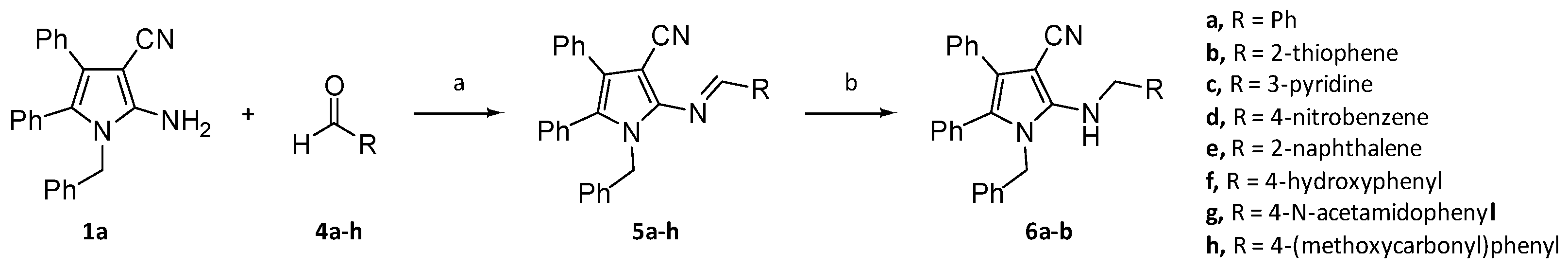
2.1. Syntheses of a series of derivatives of compounds 1a and 1b
2.2. Assessment of the inhibitory effect using kinetic assays
2.3. Checkerboard sensitivity assays
3. Conclusions
4. Materials and methods
4.1. Chemical synthesis for preparation of derivatives of compounds 1a and 1b
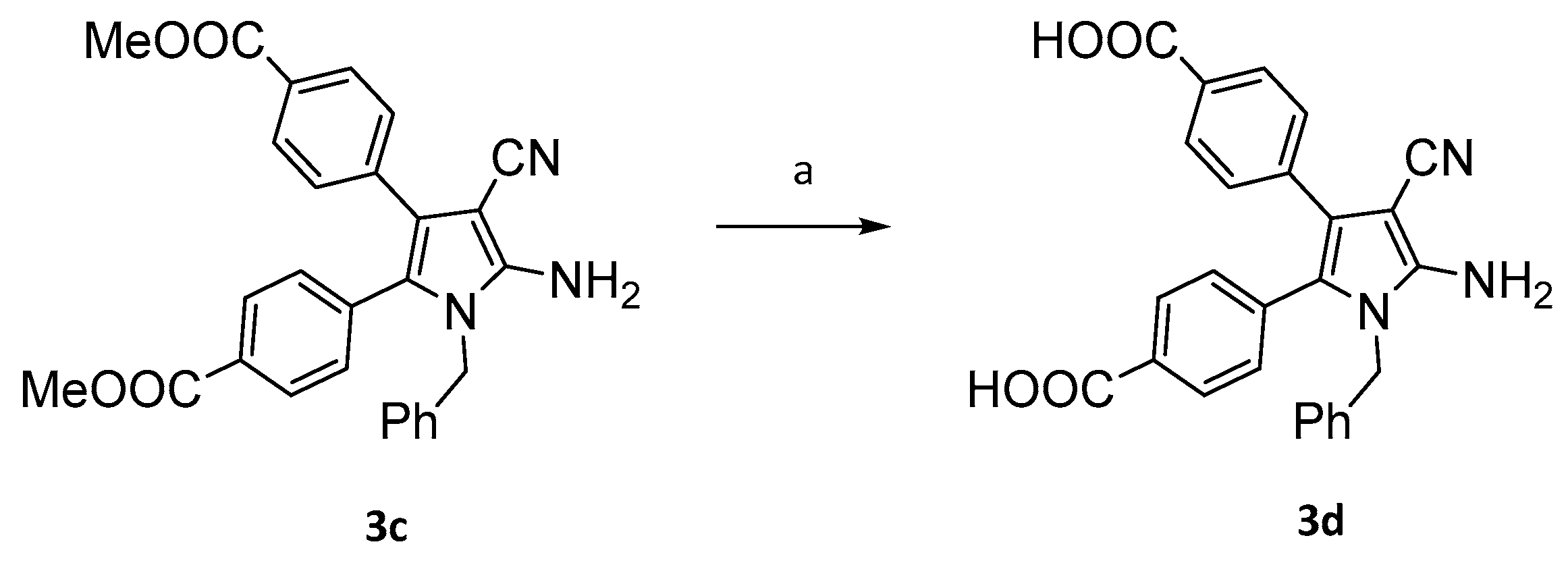
4.2. Synthesis of compounds 3b-3d
4.3. General procedure for the preparation of imine derivatives 5a-5i
4.4. General Procedure for the reduction of imines to amines
4.5. Enzyme activity and inhibition assays
4.6. Checkboard sensitivity test
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Fisher, J.F.; Meroueh, S.O.; Mobashery, S. Bacterial resistance to β-lactam antibiotics: compelling opportunism, compelling opportunity. Chem. Rev. 2005, 105, 395–424. [Google Scholar] [CrossRef]
- Allen, H.K.; Donato, J.; Wang, H.; Cloud-Hansen, K.A.; Davies, J.; Handelsman, J. Call of the wild: antibiotic resistance genes in natural environments. Nat. Rev. Microbiol. 2010. [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010. [CrossRef]
- Hawkey, P.M. Multidrug-resistant Gram-negative bacteria: a product of globalization. J. Hosp. Infect. 2015, 89, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrobial. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Pedroso, M.M.; Waite, D.W.; Melse, O.; Wilson, L.; Miti, N.; McGeary, R.P.; Antes, I.; Guddat, L.W.; Hugenholtz, P.; Schenk, G. Broad spectrum antibiotic-degrading metallo-β-lactamases are phylogenetically diverse. Protein Cell. 2020, 11, 613–617. [Google Scholar] [CrossRef]
- Khan, A.U.; Maryam, L.; Zarrilli, R. Structure, Genetics and Worldwide Spread of New Delhi Metallo-β-lactamase (NDM): a threat to public health. BMC Microbiol. 2017, 17, 101. [Google Scholar] [CrossRef]
- Moellering, R.C. NDM-1 — A Cause for Worldwide Concern. N. Eng. J. Med. 2010, 363, 2377–2379. [Google Scholar] [CrossRef]
- Sun, Z.; Mehta, S.C.; Adamski, C.J.; Gibbs, R.A.; Palzkill, T. Deep Sequencing of Random Mutant Libraries Reveals the Active Site of the Narrow Specificity CphA Metallo-β-Lactamase is Fragile to Mutations. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Miraula, M.; Schenk, G.; Mitić, N. Promiscuous metallo-β-lactamases: MIM-1 and MIM-2 may play an essential role in quorum sensing networks. J. Inorg. Biochem. 2016, 162, 366–375. [Google Scholar] [CrossRef]
- Miraula, M.; Whitaker, J.J.; Schenk, G.; Mitić, N. β-Lactam antibiotic-degrading enzymes from non-pathogenic marine organisms: a potential threat to human health. J. Inorg. Biochem. 2015, 20, 639–651. [Google Scholar] [CrossRef] [PubMed]
