Submitted:
28 March 2023
Posted:
28 March 2023
Read the latest preprint version here
Abstract
Keywords:
1.30. years of the amyloid cascade hypothesis
2. Mutations are more likely to destroy function than improve it
3. The current “understanding” of fAD genetics
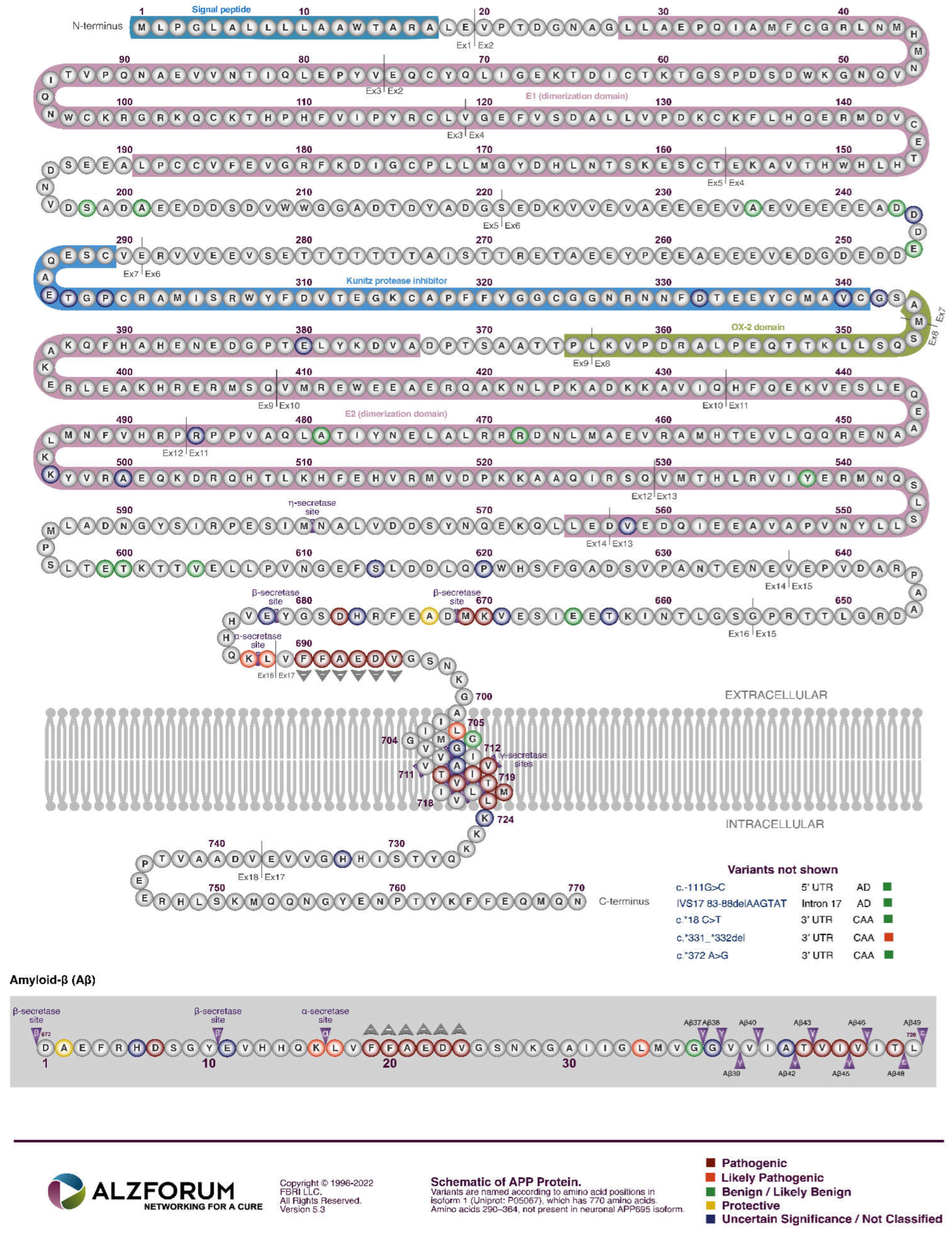
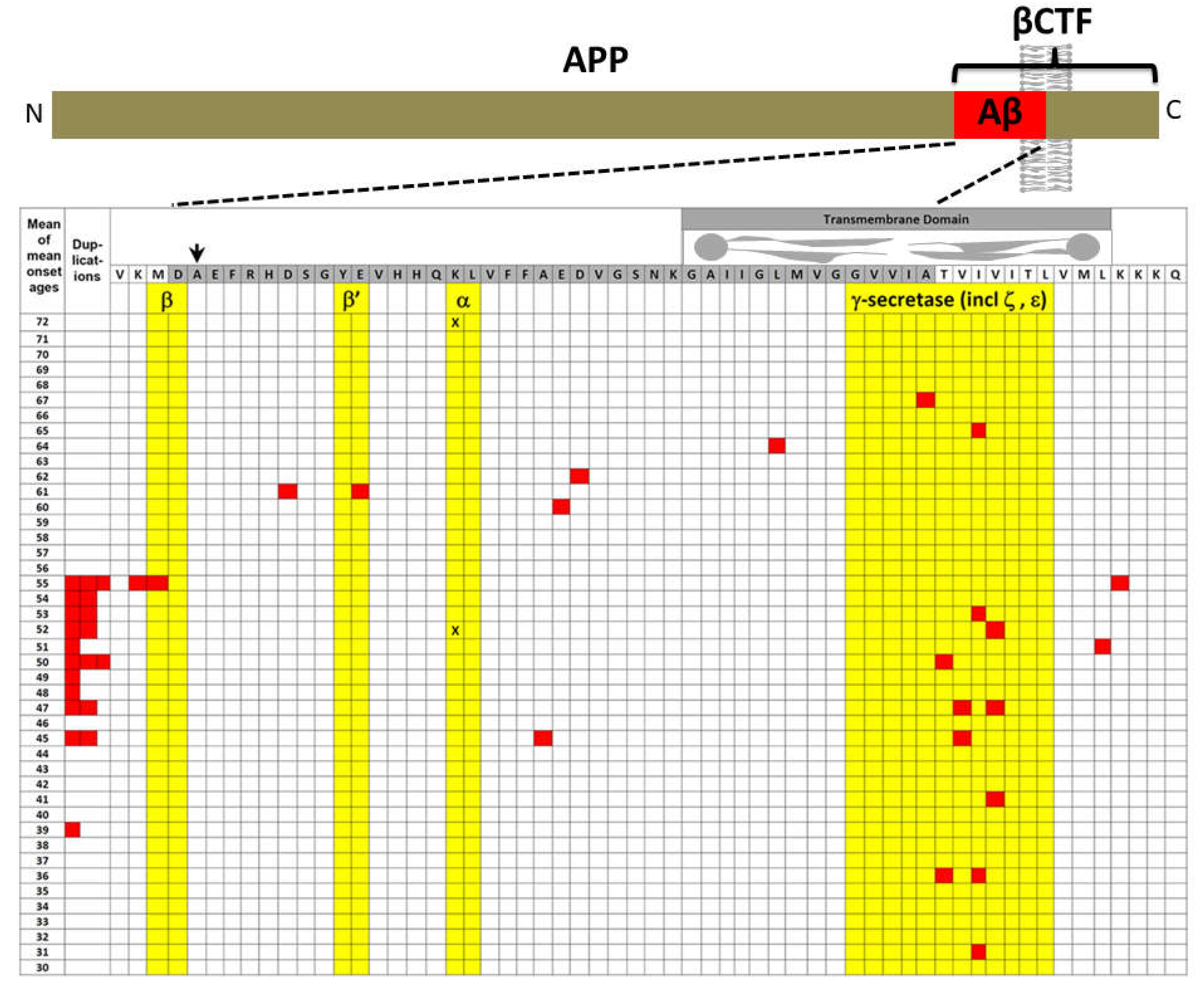
APP
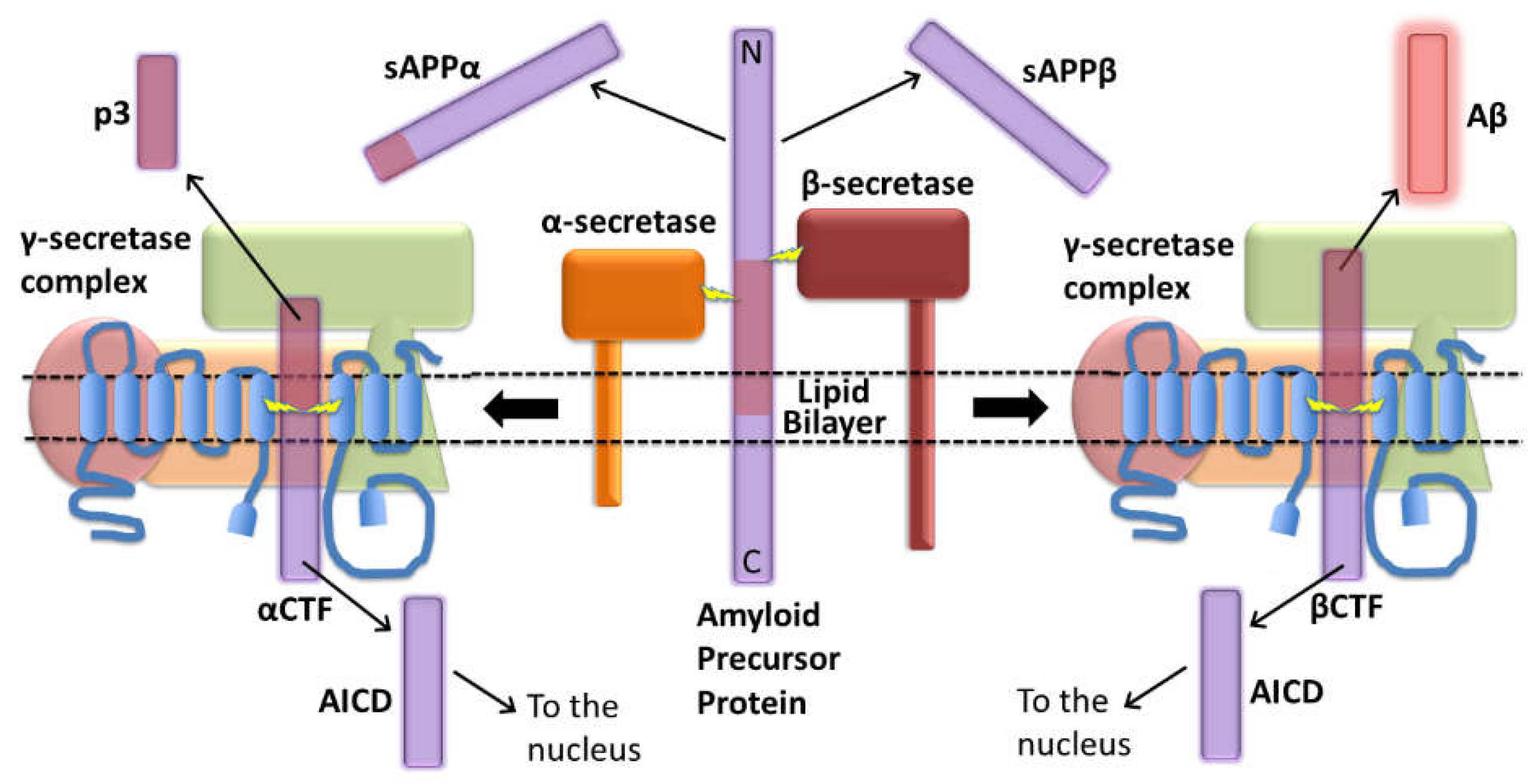
The explanatory power of increased βCTF levels as the AD-causative effect of mutations in APP
3. The original amyloid cascade hypothesis included βCTF as a candidate pathogenic agent
“Our cascade hypothesis states that AβP itself, or APP cleavage products containing
AβP, are neurotoxic and lead to neurofibrillary tangle formation and cell death. Thus, two successive events are needed to produce Alzheimer’s pathology. First, AβP must be generated as an intact entity, either by accumulation of AβP or as an AβP-containing fragment of APP. Second, this molecule must facilitate or cause neuronal death and neurofibrillary tangle formation. Neve and her colleagues have reported that the AβP-containing COOH-terminal fragment is toxic to cultured neurons(18)…
“… The evidence we have described supports the hypothesis that the AβP molecule initiates the pathological cascade of Alzheimer’s disease. AβP-containing COOH-terminal derivatives of APP seem the most likely molecular candidates for initiation of the cascade, with the process presumably taking several decades to produce the full-blown pathology of the disease.”
