1. Introduction
Synucleinopathies are neurodegenerative disorders that are characterized by the loss of neurons and deposition of misfolded α-synuclein (αSyn) in various cell types. αSyn is a 140-amino acid cytoplasmic protein encoded by the
SNCA gene and is localized in presynaptic terminals of neurons[
1]. Its pathogenic form is composed of insoluble β-sheet-rich structures and phosphorylation at the Ser129 residue[
2,
3]. Synucleinopathies comprise Parkinson’s Disease (PD), Parkinson’s Disease with dementia (PDD) and dementia with Lewy body (DLB), together grouped as Lewy body diseases (LBDs), and multiple system atrophy (MSA). Neuropathologically, LBDs are characterized by inclusion body formation in neurons, specifically in Lewy bodies (LB) and Lewy neurites[
4,
5], collectively referred to as Lewy pathology. Clinical symptoms of LBDs include bradykinesia, rigidity, resting tremor, postural instability, and other non-motor symptoms. On the other hand, MSA is neuropathologically characterized by the accumulation of αSyn in oligodendrocytes, termed glial cytoplasmic inclusions (GCI)[
6]. Clinically, patients with MSA have more rapid progression of disease, with motor and cerebellar symptoms, and autonomic failure[
7].
This diverse nature of synucleinopathies stem from the same αSyn protein, but recent discoveries on the structural and biochemical differences have led to the hypothesis that distinct conformations of misfolded αSyn result in different polymorphs or strains[
8,
9,
10,
11]. Distinct polymorphs have been shown to exhibit different rates of cell-to-cell propagation, postulated to take part in determining the clinical and neuropathological phenotypes in synucleinopathies, as occurs in prion disorders[
12,
13]. Based on this, the seeding behavior of misfolded αSyn in LBD and MSA has been recapitulated
in vitro using seed amplification assays (SAAs), such as real-time quaking-induced conversion (RT-QuIC) or protein misfolding cyclic amplification (PMCA). SAAs are shaking-based cyclic amplification techniques that exploit the property of self-propagation, amplifying αSyn seeds that can be detected and characterized with high sensitivity and specificity in post-mortem brain tissue and
in vivo samples like cerebrospinal fluid and skin[
14,
15]. SAAs allow real-time detection of aggregated αSyn using thioflavin T (ThT) that binds to β-sheet structures of misfolded αSyn. ThT fluorescence is measured at multiple time points, allowing the analysis of kinetic differences between different subjects[
16]. Different temperature, pH, salt and buffer composition can alter the
in vitro fibril growth and sensitivity of the assay, further enabling a selective detection of pathogenic seeds[
17,
18,
19]. Recent studies from our group have identified optimal assay conditions that favor either LBD or MSA seeding activity, and demonstrated that not only seeding differs between synucleinopathies but also between regions within subjects[
20,
21].
Despite the great sensitivity and specificity shown by these assays, post-mortem frozen brain tissues are less available with the widespread decrease in autopsy rates and the rising cost of storage of these specimens in ultra-low temperature freezers. To overcome this limitation, FFPE tissues can be utilized in seeding assays to allow further evaluation of large cohorts. FFPE remains the gold standard for tissue preservation that is widely used in hospitals, brain banks and research laboratories, allowing neuropathological evaluations of the human post-mortem brain. However, the formaldehyde-fixed tissues have various crosslinks between macromolecules and most notably, Schiff base formation between proteins and formaldehyde[
22]. These inter- and intramolecular crosslinks improve the stability of proteins which is crucial for tissue preservation. Although FFPE tissues retain biological information that can allow understanding of underlying disease mechanisms, they are limited to histological analysis once tissues are fixed. Despite this limitation, several studies have reported the successful extraction and recovery of disease-associated prion protein (PrP) from FFPE or formaldehyde-fixed tissues[
23,
24]. Because the immuno-detection of the misfolded PrP may be difficult in archival tissues, some groups have used FFPE tissues to sensitively detect misfolded PrP in chronic wasting disease (CWD) and sporadic Creutzfeldt–Jakob disease (sCJD) using SAAs[
25,
26]. Although formaldehyde-fixation decreases protein extraction efficiency, extraction of misfolded PrP from FFPE tissue was sufficient using a simple PBS buffer conventionally used to homogenize frozen tissue, and subsequent SAAs were not limited by the low extraction efficiency, given the high infectivity profile of misfolded prion proteins[
27].