- Selleck, C.L., J. L., Harmer, J.; Guddat, L. W.; Mitić, N.; Helweh, W.; Ollis, D. L.; Craig, W. A.; Tierney, D. L.; Pedroso, M. M.; Schenk, G. AIM-1: An Antibiotic-Degrading Metallohydrolase That Displays Mechanistic Flexibility. Chem. Eur. J. 2016, 22, 17704–17714. [CrossRef] [PubMed]
- Pedroso, M.M.; Selleck, C.; Enculescu, C.; Harmer, J.R.; Mitić, N.; Craig, W.R.; Helweh, W.; Hugenholtz, P.; Tyson, G.W.; Tierney, D.L.; et al. Characterization of a highly efficient antibiotic-degrading metallo-β-lactamase obtained from an uncultured member of a permafrost community. Metallomics 2017. [CrossRef] [PubMed]
- Wilson, L.A.; Knaven, E.G.; Morris, M.T.; Pedroso, M.M.; Schofield, C.J.; Brück, T.; Moden, M.; Waite, D.W.; Hugenholtz, P.; Guddat, L.W.; Schenk, G. SIE-1 from Sphingobium indicum, the first example of a B3 metallo-β-lactamase with an active site glutamic acid ligand. Antimicrob. Agents. Chemther. 2021, 65, 100015. [Google Scholar] [CrossRef]
- Bahr, G.; González, L.J.; Vila, A.J. Metallo-β-lactamases in the Age of Multidrug Resistance: From Structure and Mechanism to Evolution, Dissemination, and Inhibitor Design. Chem. Rev. 2021. [CrossRef] [PubMed]
- McGeary, R.P..; Schenk, G.; Guddat, L.W. The Applications of Binuclear Metallohydrolases in Medicine: Recent Advances in the Design and Development of Novel Drug Leads for Purple Acid Phosphatases, Metallo-β-Lactamases and Arginases. Eur. J. Med. Chem. 2014, 76, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Brem, J.; Van Berkel, S.S.; Zollman, D.; Lee, S.Y.; Gileadi, O.; McHugh, P.J.; Walsh, T.R.; McDonough, M.A.; Schofield, C.J. Structural basis of metallo-β-lactamase inhibition by captopril stereoisomers. Antimicrob. Agents Chemother. 2016, 60, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.M.; Fatahala, S.S.; Mohamed, Z.M.; McGeary, R.P.; Schenk, G.; Ollis, D.L.; Mohamed, M.S. Synthesis and Kinetic Testing of Tetrahydropyrimidine-2-thione and Pyrrole Derivatives as Inhibitors of the Metallo-β-lactamase from Klebsiella pneumonia and Pseudomonas aeruginosa. Chem. Biol. Drug. Des. 2012, 80, 500–515. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Hussein, W.M.; McGeary, R.P.; Vella, P.; Schenk, G.; Abd El-hameed, R.H. Synthesis and kinetic testing of new inhibitors for a metallo-β-lactamase from Klebsiella pneumonia and Pseudomonas aeruginosa. Eur. J. Med. Chem. 2011, 46, 6075–6082. [Google Scholar] [CrossRef]
- McGeary, R.P.; Tan, D.T.C.; Selleck, C.; Monteiro Pedroso, M.; Sidjabat, H.E.; Schenk, G. Structure-activity relationship study and optimisation of 2-aminopyrrole-1-benzyl-4,5-diphenyl-1H-pyrrole-3-carbonitrile as a broad spectrum metallo-β-lactamase inhibitor. Eur. J. Med. Chem. 2017, 137, 351–364. [Google Scholar] [CrossRef]