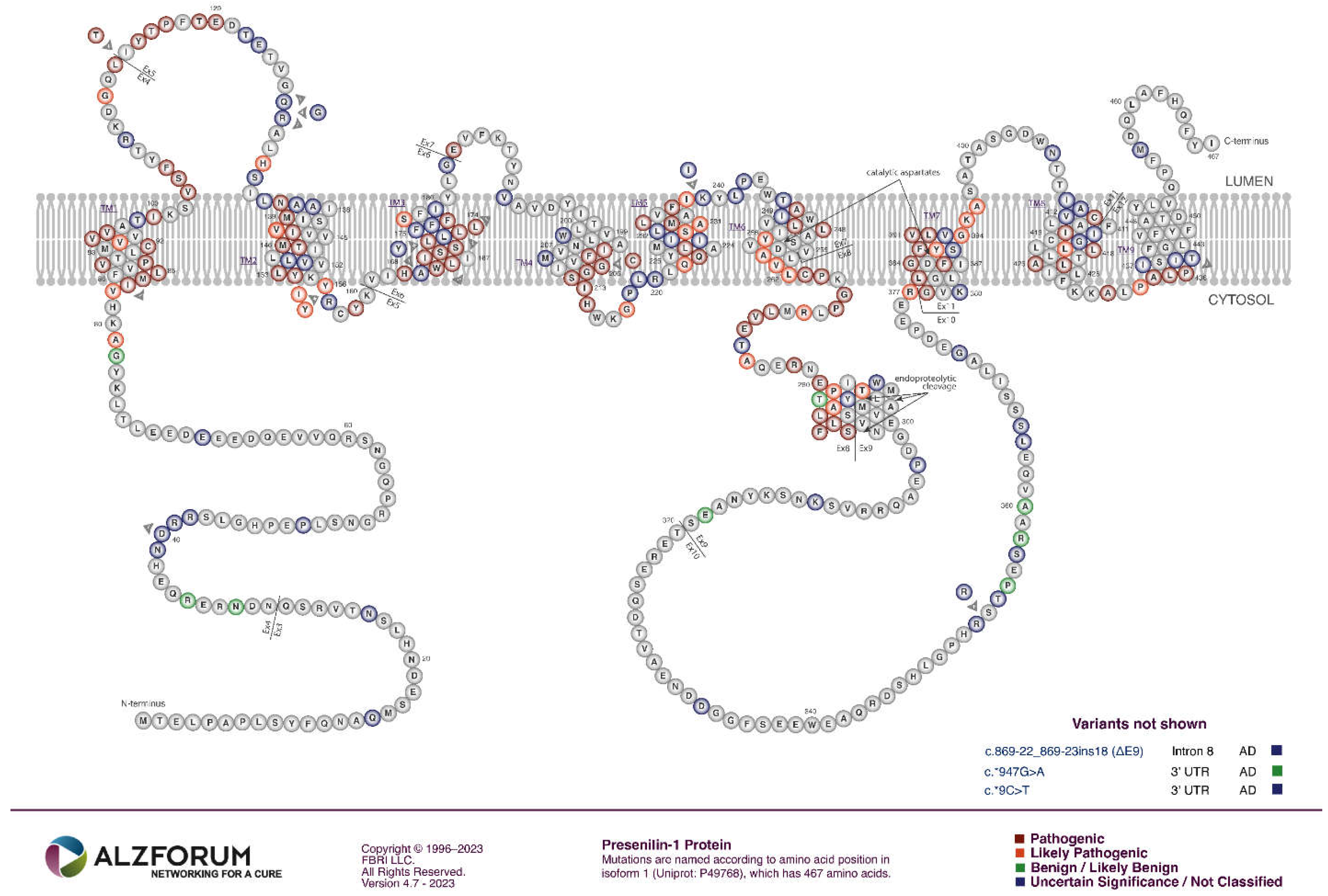
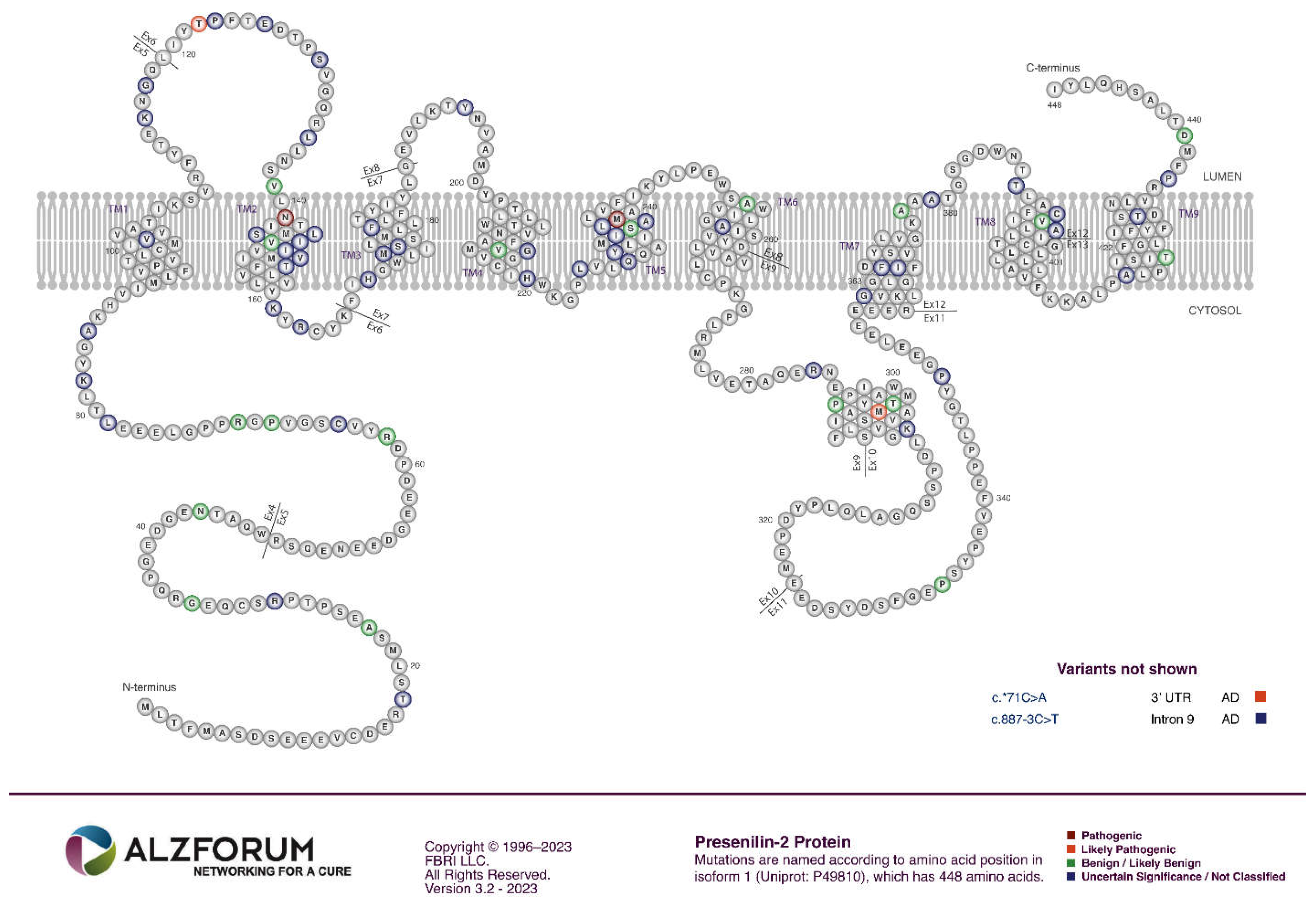
PSEN1 and PSEN2
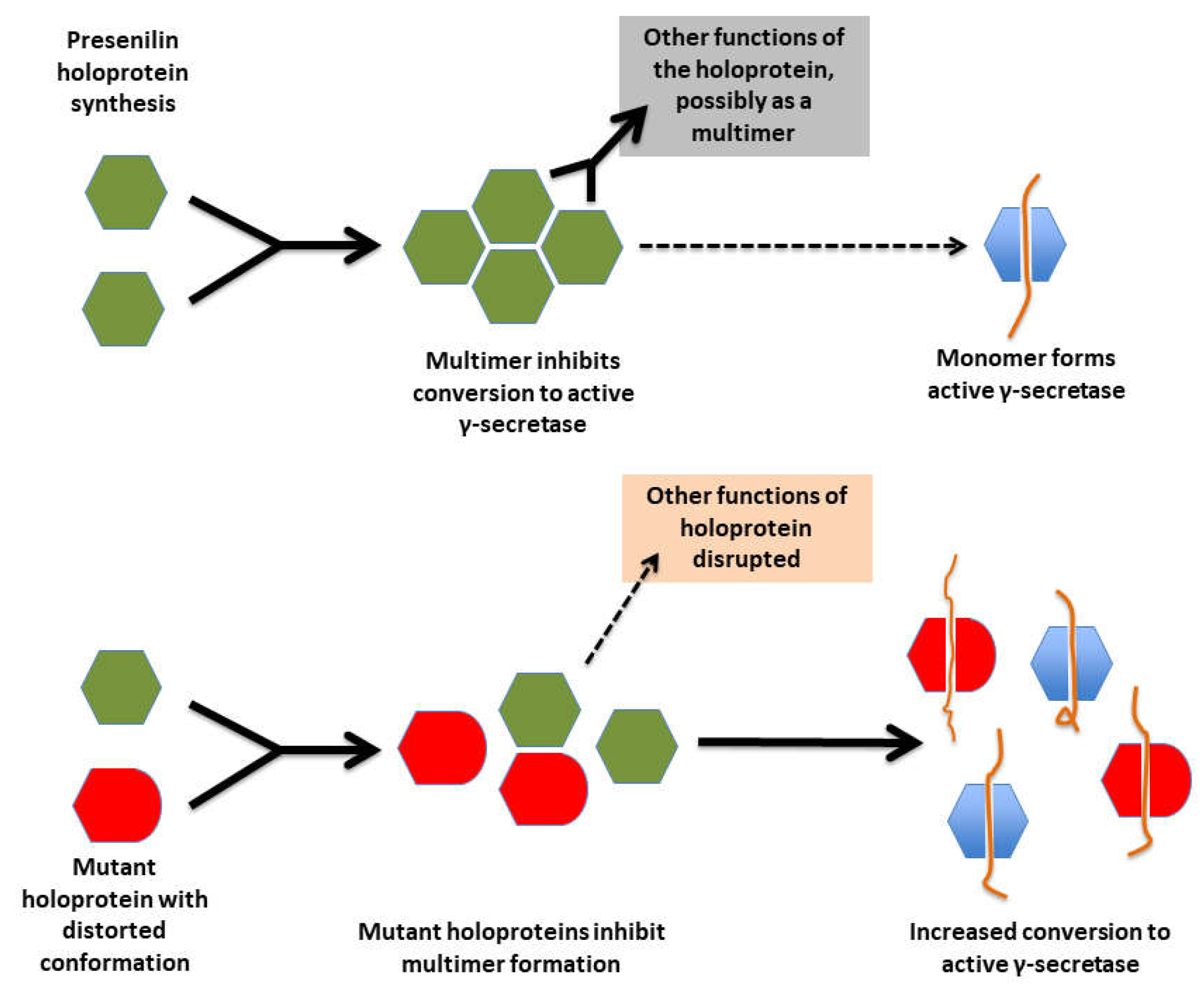
- The great majority of fAD mutations in PSEN1 and PSEN2 are missense mutations altering the protein coding sequence. Even those mutations causing changes in transcript splicing (e.g. PSEN1 L113_I114insT [90]) always produce at least one transcript form that preserves the open reading frame to code for a “full-length” protein. We addressed this issue in a previous review [16], describing it as the reading-frame preservation rule. Consistent with this (with the exception of one unique and important gain-of-function mutation discussed later [91]), no mutations or genetic variants affecting PSEN1 or PSEN2 transcript regulation (without changing protein-coding sequences) have been discovered that increase the risk of AD [92].