In addition, a handful of studies have successfully detected and amplified misfolded αSyn in FFPE skin, submandibular glands, and gastrointestinal tract[
28,
29,
30]. However, the authors consistently reported limitations in the extraction efficiency of proteins, presumably due to formaldehyde crosslinks formed between proteins and other macromolecules during fixation[
31,
32,
33], remnant paraffin residues in tissue or the low amount of tissue used (5-6-µm tissue sections). Different techniques have been reported to remove paraffin residues and reverse formaldehyde crosslinks using various protein extraction buffers[
34,
35,
36,
37,
38]. Among the different techniques, the application of thermal energy via antigen retrieval and extraction buffers corrected for ionic strength differences were determined to be the key elements for efficient protein extraction[
39,
40,
41,
42].
Considering that FFPE tissues can provide the advantage of linking seeding behavior with neuropathologically characterized brain regions, and allow the investigation of larger cohorts, we aimed to establish a streamlined protein extraction protocol specific for the evaluation of αSyn seeding in FFPE human brain tissue.
2. Materials and Methods
2.1. Case Selection
6 subjects with LBD (4 females), 2 subjects with MSA (1 female) and 1 subject lacking pathology (1 female) were selected from the University Health Network-Neurodegenerative Brain Collection (UHN-NBC, Toronto, Canada). The temporal cortex of 1 LBD subject was used for optimization and the substantia nigra was used for validation. For the rest of the experiments, the temporal cortex of LBD subjects and cerebellum of MSA subjects were used in this study, in addition to the parietal cortex of a control subject. Case selection was based on a systematic neuropathological evaluation using the diagnostic criteria of neurodegenerative conditions and co-pathologies[
4,
43]. Demographics and neuropathological evaluations are summarized in
Table 1. Human brain tissues were collected during autopsy with informed consent from patients or their relatives and approved by institutional review boards. All tissues were fixed in formaldehyde for 2 weeks and embedded in paraffin for staining and storage for research purposes. This study was approved by the University Health Network Research Ethics Board (Nr. 20-5258).
CAA, cerebral amyloid angiopathy; LATE-NC, limbic-predominant age-related TDP-43 encephalopathy-neuropathological change; LBD, Lewy body disease; Aβ, amyloid-β; ARTAG, age-related tau astrogliopathy; GM, grey matter; WM, white matter; MSA, multiple system atrophy; PART, primary age-related tauopathy; AGD, argyrophilic grain disease.
2.2. Immunohistochemistry
FFPE tissues were cut into 4.5-µm-thick sections using a microtome and immuno-stained with the monoclonal 5G4 anti-mouse antibody[
44], that labels only the disease-associated αSyn (1:4000; 5 min pre-treatment with 80% formic acid; Roboscreen, Leipzig, Germany). Target retrieval was performed using the DAKO EnVision FLEX Target Retrieval Solution. The DAKO EnVision detection kit, EnVision FLEX peroxidase-blocking solution, 3,3'-Diaminobenzidine chromogen and EnVision FLEX+ mouse linker (Dako, Glostrup, Denmark) was used to visualize the antibody reactions.
Digital images were obtained with TissueScope
TM LE120 and TissueSnap
TM (Huron, Saint Jacobs, Canada) and cropped using HuronViewer software (
Figure S1).
2.3. Sample Collection
To test the protein extraction efficiency, FFPE tissues were prepared by 1) cutting 4.5-µm-thick sections on positively charged glass slides totalling up to 45-µm of tissue, 2) cutting 4.5-µm-thick scrolls in 1.5 mL low protein binding tubes (Sarstedt, Nümbrecht, Germany) totalling up to 45-µm of tissue, 3) collecting 4-mm micro-punch of the of the FFPE block, 4) collecting 2-mm micro-punch of the FFPE block, and 5) collecting 4-mm micro-punch of the formaldehyde-fixed tissue. To ensure that FFPE blocks were re-usable after micro-puncture, paraffin was melted prior to micro-punch collection and the rest of the tissue was re-embedded. To melt the wax, FFPE blocks were placed on Tissue-Tek® base molds and incubated in the paraffin-melting chamber of the embedding machine (Sakura Finetek, Nagano, Japan) for 10 minutes at 62°C. Disposable biopsy punch with plunger (Integra Miltex, York, PA, USA), conventionally used for skin biopsies, was used to collect micro-punches of the FFPE tissue block. Biopsy punches were safely discarded after collecting each sample to avoid cross-contamination.