- Spellberg, B. The future of antibiotics. Crit. Care 2014, 18, 228–228. [Google Scholar] [CrossRef]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J. The Epidemic of Antibiotic-Resistant Infections: A Call to Action for the Medical Community from the Infectious Diseases Society of America. Clin. Infec. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1–1. [Google Scholar] [CrossRef]
- Brennan-Krohn, T.; Kirby, J.E. Antimicrobial Synergy Testing by the Inkjet Printer-assisted Automated Checkerboard Array and the Manual Time-kill Method. J. Visual. Exp. 2019. [CrossRef]
- Mitic, N.; Miraula, M.; Selleck, C.; Hadler, K.S.; Uribe, E.; Pedroso, M.M.; Schenk, G. Catalytic mechanisms of metallohydrolases containing two metal ions. Adv. Protein Chem. Struct. Biol. 2014, 97, 49–81. [Google Scholar] [CrossRef]
- McGeary, R.P.; Tan, D.T.; Schenk, G. Progress toward inhibitors of metallo-β-lactamases. Future Med. Chem. 2017, 9, 673–691. [Google Scholar] [CrossRef]
- Arjomandi, O.K.; Hussein, W.M.; Vella, P.; Yusof, Y.; Sidjabat, H.E.; Schenk, G.; McGeary, R.P. Design, synthesis, and in vitro and biological evaluation of potent amino acid-derived thiol inhibitors of the metallo-β-lactamase IMP-1. Eur. J. Med. Chem. 2016, 114, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Segel, I.H. Enzyme kinetics: Behavior and analysis of rapid equilibrium and steady-state enzyme systems; John Wiley & Sons: United States of America, 1993; p. 985. [Google Scholar]
- Valizadeh, M.; Schenk, G.; Nash, K.; Oddie, G.W.; Guddat, L.W.; Hume, D.A.; de Jersey, J.; Burke, T.R.; Hamilton, S. Phosphotyrosyl Peptides and Analogues as Substrates and Inhibitors of Purple Acid Phosphatases. Arch. Biochem. Biophys. 2004, 424, 154–162. [Google Scholar] [CrossRef] [PubMed]
| Inhibitor | NDM-1 (B1) | AIM-1 (B3) |
|---|---|---|
| 1b | 47 ± 6 | 42 ± 2 |
| 5a | 38 ± 2 | 42 ± 3 |
| 5b | 57 ± 8 | 42 ± 3 |
| 5c | 28 ± 4 | 11 ± 2 |
| 5d | 45 ± 1 | 40 ± 3 |
| 5e | 50 ± 3 | 26 ± 4 |
| 5f | 49 ± 8 | 61 ± 3 |
| 5g | 14 ± 3 | 10 ± 4 |
| 5h | 27 ± 4 | 19 ± 6 |
| 5i | 40 ± 7 | 39 ± 1 |
| 6a | 41 ± 2 | 32 ± 3 |
| 6b | 29 ± 4 | 25 ± 1 |
| COMPOUND | NDM-1 | CPHA | AIM-1 | ||
|---|---|---|---|---|---|
| Kic | Kiuc | IC50 | Kic | Kiuc | |
| 1B | 9 ± 14 | 5 ± 1 | n/d | 11 ± 3.0 | 19 ± 3 |
| 5A | 3.2 ± 1.0 | 6 ± 1 | 75 ± 3 | 3.6 ± 0.5 | 11 ± 3 |
| 6A | - | 2.7 ± 0.3 | n/d | n/d | n/d |
| 5B | 1.7 ± 0.6 | 11 ± 6.3 | 57 ± 2 | 4.5 ± 1.0 | 35 ± 28 |
| 5F | 1.3 ± 0.7 | 0.9 ± 0.1 | 45 ± 2 | 4.4 ± 0.4 | 4.7 ± 1.4 |
| 5H | 34 ± 113 | 4.4 ± 0.8 | n/d | 6.4 ± 1.1 | 72 ± 37 |
| Compounds | NDM-1 | CphA | AIM-1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| PenG | CXM | MEM | PenG | CXM | MEM | PenG | CXM | MEM | |
| 1b | S | S | A | S | S | S | A | A | A |
| 5a | S | S | A | S | S | S | S | S | S |
| 5b | S | S | A | S | S | S | S | A | A |
| 5f | S | S | A | S | S | S | S | S | S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