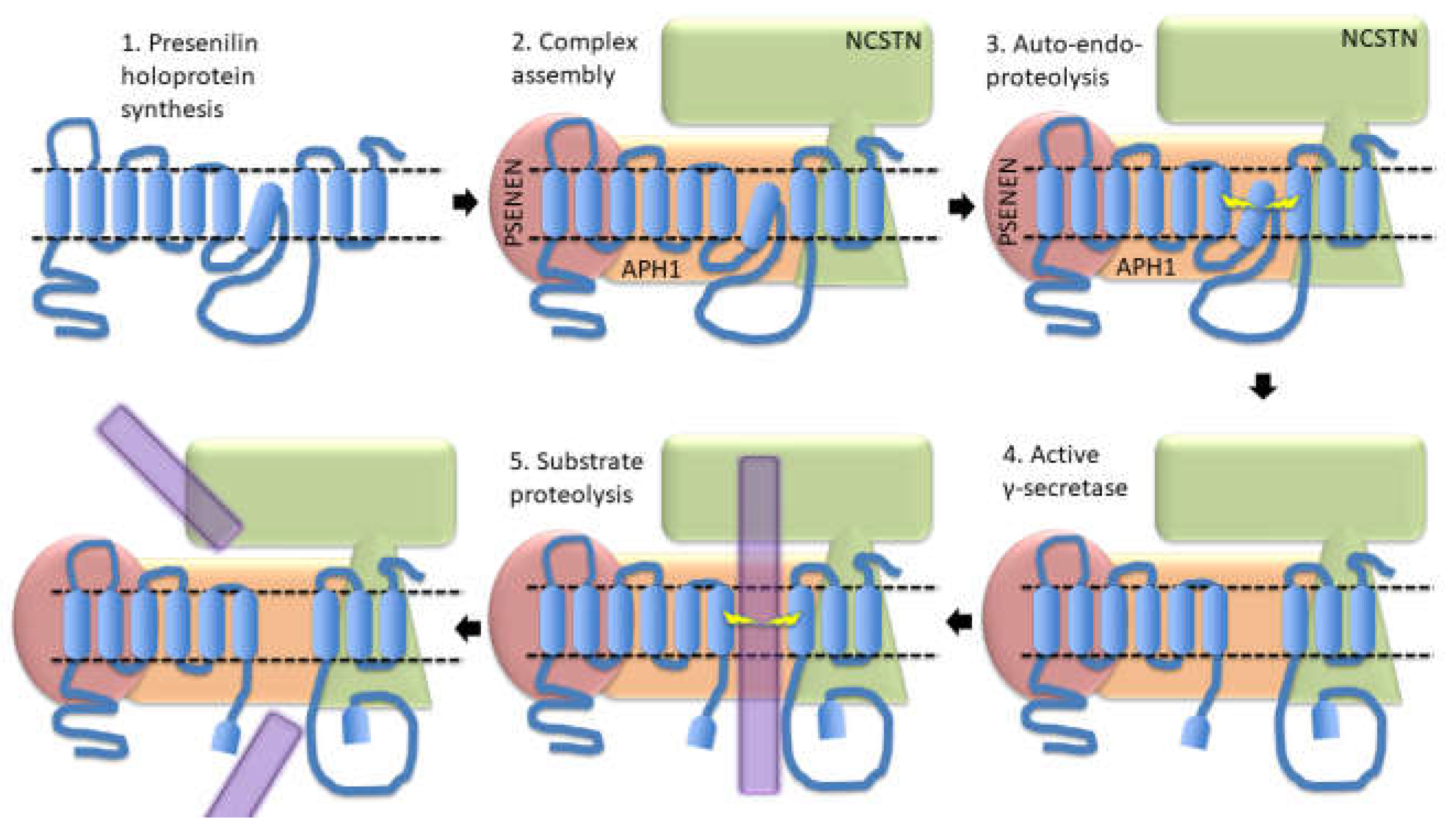
- Loss-of-function mutations that would be expected simply to reduce γ-secretase activity, without otherwise distorting it, do not cause fAD. This is dramatically illustrated by the existence of frameshift mutations in PSEN1 that truncate the open reading frame and do not cause fAD while causing a completely unrelated disease of the skin, Acne Inversa, familial 3, (ACNINV3, also known as hidradenitis suppurativa) [93-95]. Unlike fAD, familial Acne Inversa can also be caused by mutations in genes encoding two other components of the γ-secretase enzyme complex, NICASTRIN (NCSTN), and PRESENILIN ENHANCER, GAMMA-SECRETASE SUBUNIT (PSENEN, formerly known as PEN2) [93] (See also Figure 7). All these mutations affecting different components of the γ-secretase enzyme complex almost certainly act through reduction of cellular γ-secretase activity but mutations causing fAD only occur in PSEN1 and PSEN2 and not in genes encoding other γ-secretase complex components. Therefore, fAD cannot be due to a simple loss of γ-secretase activity. The reading frame preservation rule implies that a gain-of-function mechanism is involved.
Presenilin Holoproteins
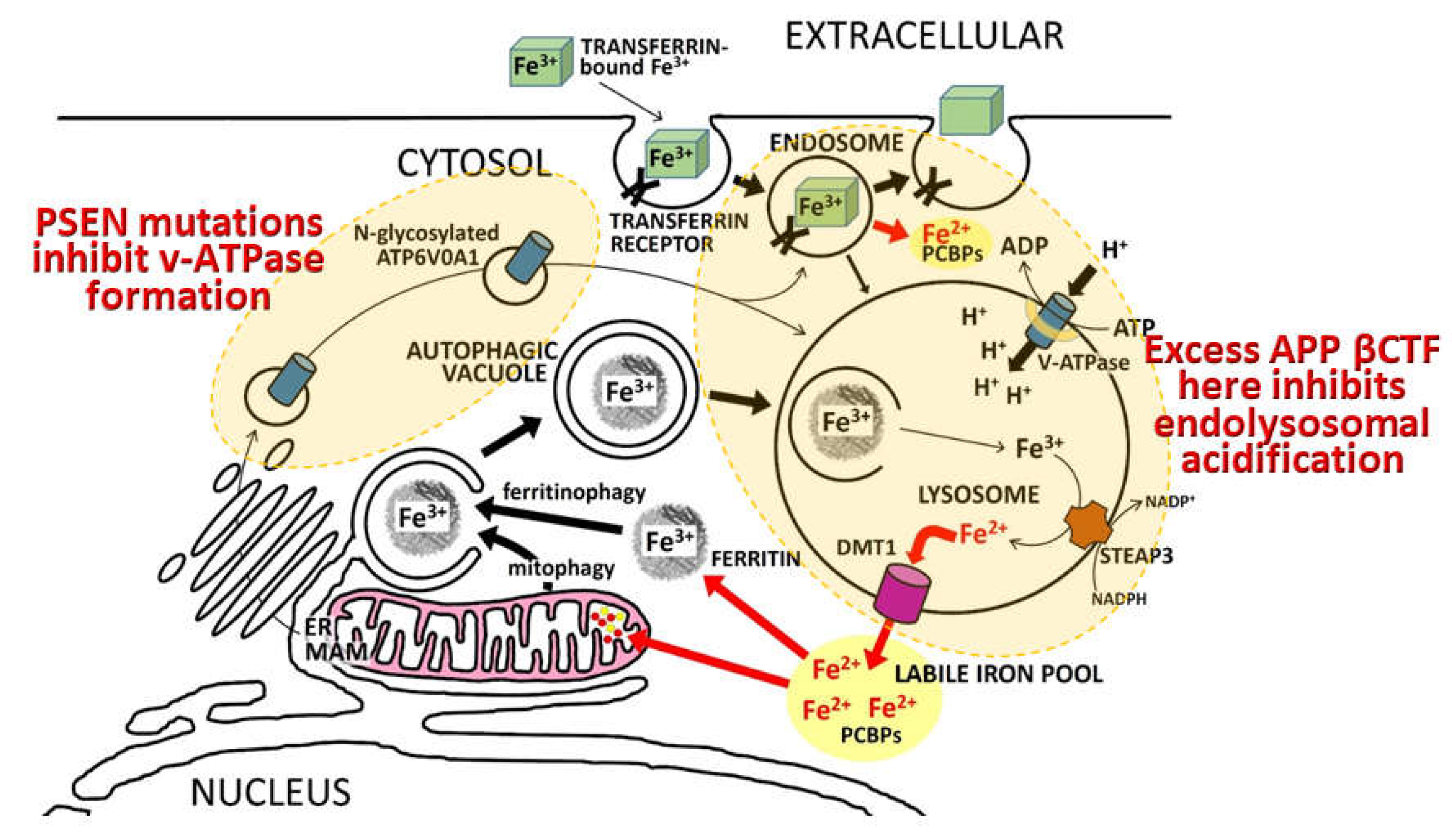
“…identified a direct physical interaction of lysosomal-associated APP-βCTF with the cytosol-exposed domain of the transmembrane V0a1 subunit of the vATPase. Competition by APP-βCTF with specific V1 subunits for binding to the V0a1 subunit impedes association of the V1 and V0 sectors of the complex….Impaired vATPase assembly … is rescued by specifically lowering APP-βCTF levels”
4. Presenilin holoproteins and/or γ-secretase complexes form multimers
“To gain a better understanding of the moderate dominant negative effect, the expression level of the WT and mutant PSEN1 alleles, as well as the oligomerization state of γ-secretases, should be carefully examined under in vivo circumstances, especially in patients’ brains.”
“We wish to stress that our experimental data provide no supporting evidence for a potential role of γ-secretase in the development of AD. In fact, a number of the γ-secretase variants with pathogenic PS1 mutations, exemplified by S365A, have WT-level proteolytic activity in terms of Aβ42 and Aβ40 production (18). The development of AD in the patients with these PS1 mutations cannot be explained by the WT-level proteolytic activity of these γ-secretase variants in vitro. Nonetheless, the dominant negative effect of these PS1 mutations in patients with AD still applies, suggesting such an effect may not be recapitulated by the catalytic function of PS1 in γ-secretase. We speculate that, for the vast majority of patients with AD, the dominant negative effect of PS1 is perhaps effected through other mechanisms that are independent of γ-secretase.”
5. A parsimonious model to account for superficially inconsistent/conflicting presenilin mutation data
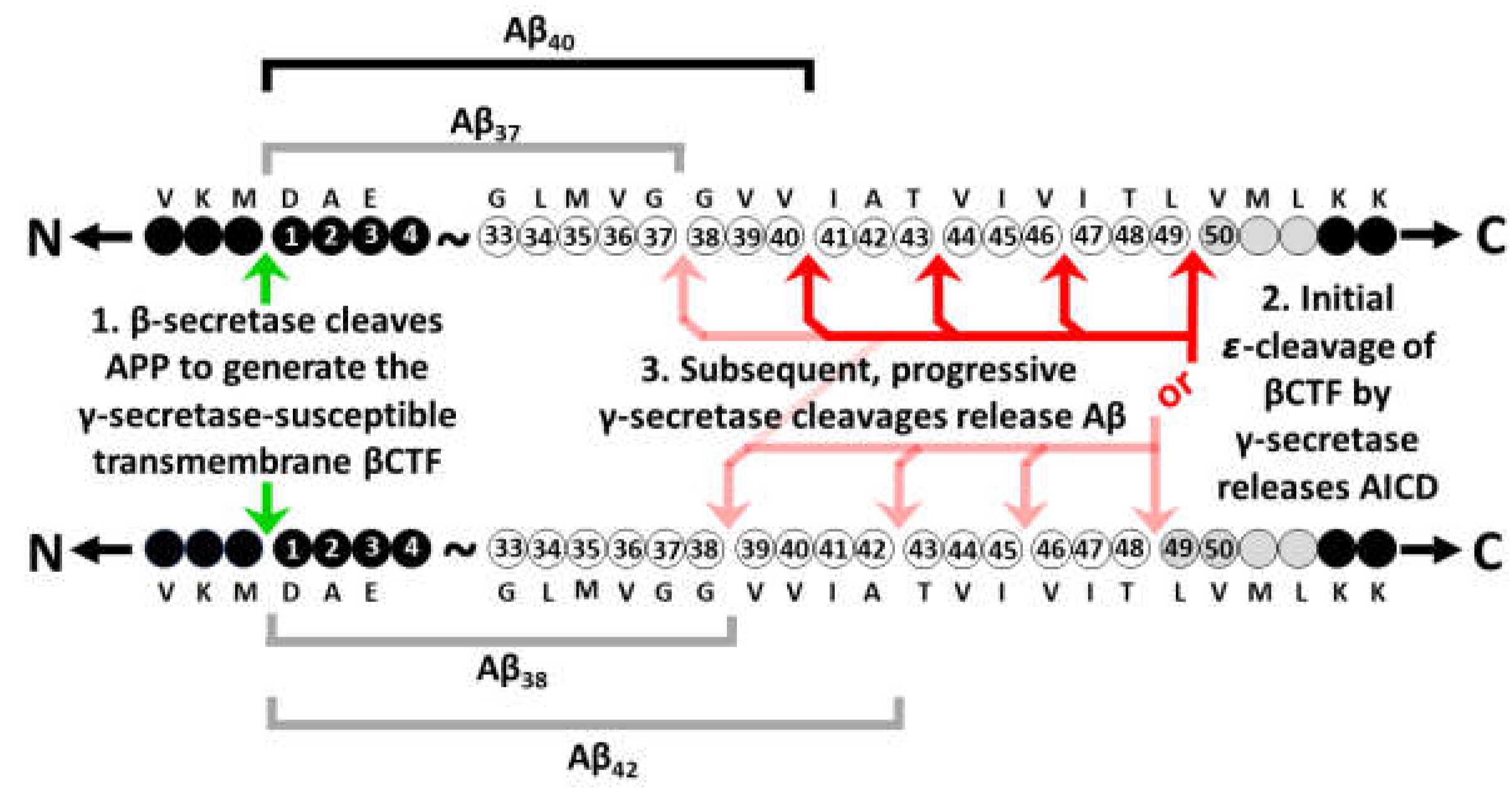
6. Additional evidence consistent with increased γ-secretase activity due to fAD mutations
7. Notes on the importance of iron and hypoxia in fAD pathogenesis
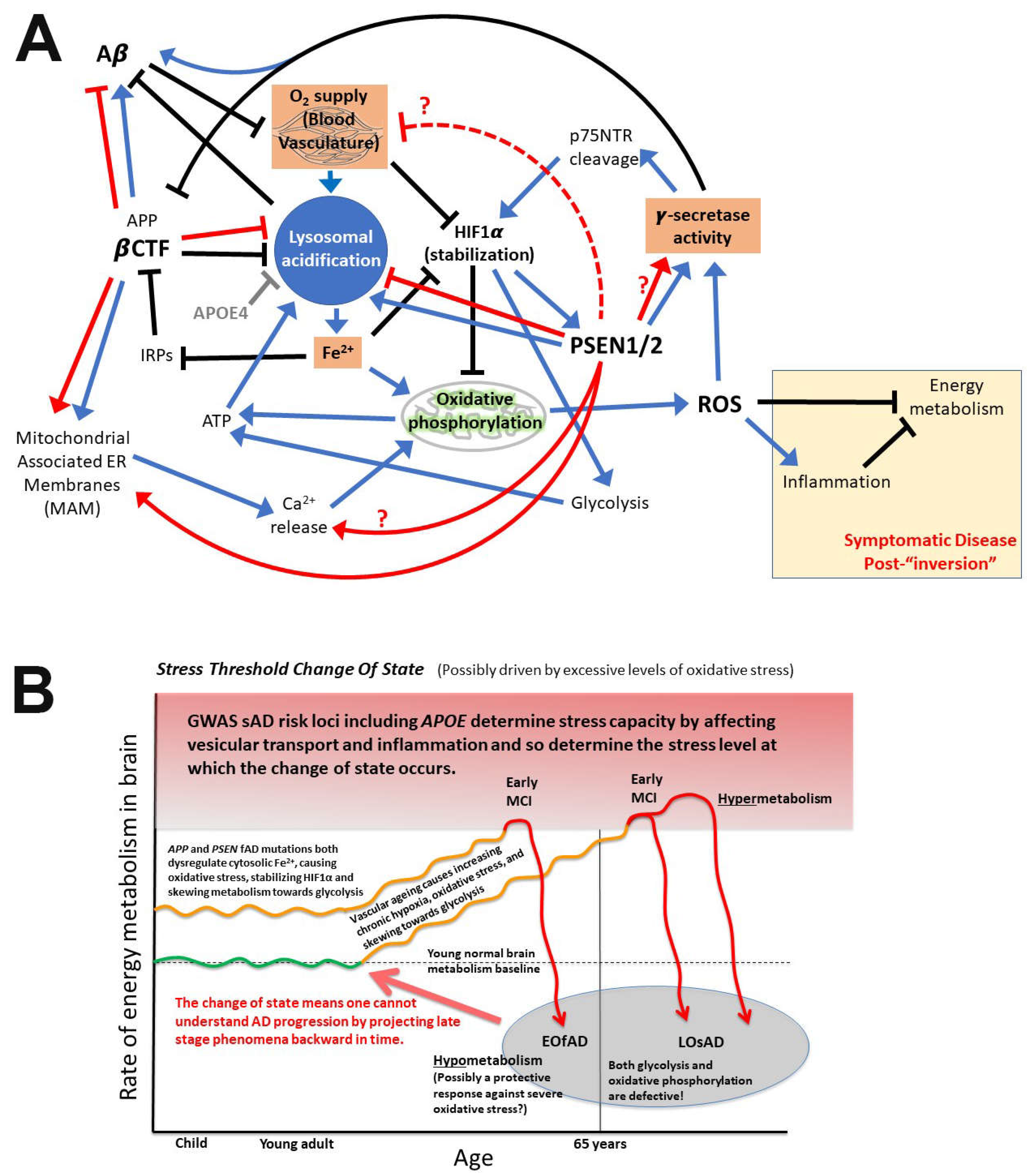
8. What is APP’s role in iron homeostasis?
9. Predictions arising from these proposed mechanisms
10. Conclusions
Supplementary Files
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lumsden AL, Rogers JT, Majd S, Newman M, Sutherland GT, Verdile G, Lardelli M. Dysregulation of Neuronal Iron Homeostasis as an Alternative Unifying Effect of Mutations Causing Familial Alzheimer’s Disease. Front Neurosci 2018, 12, 533. [Google Scholar] [CrossRef]
- Petit D, Fernandez SG, Zoltowska KM, Enzlein T, Ryan NS, O’Connor A, Szaruga M, Hill E, Vandenberghe R, Fox NC, Chavez-Gutierrez L. Abeta profiles generated by Alzheimer’s disease causing PSEN1 variants determine the pathogenicity of the mutation and predict age at disease onset. Mol Psychiatry 2022, 27, 2821–2832. [Google Scholar] [CrossRef]
- Gama Sosa MA, Gasperi RD, Rocher AB, Wang AC, Janssen WG, Flores T, Perez GM, Schmeidler J, Dickstein DL, Hof PR, Elder GA. Age-related vascular pathology in transgenic mice expressing presenilin 1-associated familial Alzheimer’s disease mutations. Am J Pathol 2010, 176, 353–368. [Google Scholar] [CrossRef]
- Berchtold NC, Sabbagh MN, Beach TG, Kim RC, Cribbs DH, Cotman CW Brain gene expression patterns differentiate mild cognitive impairment from normal aged and Alzheimer’s disease. Neurobiol Aging 2014, 35, 1961–1972. [CrossRef]
- Donertas HM, Izgi H, Kamacioglu A, He Z, Khaitovich P, Somel M. Gene expression reversal toward pre-adult levels in the aging human brain and age-related loss of cellular identity. Sci Rep 2017, 7, 5894. [Google Scholar] [CrossRef]
- Roberts JA, Varma VR, An Y, Varma S, Candia J, Fantoni G, Tiwari V, Anerillas C, Williamson A, Saito A, Loeffler T, Schilcher I, Moaddel R, Khadeer M, Lovett J, Tanaka T, Pletnikova O, Troncoso JC, Bennett DA, Albert MS, Yu K, Niu M, Haroutunian V, Zhang B, Peng J, Croteau DL, Resnick SM, Gorospe M, Bohr VA, Ferrucci L, Thambisetty M. A brain proteomic signature of incipient Alzheimer’s disease in young APOE epsilon4 carriers identifies novel drug targets. Sci Adv 2021, 7, eabi8178. [CrossRef]
- Sutphen CL, McCue L, Herries EM, Xiong C, Ladenson JH, Holtzman DM, Fagan AM, Adni. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimers Dement 2018, 14, 869–879. [Google Scholar] [CrossRef]
- Merlo S, Spampinato SF, Sortino MA. Early compensatory responses against neuronal injury: A new therapeutic window of opportunity for Alzheimer’s Disease? CNS Neurosci Ther 2019, 25, 5–13. [Google Scholar] [CrossRef]
- Hardy JA, Higgins GA. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Maloney JA, Bainbridge T, Gustafson A, Zhang S, Kyauk R, Steiner P, van der Brug M, Liu Y, Ernst JA, Watts RJ, Atwal JK. Molecular mechanisms of Alzheimer disease protection by the A673T allele of amyloid precursor protein. J Biol Chem 2014, 289, 30990–31000. [Google Scholar] [CrossRef] [PubMed]
- Wang LS, Naj AC, Graham RR, Crane PK, Kunkle BW, Cruchaga C, Murcia JD, Cannon-Albright L, Baldwin CT, Zetterberg H; et al. Rarity of the Alzheimer disease-protective APP A673T variant in the United States. JAMA Neurol 2015, 72, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ting SK, Chong MS, Kandiah N, Hameed S, Tan L, Au WL, Prakash KM, Pavanni R, Lee TS, Foo JN, Bei JX, Yu XQ, Liu JJ, Zhao Y, Lee WL, Tan EK. Absence of A673T amyloid-beta precursor protein variant in Alzheimer’s disease and other neurological diseases. Neurobiol Aging 2013, 34, 2441.e2447–2448. [Google Scholar] [CrossRef]
- Mengel-From J, Jeune B, Pentti T, McGue M, Christensen K, Christiansen L. The APP A673T frequency differs between Nordic countries. Neurobiol Aging 2015, 36, 2909.e2901–2904. [Google Scholar] [CrossRef]
- Kero M, Paetau A, Polvikoski T, Tanskanen M, Sulkava R, Jansson L, Myllykangas L, Tienari PJ. Amyloid precursor protein (APP) A673T mutation in the elderly Finnish population. Neurobiol Aging 2013, 34, 1518.e1511–e1513. [CrossRef]
- Bamne MN, Demirci FY, Berman S, Snitz BE, Rosenthal SL, Wang X, Lopez OL, Kamboh MI. Investigation of an amyloid precursor protein protective mutation (A673T) in a North American case-control sample of late-onset Alzheimer’s disease. Neurobiol Aging 2014, 35, 1779.e1715–1776. [Google Scholar] [CrossRef]
- Jayne T, Newman M, Verdile G, Sutherland G, Munch G, Musgrave I, Moussavi Nik SH, Lardelli M. Evidence For and Against a Pathogenic Role of Reduced gamma-Secretase Activity in Familial Alzheimer’s Disease. J Alzheimers Dis 2016, 52, 781–799. [Google Scholar] [CrossRef]
- Pottier C, Hannequin D, Coutant S, Rovelet-Lecrux A, Wallon D, Rousseau S, Legallic S, Paquet C, Bombois S, Pariente J, Thomas-Anterion C, Michon A, Croisile B, Etcharry-Bouyx F, Berr C, Dartigues JF, Amouyel P, Dauchel H, Boutoleau-Bretonniere C, Thauvin C, Frebourg T, Lambert JC, Campion D, Collaborators PG. High frequency of potentially pathogenic SORL1 mutations in autosomal dominant early-onset Alzheimer disease. Mol Psychiatry 2012, 17, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Verheijen J, Van den Bossche T, van der Zee J, Engelborghs S, Sanchez-Valle R, Llado A, Graff C, Thonberg H, Pastor P, Ortega-Cubero S, Pastor MA, Benussi L, Ghidoni R, Binetti G, Clarimon J, Lleo A, Fortea J, de Mendonca A, Martins M, Grau-Rivera O, Gelpi E, Bettens K, Mateiu L, Dillen L, Cras P, De Deyn PP, Van Broeckhoven C, Sleegers K. A comprehensive study of the genetic impact of rare variants in SORL1 in European early-onset Alzheimer’s disease. Acta Neuropathol 2016, 132, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Bellenguez C, Charbonnier C, Grenier-Boley B, Quenez O, Le Guennec K, Nicolas G, Chauhan G, Wallon D, Rousseau S, Richard AC, Boland A, Bourque G, Munter HM, Olaso R, Meyer V, Rollin-Sillaire A, Pasquier F, Letenneur L, Redon R, Dartigues JF, Tzourio C, Frebourg T, Lathrop M, Deleuze JF, Hannequin D, Genin E, Amouyel P, Debette S, Lambert JC, Campion D, collaborators CM. Contribution to Alzheimer’s disease risk of rare variants in TREM2, SORL1, and ABCA7 in 1779 cases and 1273 controls. Neurobiol Aging 2017, 59, 220.e221–220.e229. [Google Scholar] [CrossRef]
- Barthelson K, Newman M, Lardelli M. Sorting Out the Role of the Sortilin-Related Receptor 1 in Alzheimer’s Disease. J Alzheimers Dis Rep 2020, 4, 123–140. [Google Scholar] [CrossRef]
- Fortea J, Zaman SH, Hartley S, Rafii MS, Head E, Carmona-Iragui M. Alzheimer’s disease associated with Down syndrome: A genetic form of dementia. Lancet Neurol 2021, 20, 930–942. [Google Scholar] [CrossRef]
- Rovelet-Lecrux A, Hannequin D, Raux G, Le Meur N, Laquerrière A, Vital A, Dumanchin C, Feuillette S, Brice A, Vercelletto M, Dubas F, Frebourg T, Campion D. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nat Genet 2006, 38, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Xu TH, Yan Y, Kang Y, Jiang Y, Melcher K, Xu HE. Alzheimer’s disease-associated mutations increase amyloid precursor protein resistance to gamma-secretase cleavage and the Abeta42/Abeta40 ratio. Cell Discov 2016, 2, 16026. [Google Scholar] [CrossRef]
- Haass C, Hung AY, Selkoe DJ, Teplow DB. Mutations associated with a locus for familial Alzheimer’s disease result in alternative processing of amyloid beta-protein precursor. J Biol Chem 1994, 269, 17741–17748. [Google Scholar] [CrossRef]
- Zhou L, Brouwers N, Benilova I, Vandersteen A, Mercken M, Van Laere K, Van Damme P, Demedts D, Van Leuven F, Sleegers K, Broersen K, Van Broeckhoven C, Vandenberghe R, De Strooper B. Amyloid precursor protein mutation E682K at the alternative beta-secretase cleavage beta’-site increases Abeta generation. EMBO Mol Med 2011, 3, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Mullan M, Crawford F, Axelman K, Houlden H, Lilius L, Winblad B, Lannfelt L. A pathogenic mutation for probable Alzheimer’s disease in the APP gene at the N-terminus of beta-amyloid. Nat Genet 1992, 1, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Cai XD, Golde TE, Younkin SG. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science 1993, 259, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Jiang Y, Sato Y, Im E, Berg M, Bordi M, Darji S, Kumar A, Mohan PS, Bandyopadhyay U, Diaz A, Cuervo AM, Nixon RA. Lysosomal Dysfunction in Down Syndrome Is APP-Dependent and Mediated by APP-betaCTF (C99). J Neurosci 2019, 39, 5255–5268. [Google Scholar] [CrossRef] [PubMed]
- Chen WT, Hong CJ, Lin YT, Chang WH, Huang HT, Liao JY, Chang YJ, Hsieh YF, Cheng CY, Liu HC, Chen YR, Cheng IH. Amyloid-beta (Abeta) D7H mutation increases oligomeric Abeta42 and alters properties of Abeta-zinc/copper assemblies. PLoS ONE 2012, 7, e35807. [Google Scholar] [CrossRef]
- Bugiani O, Giaccone G, Rossi G, Mangieri M, Capobianco R, Morbin M, Mazzoleni G, Cupidi C, Marcon G, Giovagnoli A, Bizzi A, Di Fede G, Puoti G, Carella F, Salmaggi A, Romorini A, Patruno GM, Magoni M, Padovani A, Tagliavini F. Hereditary cerebral hemorrhage with amyloidosis associated with the E693K mutation of APP. Arch Neurol 2010, 67, 987–995. [Google Scholar] [CrossRef]
- Tagliavini J, Williot P, Congiu L, Chicca M, Lanfredi M, Rossi R, Fontana F. Molecular cytogenetic analysis of the karyotype of the European Atlantic sturgeon, Acipenser sturio. Heredity (Edinb) 1999, 83, 520–525. [Google Scholar] [CrossRef]
- Obici L, Demarchi A, de Rosa G, Bellotti V, Marciano S, Donadei S, Arbustini E, Palladini G, Diegoli M, Genovese E, Ferrari G, Coverlizza S, Merlini G. A novel AbetaPP mutation exclusively associated with cerebral amyloid angiopathy. Ann Neurol 2005, 58, 639–644. [Google Scholar] [CrossRef]
- Haapasalo A, Kovacs DM. The many substrates of presenilin/gamma-secretase. J Alzheimers Dis 2011, 25, 3–28. [Google Scholar] [CrossRef]
- Guner G, Lichtenthaler SF. The substrate repertoire of gamma-secretase/presenilin. Semin Cell Dev Biol 2020, 105, 27–42. [Google Scholar] [CrossRef]
- Yan Y, Xu TH, Melcher K, Xu HE. Defining the minimum substrate and charge recognition model of gamma-secretase. Acta Pharmacol Sin 2017, 38, 1412–1424. [Google Scholar] [CrossRef]
- Takami M, Nagashima Y, Sano Y, Ishihara S, Morishima-Kawashima M, Funamoto S, Ihara Y. gamma-Secretase: Successive tripeptide and tetrapeptide release from the transmembrane domain of beta-carboxyl terminal fragment. J Neurosci 2009, 29, 13042–13052. [Google Scholar] [CrossRef]
- Qi-Takahara Y, Morishima-Kawashima M, Tanimura Y, Dolios G, Hirotani N, Horikoshi Y, Kametani F, Maeda M, Saido TC, Wang R, Ihara Y. Longer forms of amyloid beta protein: Implications for the mechanism of intramembrane cleavage by gamma-secretase. J Neurosci 2005, 25, 436–445. [Google Scholar] [CrossRef]
- Chavez-Gutierrez L, Bammens L, Benilova I, Vandersteen A, Benurwar M, Borgers M, Lismont S, Zhou L, Van Cleynenbreugel S, Esselmann H, Wiltfang J, Serneels L, Karran E, Gijsen H, Schymkowitz J, Rousseau F, Broersen K, De Strooper B. The mechanism of gamma-Secretase dysfunction in familial Alzheimer disease. EMBO J 2012, 31, 2261–2274. [Google Scholar] [CrossRef]
- Tan J, Mao G, Cui MZ, Kang SC, Lamb B, Wong BS, Sy MS, Xu X. Effects of gamma-secretase cleavage-region mutations on APP processing and Abeta formation: Interpretation with sequential cleavage and alpha-helical model. J Neurochem 2008, 107, 722–733. [Google Scholar] [CrossRef]
- Suarez-Calvet M, Belbin O, Pera M, Badiola N, Magrane J, Guardia-Laguarta C, Munoz L, Colom-Cadena M, Clarimon J, Lleo A. Autosomal-dominant Alzheimer’s disease mutations at the same codon of amyloid precursor protein differentially alter Abeta production. J Neurochem 2014, 128, 330–339. [Google Scholar] [CrossRef]
- Jarrett JT, Lansbury PT, Jr. Seeding "one-dimensional crystallization" of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell 1993, 73, 1055–1058. [Google Scholar] [CrossRef]
- Tamaoka A, Odaka A, Ishibashi Y, Usami M, Sahara N, Suzuki N, Nukina N, Mizusawa H, Shoji S, Kanazawa I; et al. APP717 missense mutation affects the ratio of amyloid beta protein species (A beta 1-42/43 and a beta 1-40) in familial Alzheimer’s disease brain. J Biol Chem 1994, 269, 32721–32724. [Google Scholar] [CrossRef]
- Borchelt DR, Thinakaran G, Eckman CB, Lee MK, Davenport F, Ratovitsky T, Prada CM, Kim G, Seekins S, Yager D, Slunt HH, Wang R, Seeger M, Levey AI, Gandy SE, Copeland NG, Jenkins NA, Price DL, Younkin SG, Sisodia SS. Familial Alzheimer’s disease-linked presenilin 1 variants elevate Abeta1-42/1-40 ratio in vitro and in vivo. Neuron 1996, 17, 1005–1013. [Google Scholar] [CrossRef]
- Lichtenthaler SF, Ida N, Multhaup G, Masters CL, Beyreuther K. Mutations in the transmembrane domain of APP altering gamma-secretase specificity. Biochemistry 1997, 36, 15396–15403. [Google Scholar] [CrossRef]
- Naslund J, Schierhorn A, Hellman U, Lannfelt L, Roses AD, Tjernberg LO, Silberring J, Gandy SE, Winblad B, Greengard P; et al. Relative abundance of Alzheimer A beta amyloid peptide variants in Alzheimer disease and normal aging. Proc Natl Acad Sci USA 1994, 91, 8378–8382. [Google Scholar] [CrossRef]
- Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med 1996, 2, 864–870. [Google Scholar] [CrossRef]
- Citron M, Westaway D, Xia W, Carlson G, Diehl T, Levesque G, Johnson-Wood K, Lee M, Seubert P, Davis A, Kholodenko D, Motter R, Sherrington R, Perry B, Yao H, Strome R, Lieberburg I, Rommens J, Kim S, Schenk D, Fraser P, St George Hyslop P, Selkoe DJ. Mutant presenilins of Alzheimer’s disease increase production of 42-residue amyloid beta-protein in both transfected cells and transgenic mice. Nat Med 1997, 3, 67–72. [Google Scholar] [CrossRef]
- Jayadev S, Leverenz JB, Steinbart E, Stahl J, Klunk W, Yu CE, Bird TD. Alzheimer’s disease phenotypes and genotypes associated with mutations in presenilin 2. Brain 2010, 133, 1143–1154. [Google Scholar] [CrossRef]
- Arimon M, Takeda S, Post KL, Svirsky S, Hyman BT, Berezovska O. Oxidative stress and lipid peroxidation are upstream of amyloid pathology. Neurobiol Dis 2015, 84, 109–119. [Google Scholar] [CrossRef]
- Zhu X, Raina AK, Perry G, Smith MA. Alzheimer’s disease: The two-hit hypothesis. Lancet Neurol 2004, 3, 219–226. [Google Scholar] [CrossRef]
- Martins RN, Harper CG, Stokes GB, Masters CL. Increased cerebral glucose-6-phosphate dehydrogenase activity in Alzheimer’s disease may reflect oxidative stress. J Neurochem 1986, 46, 1042–1045. [Google Scholar] [CrossRef]
- Holmes O, Paturi S, Ye W, Wolfe MS, Selkoe DJ. Effects of membrane lipids on the activity and processivity of purified gamma-secretase. Biochemistry 2012, 51, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Song Y, Mittendorf KF, Lu Z, Sanders CR. Impact of bilayer lipid composition on the structure and topology of the transmembrane amyloid precursor C99 protein. J Am Chem Soc 2014, 136, 4093–4096. [Google Scholar] [CrossRef] [PubMed]
- Montesinos J, Pera M, Larrea D, Guardia-Laguarta C, Agrawal RR, Velasco KR, Yun TD, Stavrovskaya IG, Xu Y, Koo SY, Snead AM, Sproul AA, Area-Gomez E. The Alzheimer’s disease-associated C99 fragment of APP regulates cellular cholesterol trafficking. EMBO J 2020, 39, e103791. [Google Scholar] [CrossRef] [PubMed]
- 55. Cho YY, Kwon OH, Chung S. Preferred Endocytosis of Amyloid Precursor Protein from Cholesterol-Enriched Lipid Raft Microdomains. Molecules.
- Vetrivel KS, Cheng H, Lin W, Sakurai T, Li T, Nukina N, Wong PC, Xu H, Thinakaran G. Association of gamma-secretase with lipid rafts in post-Golgi and endosome membranes. J Biol Chem 2004, 279, 44945–44954. [Google Scholar] [CrossRef] [PubMed]
- Pera M, Larrea D, Guardia-Laguarta C, Montesinos J, Velasco KR, Agrawal RR, Xu Y, Chan RB, Di Paolo G, Mehler MF, Perumal GS, Macaluso FP, Freyberg ZZ, Acin-Perez R, Enriquez JA, Schon EA, Area-Gomez E. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J 2017, 36, 3356–3371. [Google Scholar] [CrossRef] [PubMed]
- Cui JG, Fraser PE, St George-Hyslop P, Westaway D, Lukiw WJ. Potential roles for presenilin-1 in oxygen sensing and in glial-specific gene expression. Neuroreport 2004, 15, 2025–2028. [Google Scholar] [CrossRef] [PubMed]
- Moussavi Nik SH, Wilson L, Newman M, Croft K, Mori TA, Musgrave I, Lardelli M. The BACE1-PSEN-AbetaPP regulatory axis has an ancient role in response to low oxygen/oxidative stress. J Alzheimers Dis 2012, 28, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Moussavi Nik SH, Newman M, Wilson L, Ebrahimie E, Wells S, Musgrave I, Verdile G, Martins RN, Lardelli M. Alzheimer’s disease-related peptide PS2V plays ancient, conserved roles in suppression of the unfolded protein response under hypoxia and stimulation of gamma-secretase activity. Hum Mol Genet 2015, 24, 3662–3678. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress in cell culture: An under-appreciated problem? FEBS Lett 2003, 540, 3–6. [Google Scholar] [CrossRef]
- Eggert S, Gonzalez AC, Thomas C, Schilling S, Schwarz SM, Tischer C, Adam V, Strecker P, Schmidt V, Willnow TE, Hermey G, Pietrzik CU, Koo EH, Kins S. Dimerization leads to changes in APP (amyloid precursor protein) trafficking mediated by LRP1 and SorLA. Cell Mol Life Sci 2018, 75, 301–322. [Google Scholar] [CrossRef]
- Jung JI, Premraj S, Cruz PE, Ladd TB, Kwak Y, Koo EH, Felsenstein KM, Golde TE, Ran Y. Independent Relationship between Amyloid Precursor Protein (APP) Dimerization and γ-Secretase Processivity. PLoS ONE 2014, 9, e111553. [Google Scholar] [CrossRef]
- Scheuermann S, Hambsch B, Hesse L, Stumm J, Schmidt C, Beher D, Bayer TA, Beyreuther K, Multhaup G. Homodimerization of amyloid precursor protein and its implication in the amyloidogenic pathway of Alzheimer’s disease. J Biol Chem 2001, 276, 33923–33929. [Google Scholar] [CrossRef] [PubMed]
- Kwart D, Gregg A, Scheckel C, Murphy EA, Paquet D, Duffield M, Fak J, Olsen O, Darnell RB, Tessier-Lavigne M. A Large Panel of Isogenic APP and PSEN1 Mutant Human iPSC Neurons Reveals Shared Endosomal Abnormalities Mediated by APP beta-CTFs, Not Abeta. Neuron 2019, 104, 1022. [Google Scholar] [CrossRef] [PubMed]
- Laird, J. The Law of Parsimony. The Monist 1919, 29, 321–344. [Google Scholar] [CrossRef]
- Morris GP, Clark IA, Vissel B. Questions concerning the role of amyloid-beta in the definition, aetiology and diagnosis of Alzheimer’s disease. Acta Neuropathol 2018, 136, 663–689. [Google Scholar] [CrossRef] [PubMed]
- Castellani RJ, Lee HG, Siedlak SL, Nunomura A, Hayashi T, Nakamura M, Zhu X, Perry G, Smith MA. Reexamining Alzheimer’s disease: Evidence for a protective role for amyloid-beta protein precursor and amyloid-beta. J Alzheimers Dis 2009, 18, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Castellani RJ, Smith MA. Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is ‘too big to fail’. J Pathol 2011, 224, 147–152. [Google Scholar] [CrossRef]
- Mondragon-Rodriguez S, Basurto-Islas G, Lee HG, Perry G, Zhu X, Castellani RJ, Smith MA. Causes versus effects: The increasing complexities of Alzheimer’s disease pathogenesis. Expert Rev Neurother 2010, 10, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Kepp, KP. Alzheimer’s disease due to loss of function: A new synthesis of the available data. Prog Neurobiol 2016, 143, 36–60. [Google Scholar] [CrossRef]
- Perneczky R, Jessen F, Grimmer T, Levin J, Floel A, Peters O, Froelich L. Anti-amyloid antibody therapies in Alzheimer’s disease. Brain. [CrossRef]
- Richards RI, Robertson SA, O’Keefe LV, Fornarino D, Scott A, Lardelli M, Baune BT. The Enemy within: Innate Surveillance-Mediated Cell Death, the Common Mechanism of Neurodegenerative Disease. Front Neurosci 2016, 10, 193. [Google Scholar] [CrossRef]
- Ihnatovych I, Birkaya B, Notari E, Szigeti K. iPSC-Derived Microglia for Modeling Human-Specific DAMP and PAMP Responses in the Context of Alzheimer’s Disease. Int J Mol Sci 2020, 21, 9668. [CrossRef]
- Kolata, G. An Alzheimer’s Treatment Fails: ‘We Don’t Have Anything Now’. The New York Times. 2020.
- Yokoyama M, Kobayashi H, Tatsumi L, Tomita T. Mouse Models of Alzheimer’s Disease. Front Mol Neurosci 2022, 15, 912995. [Google Scholar] [CrossRef] [PubMed]
- Pulina MV, Hopkins M, Haroutunian V, Greengard P, Bustos V. C99 selectively accumulates in vulnerable neurons in Alzheimer’s disease. Alzheimers Dement 2020, 16, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Vaillant-Beuchot L, Mary A, Pardossi-Piquard R, Bourgeois A, Lauritzen I, Eysert F, Kinoshita PF, Cazareth J, Badot C, Fragaki K, Bussiere R, Martin C, Mary R, Bauer C, Pagnotta S, Paquis-Flucklinger V, Buee-Scherrer V, Buee L, Lacas-Gervais S, Checler F, Chami M. Accumulation of amyloid precursor protein C-terminal fragments triggers mitochondrial structure, function, and mitophagy defects in Alzheimer’s disease models and human brains. Acta Neuropathol 2021, 141, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 2008, 105, 6415–6420. [Google Scholar] [CrossRef] [PubMed]
- Checler F, Afram E, Pardossi-Piquard R, Lauritzen I. Is gamma-secretase a beneficial inactivating enzyme of the toxic APP C-terminal fragment C99? J Biol Chem 2021, 296, 100489. [Google Scholar]
- Gao S, Casey AE, Sargeant TJ, Mäkinen VP. Genetic variation within endolysosomal system is associated with late-onset Alzheimer’s disease. Brain 2018, 141, 2711–2720. [Google Scholar] [CrossRef]
- Szabo MP, Mishra S, Knupp A, Young JE. The role of Alzheimer’s disease risk genes in endolysosomal pathways. Neurobiol Dis 2022, 162, 105576. [Google Scholar] [CrossRef]
- Prasad H, Rao R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc Natl Acad Sci U S A 2018, 115, E6640–E6649. [Google Scholar] [CrossRef]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef]
- Csordas G, Hajnoczky G. SR/ER-mitochondrial local communication: Calcium and ROS. Biochim Biophys Acta 2009, 1787, 1352–1362. [Google Scholar] [CrossRef] [PubMed]
- Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA. Upregulated function of mitochondria-associated ER membranes in Alzheimer disease. EMBO J 2012, 31, 4106–4123. [Google Scholar] [CrossRef] [PubMed]
- Area-Gomez E, de Groof AJC, Boldogh I, Bird TD, Gibson GE, Koehler CM, Yu WH, Duff KE, Yaffe MP, Pon LA, Schon EA. Presenilins Are Enriched in Endoplasmic Reticulum Membranes Associated with Mitochondria. The American Journal of Pathology 2009, 175, 1810–1816. [Google Scholar] [CrossRef] [PubMed]
- Lanoiselee HM, Nicolas G, Wallon D, Rovelet-Lecrux A, Lacour M, Rousseau S, Richard AC, Pasquier F, Rollin-Sillaire A, Martinaud O, Quillard-Muraine M, de la Sayette V, Boutoleau-Bretonniere C, Etcharry-Bouyx F, Chauvire V, Sarazin M, le Ber I, Epelbaum S, Jonveaux T, Rouaud O, Ceccaldi M, Felician O, Godefroy O, Formaglio M, Croisile B, Auriacombe S, Chamard L, Vincent JL, Sauvee M, Marelli-Tosi C, Gabelle A, Ozsancak C, Pariente J, Paquet C, Hannequin D, Campion D, collaborators of the CNRMAJp. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med 2017, 14, e1002270. [Google Scholar] [CrossRef]
- Ryman DC, Acosta-Baena N, Aisen PS, Bird T, Danek A, Fox NC, Goate A, Frommelt P, Ghetti B, Langbaum JB, Lopera F, Martins R, Masters CL, Mayeux RP, McDade E, Moreno S, Reiman EM, Ringman JM, Salloway S, Schofield PR, Sperling R, Tariot PN, Xiong C, Morris JC, Bateman RJ, Dominantly Inherited Alzheimer N. Symptom onset in autosomal dominant Alzheimer disease: A systematic review and meta-analysis. Neurology 2014, 83, 253–260. [Google Scholar] [CrossRef] [PubMed]
- De Jonghe C, Cruts M, Rogaeva EA, Tysoe C, Singleton A, Vanderstichele H, Meschino W, Dermaut B, Vanderhoeven I, Backhovens H, Vanmechelen E, Morris CM, Hardy J, Rubinsztein DC, St George-Hyslop PH, Van Broeckhoven C. Aberrant splicing in the presenilin-1 intron 4 mutation causes presenile Alzheimer’s disease by increased Abeta42 secretion. Hum Mol Genet 1999, 8, 1529–1540. [Google Scholar] [CrossRef]
- Pang Y, Li T, Wang Q, Qin W, Li Y, Wei Y, Jia L. A Rare Variation in the 3’ Untranslated Region of the Presenilin 2 Gene Is Linked to Alzheimer’s Disease. Mol Neurobiol 2021, 58, 4337–4347. [Google Scholar] [CrossRef] [PubMed]
- Brenowitz WD, Fornage M, Launer LJ, Habes M, Davatzikos C, Yaffe K. Alzheimer’s Disease Genetic Risk, Cognition, and Brain Aging in Midlife. Ann Neurol, 2022. [CrossRef]
- Wang Z, Yan Y, Wang B. gamma-Secretase Genetics of Hidradenitis Suppurativa: A Systematic Literature Review. Dermatology 2021, 237, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Duchatelet S, Miskinyte S, Delage M, Ungeheuer MN, Lam T, Benhadou F, Del Marmol V, Vossen A, Prens EP, Cogrel O, Beylot-Barry M, Girard C, Vidil J, Join-Lambert O, Parisot M, Nitschke P, Hanein S, Fraitag S, Van der Zee HH, Bessis D, Damiani G, Altomare A, Liao YH, Nikolakis G, Zouboulis CC, Nassif A, Hovnanian A. Low Prevalence of GSC Gene Mutations in a Large Cohort of Predominantly Caucasian Patients with Hidradenitis Suppurativa. J Invest Dermatol 2020, 140, 2085–2088. [Google Scholar] [CrossRef]
- Wang B, Yang W, Wen W, Sun J, Su B, Liu B, Ma D, Lv D, Wen Y, Qu T, Chen M, Sun M, Shen Y, Zhang X. Gamma-secretase gene mutations in familial acne inversa. Science 2010, 330, 1065. [Google Scholar] [CrossRef]
- Duering M, Grimm MO, Grimm HS, Schroder J, Hartmann T. Mean age of onset in familial Alzheimer’s disease is determined by amyloid beta 42. Neurobiol Aging 2005, 26, 785–788. [Google Scholar] [CrossRef] [PubMed]
- Kumar-Singh S, Theuns J, Van Broeck B, Pirici D, Vennekens K, Corsmit E, Cruts M, Dermaut B, Wang R, Van Broeckhoven C. Mean age-of-onset of familial alzheimer disease caused by presenilin mutations correlates with both increased Abeta42 and decreased Abeta40. Hum Mutat 2006, 27, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Sun L, Zhou R, Yang G, Shi Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc Natl Acad Sci U S A 2017, 114, E476–E485. [Google Scholar] [CrossRef]
- Zhou R, Yang G, Shi Y. Dominant negative effect of the loss-of-function gamma-secretase mutants on the wild-type enzyme through heterooligomerization. Proc Natl Acad Sci U S A 2017, 114, 12731–12736. [Google Scholar] [CrossRef] [PubMed]
- Szaruga M, Munteanu B, Lismont S, Veugelen S, Horre K, Mercken M, Saido TC, Ryan NS, De Vos T, Savvides SN, Gallardo R, Schymkowitz J, Rousseau F, Fox NC, Hopf C, De Strooper B, Chavez-Gutierrez L. Alzheimer’s-causing mutations shift Abeta length by destabilizing gamma-secretase-Abetan interactions. Cell 2021, 184, 2257–2258. [Google Scholar] [CrossRef] [PubMed]
- Liu L, Lauro BM, He A, Lee H, Bhattarai S, Wolfe MS, Bennett DA, Karch CM, Young-Pearse T, Dominantly Inherited Alzheimer N, Selkoe DJ. Identification of the Abeta37/42 peptide ratio in CSF as an improved Abeta biomarker for Alzheimer’s disease. Alzheimers Dement 2023, 19, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Fukumori A, Fluhrer R, Steiner H, Haass C. Three-amino acid spacing of presenilin endoproteolysis suggests a general stepwise cleavage of gamma-secretase-mediated intramembrane proteolysis. J Neurosci 2010, 30, 7853–7862. [Google Scholar] [CrossRef] [PubMed]
- Steiner H, Romig H, Grim MG, Philipp U, Pesold B, Citron M, Baumeister R, Haass C. The biological and pathological function of the presenilin-1 Deltaexon 9 mutation is independent of its defect to undergo proteolytic processing. J Biol Chem 1999, 274, 7615–7618. [Google Scholar] [CrossRef] [PubMed]
- Xia D, Watanabe H, Wu B, Lee SH, Li Y, Tsvetkov E, Bolshakov VY, Shen J, Kelleher RJ, 3rd. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer’s disease. Neuron 2015, 85, 967–981. [Google Scholar] [CrossRef]
- Heilig EA, Gutti U, Tai T, Shen J, Kelleher RJ, 3rd. Trans-dominant negative effects of pathogenic PSEN1 mutations on gamma-secretase activity and Abeta production. J Neurosci 2013, 33, 11606–11617. [Google Scholar] [CrossRef]
- Shen L, Qin W, Wu L, Zhou A, Tang Y, Wang Q, Jia L, Jia J. Two novel presenilin-1 mutations (I249L and P433S) in early onset Chinese Alzheimer’s pedigrees and their functional characterization. Biochemical and Biophysical Research Communications 2019, 516, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Kretner B, Trambauer J, Fukumori A, Mielke J, Kuhn PH, Kremmer E, Giese A, Lichtenthaler SF, Haass C, Arzberger T, Steiner H. Generation and deposition of Abeta43 by the virtually inactive presenilin-1 L435F mutant contradicts the presenilin loss-of-function hypothesis of Alzheimer’s disease. EMBO Mol Med 2016, 8, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Steiner H, Capell A, Pesold B, Citron M, Kloetzel PM, Selkoe DJ, Romig H, Mendla K, Haass C. Expression of Alzheimer’s disease-associated presenilin-1 is controlled by proteolytic degradation and complex formation. J Biol Chem 1998, 273, 32322–32331. [Google Scholar] [CrossRef] [PubMed]
- Dewji NN, Do C, Singer SJ. On the spurious endoproteolytic processing of the presenilin proteins in cultured cells and tissues. Proc Natl Acad Sci U S A 1997, 94, 14031–14036. [Google Scholar] [CrossRef] [PubMed]
- Raut S, Patel R, Al-Ahmad AJ. Presence of a mutation in PSEN1 or PSEN2 gene is associated with an impaired brain endothelial cell phenotype in vitro. Fluids Barriers CNS 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Vidoni C, Follo C, Savino M, Melone MAB, Isidoro C. The Role of Cathepsin D in the Pathogenesis of Human Neurodegenerative Disorders. Medicinal Research Reviews 2016, 36, 845–870. [Google Scholar] [CrossRef] [PubMed]
- Lee JH, Yang DS, Goulbourne CN, Im E, Stavrides P, Pensalfini A, Chan H, Bouchet-Marquis C, Bleiwas C, Berg MJ, Huo C, Peddy J, Pawlik M, Levy E, Rao M, Staufenbiel M, Nixon RA. Faulty autolysosome acidification in Alzheimer’s disease mouse models induces autophagic build-up of Abeta in neurons, yielding senile plaques. Nat Neurosci 2022, 25, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Kim SH, Cho YS, Kim Y, Park J, Yoo SM, Gwak J, Kim Y, Gwon Y, Kam TI, Jung YK. Endolysosomal impairment by binding of amyloid beta or MAPT/Tau to V-ATPase and rescue via the HYAL-CD44 axis in Alzheimer disease. Autophagy, 1–20. [CrossRef]
- Mustaly-Kalimi S, Gallegos W, Marr RA, Gilman-Sachs A, Peterson DA, Sekler I, Stutzmann GE. Protein mishandling and impaired lysosomal proteolysis generated through calcium dysregulation in Alzheimer’s disease. Proc Natl Acad Sci U S A 2022, 119, e2211999119. [Google Scholar] [CrossRef] [PubMed]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep 2015, 12, 1430–1444. [Google Scholar] [CrossRef]
- Schroeter EH, Ilagan MX, Brunkan AL, Hecimovic S, Li YM, Xu M, Lewis HD, Saxena MT, De Strooper B, Coonrod A, Tomita T, Iwatsubo T, Moore CL, Goate A, Wolfe MS, Shearman M, Kopan R. A presenilin dimer at the core of the gamma-secretase enzyme: Insights from parallel analysis of Notch 1 and APP proteolysis. Proc Natl Acad Sci U S A 2003, 100, 13075–13080. [Google Scholar] [CrossRef]
- Brautigam H, Moreno CL, Steele JW, Bogush A, Dickstein DL, Kwok JB, Schofield PR, Thinakaran G, Mathews PM, Hof PR, Gandy S, Ehrlich ME. Physiologically generated presenilin 1 lacking exon 8 fails to rescue brain PS1-/- phenotype and forms complexes with wildtype PS1 and nicastrin. Sci Rep 2015, 5, 17042. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Ayala AA, Sannerud R, Mondin M, Poersch K, Vermeire W, Paparelli L, Berlage C, Koenig M, Chavez-Gutierrez L, Ulbrich MH, Munck S, Mizuno H, Annaert W. Super-resolution microscopy reveals majorly mono- and dimeric presenilin1/gamma-secretase at the cell surface. Elife 2020, 9. [CrossRef]
- Valapala M, Hose S, Gongora C, Dong L, Wawrousek EF, Samuel Zigler J, Jr. , Sinha D. Impaired endolysosomal function disrupts Notch signalling in optic nerve astrocytes. Nat Commun 2013, 4, 1629. [Google Scholar] [CrossRef] [PubMed]
- Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 2010, 137, 1825–1832. [Google Scholar] [CrossRef] [PubMed]
- Yan Y, Denef N, Schupbach T. The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 2009, 17, 387–402. [Google Scholar] [CrossRef]
- Maesako M, Houser MCQ, Turchyna Y, Wolfe MS, Berezovska O. Presenilin/gamma-Secretase Activity Is Located in Acidic Compartments of Live Neurons. J Neurosci 2022, 42, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Le Moan N, Houslay DM, Christian F, Houslay MD, Akassoglou K. Oxygen-dependent cleavage of the p75 neurotrophin receptor triggers stabilization of HIF-1alpha. Mol Cell 2011, 44, 476–490. [Google Scholar] [CrossRef]
- Villa JC, Chiu D, Brandes AH, Escorcia FE, Villa CH, Maguire WF, Hu CJ, de Stanchina E, Simon MC, Sisodia SS, Scheinberg DA, Li YM. Nontranscriptional role of Hif-1alpha in activation of gamma-secretase and notch signaling in breast cancer. Cell Rep 2014, 8, 1077–1092. [Google Scholar] [CrossRef] [PubMed]
- De Gasperi R, Sosa MA, Dracheva S, Elder GA. Presenilin-1 regulates induction of hypoxia inducible factor-1alpha: Altered activation by a mutation associated with familial Alzheimer’s disease. Mol Neurodegener 2010, 5, 38. [Google Scholar] [CrossRef] [PubMed]
- Newman M, Nik HM, Sutherland GT, Hin N, Kim WS, Halliday GM, Jayadev S, Smith C, Laird AS, Lucas CW, Kittipassorn T, Peet DJ, Lardelli M. Accelerated loss of hypoxia response in zebrafish with familial Alzheimer’s disease-like mutation of presenilin 1. Hum Mol Genet 2020, 29, 2379–2394. [Google Scholar] [CrossRef]
- Barthelson K, Dong Y, Newman M, Lardelli M. PRESENILIN 1 mutations causing early-onset familial Alzheimer’s disease or familial acne inversa differ in their effects on genes facilitating energy metabolism and signal transduction. Journal of Alzheimer’s Disease 2021, 82, 327–347. [Google Scholar] [CrossRef]
- Cervantes S, Gonzalez-Duarte R, Marfany G. Homodimerization of presenilin N-terminal fragments is affected by mutations linked to Alzheimer’s disease. FEBS Lett 2001, 505, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Hebert SS, Godin C, Tomiyama T, Mori H, Levesque G. Dimerization of presenilin-1 in vivo: Suggestion of novel regulatory mechanisms leading to higher order complexes. Biochem Biophys Res Commun 2003, 301, 119–126. [Google Scholar] [CrossRef]
- Manabe T, Katayama T, Sato N, Gomi F, Hitomi J, Yanagita T, Kudo T, Honda A, Mori Y, Matsuzaki S, Imaizumi K, Mayeda A, Tohyama M. Induced HMGA1a expression causes aberrant splicing of Presenilin-2 pre-mRNA in sporadic Alzheimer’s disease. Cell Death and Differentiation 2003, 10, 698–708. [Google Scholar] [CrossRef]
- Manabe T, Ohe K, Katayama T, Matsuzaki S, Yanagita T, Okuda H, Bando Y, Imaizumi K, Reeves R, Tohyama M, Mayeda A. HMGA1a: Sequence-specific RNA-binding factor causing sporadic Alzheimer’s disease-linked exon skipping of presenilin-2 pre-mRNA. Genes to Cells 2007, 12, 1179–1191. [Google Scholar] [CrossRef]
- Sharman MJ, Moussavi Nik SH, Chen MM, Ong D, Wijaya L, Laws SM, Taddei K, Newman M, Lardelli M, Martins RN, Verdile G. The Guinea Pig as a Model for Sporadic Alzheimer’s Disease (AD): The Impact of Cholesterol Intake on Expression of AD-Related Genes. PLoS ONE 2013, 8, e66235. [Google Scholar]
- Sato N, Imaizumi K, Manabe T, Taniguchi M, Hitomi J, Katayama T, Yoneda T, Morihara T, Yasuda Y, Takagi T, Kudo T, Tsuda T, Itoyama Y, Makifuchi T, Fraser PE, St George-Hyslop P, Tohyama M. Increased production of beta-amyloid and vulnerability to endoplasmic reticulum stress by an aberrant spliced form of presenilin 2. Journal of Biological Chemistry 2001, 276, 2108–2114. [Google Scholar] [CrossRef]
- Katayama T, Imaizumi K, Sato N, Miyoshi K, Kudo T, Hitomi J, Morihara T, Yoneda T, Gomi F, Mori Y, Nakano Y, Takeda J, Tsuda T, Itoyama Y, Murayama O, Takashima A, St George-Hyslop P, Takeda M, Tohyama M. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nature Cell Biology 1999, 1, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Katayama T, Imaizumi K, Manabe T, Hitomi J, Kudo T, Tohyama M. Induction of neuronal death by ER stress in Alzheimer’s disease. Journal of Chemical Neuroanatomy 2004, 28, 67–78. [Google Scholar] [CrossRef]
- Braggin JE, Bucks SA, Course MM, Smith CL, Sopher B, Osnis L, Shuey KD, Domoto-Reilly K, Caso C, Kinoshita C, Scherpelz KP, Cross C, Grabowski T, Nik SHM, Newman M, Garden GA, Leverenz JB, Tsuang D, Latimer C, Gonzalez-Cuyar LF, Keene CD, Morrison RS, Rhoads K, Wijsman EM, Dorschner MO, Lardelli M, Young JE, Valdmanis PN, Bird TD, Jayadev S. Alternative splicing in a presenilin 2 variant associated with Alzheimer disease. Ann Clin Transl Neurol 2019, 6, 762–777. [Google Scholar] [CrossRef]
- Mastrangelo P, Mathews PM, Chishti MA, Schmidt SD, Gu Y, Yang J, Mazzella MJ, Coomaraswamy J, Horne P, Strome B, Pelly H, Levesque G, Ebeling C, Jiang Y, Nixon RA, Rozmahel R, Fraser PE, St George-Hyslop P, Carlson GA, Westaway D. Dissociated phenotypes in presenilin transgenic mice define functionally distinct gamma-secretases. Proc Natl Acad Sci U S A 2005, 102, 8972–8977. [Google Scholar] [CrossRef]
- Sannerud R, Esselens C, Ejsmont P, Mattera R, Rochin L, Tharkeshwar AK, De Baets G, De Wever V, Habets R, Baert V, Vermeire W, Michiels C, Groot AJ, Wouters R, Dillen K, Vints K, Baatsen P, Munck S, Derua R, Waelkens E, Basi GS, Mercken M, Vooijs M, Bollen M, Schymkowitz J, Rousseau F, Bonifacino JS, Van Niel G, De Strooper B, Annaert W. Restricted Location of PSEN2/gamma-Secretase Determines Substrate Specificity and Generates an Intracellular Abeta Pool. Cell 2016, 166, 193–208. [Google Scholar] [CrossRef] [PubMed]
- Filadi R, Greotti E, Turacchio G, Luini A, Pozzan T, Pizzo P. Presenilin 2 Modulates Endoplasmic Reticulum-Mitochondria Coupling by Tuning the Antagonistic Effect of Mitofusin 2. Cell Rep 2016, 15, 2226–2238. [Google Scholar] [CrossRef]
- Yambire KF, Rostosky C, Watanabe T, Pacheu-Grau D, Torres-Odio S, Sanchez-Guerrero A, Senderovich O, Meyron-Holtz EG, Milosevic I, Frahm J, West AP, Raimundo N. Impaired lysosomal acidification triggers iron deficiency and inflammation in vivo. 2019, 8. [Google Scholar] [CrossRef]
- Smith MA, Harris PL, Sayre LM, Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc Natl Acad Sci U S A 1997, 94, 9866–9868. [Google Scholar] [CrossRef] [PubMed]
- Patel A, Malinovska L, Saha S, Wang J, Alberti S, Krishnan Y, Hyman AA. ATP as a biological hydrotrope. Science 2017, 356, 753–756. [Google Scholar] [CrossRef] [PubMed]
- David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 2010, 8, e1000450. [Google Scholar] [CrossRef]
- Zhao RZ, Jiang S, Zhang L, Yu ZB. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int J Mol Med 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Lane DJ, Merlot AM, Huang ML, Bae DH, Jansson PJ, Sahni S, Kalinowski DS, Richardson DR. Cellular iron uptake, trafficking and metabolism: Key molecules and mechanisms and their roles in disease. Biochim Biophys Acta 2015, 1853, 1130–1144. [Google Scholar] [CrossRef]
- Correia SC, Moreira PI. Oxygen Sensing and Signaling in Alzheimer’s Disease: A Breathtaking Story! Cell Mol Neurobiol 2022, 42, 3–21. [Google Scholar] [CrossRef]
- Oresic M, Hyotylainen T, Herukka SK, Sysi-Aho M, Mattila I, Seppanan-Laakso T, Julkunen V, Gopalacharyulu PV, Hallikainen M, Koikkalainen J, Kivipelto M, Helisalmi S, Lotjonen J, Soininen H. Metabolome in progression to Alzheimer’s disease. Transl Psychiatry 2011, 1, e57. [Google Scholar] [CrossRef]
- Guglielmotto M, Tamagno E, Danni O. Oxidative stress and hypoxia contribute to Alzheimer’s disease pathogenesis: Two sides of the same coin. ScientificWorldJournal 2009, 9, 781–791. [Google Scholar] [CrossRef]
- Guglielmotto M, Aragno M, Autelli R, Giliberto L, Novo E, Colombatto S, Danni O, Parola M, Smith MA, Perry G, Tamagno E, Tabaton M. The up-regulation of BACE1 mediated by hypoxia and ischemic injury: Role of oxidative stress and HIF1alpha. J Neurochem 2009, 108, 1045–1056. [Google Scholar] [CrossRef]
- Tamagno E, Guglielmotto M, Aragno M, Borghi R, Autelli R, Giliberto L, Muraca G, Danni O, Zhu X, Smith MA, Perry G, Jo DG, Mattson MP, Tabaton M. Oxidative stress activates a positive feedback between the gamma- and beta-secretase cleavages of the beta-amyloid precursor protein. J Neurochem 2008, 104, 683–695. [Google Scholar] [CrossRef]
- Kontush A, Donarski N, Beisiegel U. Resistance of human cerebrospinal fluid to in vitro oxidation is directly related to its amyloid-beta content. Free Radic Res 2001, 35, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. Amyloid-beta: An antioxidant that becomes a pro-oxidant and critically contributes to Alzheimer’s disease. Free Radic Biol Med 2001, 31, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Smith MA, Casadesus G, Joseph JA, Perry G. Amyloid-beta and tau serve antioxidant functions in the aging and Alzheimer brain. Free Radic Biol Med 2002, 33, 1194–1199. [Google Scholar] [CrossRef]
- Nadal RC, Rigby SE, Viles JH. Amyloid beta-Cu2+ complexes in both monomeric and fibrillar forms do not generate H2O2 catalytically but quench hydroxyl radicals. Biochemistry 2008, 47, 11653–11664. [Google Scholar] [CrossRef] [PubMed]
- Baruch-Suchodolsky R, Fischer B. Abeta40, either soluble or aggregated, is a remarkably potent antioxidant in cell-free oxidative systems. Biochemistry 2009, 48, 4354–4370. [Google Scholar] [CrossRef] [PubMed]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature 2004, 429, 883–891. [Google Scholar] [CrossRef]
- Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Perez JM, Evans AC, Alzheimer’s Disease Neuroimaging I. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 2016, 7, 11934. [Google Scholar] [CrossRef]
- Leenders KL, Perani D, Lammertsma AA, Heather JD, Buckingham P, Healy MJ, Gibbs JM, Wise RJ, Hatazawa J, Herold S; et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain 1990, 113, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Braz ID, Fisher JP. The impact of age on cerebral perfusion, oxygenation and metabolism during exercise in humans. J Physiol 2016, 594, 4471–4483. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio H, Rivas-Arancibia S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4(+)T Cells in Neurodegenerative Diseases. Front Cell Neurosci 2018, 12, 114. [Google Scholar] [CrossRef]
- Guay-Gagnon M, Vat S, Forget MF, Tremblay-Gravel M, Ducharme S, Nguyen QD, Desmarais P. Sleep apnea and the risk of dementia: A systematic review and meta-analysis. J Sleep Res 2022, 31, e13589. [Google Scholar] [CrossRef]
- Swinford CG, Risacher SL, Wu YC, Apostolova LG, Gao S, Bice PJ, Saykin AJ. Altered cerebral blood flow in older adults with Alzheimer’s disease: A systematic review. Brain Imaging Behav 2022. [CrossRef]
- Liu Y, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Decreased glucose transporters correlate to abnormal hyperphosphorylation of tau in Alzheimer disease. FEBS Lett 2008, 582, 359–364. [Google Scholar] [CrossRef]
- Qian L, Rawashdeh O, Kasas L, Milne MR, Garner N, Sankorrakul K, Marks N, Dean MW, Kim PR, Sharma A, Bellingham MC, Coulson EJ. Cholinergic basal forebrain degeneration due to sleep-disordered breathing exacerbates pathology in a mouse model of Alzheimer’s disease. Nature Communications 2022, 13, 6543. [Google Scholar] [CrossRef]
- Yeo TT, Chua-Couzens J, Butcher LL, Bredesen DE, Cooper JD, Valletta JS, Mobley WC, Longo FM. Absence of p75NTR causes increased basal forebrain cholinergic neuron size, choline acetyltransferase activity, and target innervation. J Neurosci 1997, 17, 7594–7605. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse PJ, Price DL, Struble RG, Clark AW, Coyle JT, Delon MR. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef]
- Zhu X, Lee HG, Perry G, Smith MA. Alzheimer disease, the two-hit hypothesis: An update. Biochim Biophys Acta 2007, 1772, 494–502. [Google Scholar] [CrossRef]
- Burns KA, Ayoub AE, Breunig JJ, Adhami F, Weng WL, Colbert MC, Rakic P, Kuan CY. Nestin-CreER mice reveal DNA synthesis by nonapoptotic neurons following cerebral ischemia hypoxia. Cereb Cortex 2007, 17, 2585–2592. [Google Scholar] [CrossRef]
- Goda N, Ryan HE, Khadivi B, McNulty W, Rickert RC, Johnson RS. Hypoxia-inducible factor 1alpha is essential for cell cycle arrest during hypoxia. Mol Cell Biol 2003, 23, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Zhou ZD, Tan EK. Iron regulatory protein (IRP)-iron responsive element (IRE) signaling pathway in human neurodegenerative diseases. Mol Neurodegener 2017, 12, 75. [Google Scholar] [CrossRef]
- Hin N, Newman M, Pederson S, Lardelli M. Iron Responsive Element-Mediated Responses to Iron Dyshomeostasis in Alzheimer’s Disease. Journal of Alzheimer’s Disease 2021, 84, 1597–1630. [Google Scholar] [CrossRef]
- Tsatsanis A, Wong BX, Gunn AP, Ayton S, Bush AI, Devos D, Duce JA. Amyloidogenic processing of Alzheimer’s disease beta-amyloid precursor protein induces cellular iron retention. Mol Psychiatry 2020, 25, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Pigoni M, Wanngren J, Kuhn PH, Munro KM, Gunnersen JM, Takeshima H, Feederle R, Voytyuk I, De Strooper B, Levasseur MD, Hrupka BJ, Muller SA, Lichtenthaler SF. Seizure protein 6 and its homolog seizure 6-like protein are physiological substrates of BACE1 in neurons. Mol Neurodegener 2016, 11, 67. [Google Scholar] [CrossRef]
- Smith MA, Perry G. Free radical damage, iron, and Alzheimer’s disease. J Neurol Sci 1995, 134, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Schubert D, Chevion M. The role of iron in beta amyloid toxicity. Biochem Biophys Res Commun 1995, 216, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Mullner EW, Kuhn LC. A stem-loop in the 3’ untranslated region mediates iron-dependent regulation of transferrin receptor mRNA stability in the cytoplasm. Cell 1988, 53, 815–825. [Google Scholar] [CrossRef]
- Casey JL, Hentze MW, Koeller DM, Caughman SW, Rouault TA, Klausner RD, Harford JB. Iron-responsive elements: Regulatory RNA sequences that control mRNA levels and translation. Science 1988, 240, 924–928. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