2.4. Deparaffinization
To test the efficiency of paraffin removal, two methods were tested: 1) xylene-ethanol, and 2) heptane-methanol. For xylene-ethanol, tissues were incubated twice in fresh xylene for 10 minutes at room temperature, then rehydrated in a series of graded ethanol (90%, 80%, 70%, 50%) and in distilled water at room temperature for 5 minutes each. Tissue on slides was placed in racks and submerged into the staining dish while for tissue in low protein binding tubes (C-tubes in optimized protocol), the supernatant was carefully removed and replaced with the next solution. For heptane-methanol, tissues were incubated in heptane for 10 minutes at room temperature, vortexed for 10 seconds in 5-minute intervals, then mixed with 5% v/v methanol, and vortexed for 10 seconds. The supernatant was then removed and tissues were dried for 5 minutes at room temperature, in a biosafety cabinet. Tissue on slides was placed in racks and dipped into the staining dish several times.
After deparaffinization and rehydration, tissue on slides was carefully scraped with a microtome blade coated in Sigmacote (Sigma, St. Louis, MI, USA) for 2 minutes and dried for 20 minutes in a biosafety cabinet. Scraped tissues were transferred to a clean 1.5 mL low protein binding tube and weighed.
2.5. Antigen Retrieval and Dissociation
The FFPE Tissue Dissociation Kit (Miltenyi BioTec, Auburn, CA, USA) was used for antigen retrieval and dissociation according to the manufacturer’s instructions with slight modifications. In brief, deparaffinized tissues were transferred to C-tubes and with the provided buffer, incubated in an 80°C water bath for 75 minutes. Once the samples were cooled, the supernatant was carefully removed, and with the provided buffer and enzymes, the samples were dissociated on the gentleMACS™ Dissociator (Miltenyi BioTec, Auburn, CA, USA). The dissociated tissues were then transferred into 1.5 mL low protein binding tubes and centrifuged at 4°C for 5 minutes at 5000 g. The supernatant was discarded and the tissue pellet was resuspended in PBS. The tubes were centrifuged once more, at 4°C for 5 minutes at 5,000 g and the supernatant was removed. The resulting tissue pellet was weighed and used for protein extraction.
2.6. Protein Extraction and Quantification
To test the efficiency of different protein extraction buffers, deparaffinized tissues were weighed and carefully transferred to Precellys® 2 mL Protein Safe Soft Tissue Homogenizing tubes containing zirconium oxide beads (CK14; Bertin Corp, Rockville, MD, USA). Ice-cold 10% weight/volume (w/v) extraction buffers (
Table 2) were added into the tube and placed in the Minilys bead homogenizer (Bertin Technologies SAS, Montigny-le-Bretonneux, France) for 30 seconds at high speed. The tubes were put on ice for 5 minutes and the homogenization was repeated 2 more times. For tissues processed with antigen retrieval and dissociation, the tissue pellet was weighed and resuspended in ice-cold 10% w/v PBS before being transferred to the homogenizing tubes. Following homogenization, the tissue homogenate was transferred to 1.5 mL low protein binding tubes, incubated for 10 minutes on ice, vortexed for 10 seconds and re-placed on ice. The extraction step was repeated a total of 4 times and following the last round of vortexing, the tubes were centrifuged for 10 minutes at 4°C 10,000
g as previously described[
20]. The supernatant was collected in a single 1.5 mL low protein binding tube and then aliquoted in 0.5 mL low protein binding tubes to avoid excessive freeze-thaw cycles.
Following protein extraction, bicinchoninic acid protein (BCA) assay (Thermo Scientific, Waltham, MA, USA) was performed to determine the concentration total protein in each sample.
2.7. αSyn SAA Protocol
SAA was performed as previously described[
20]. In brief, SAA reaction mixture was composed of 40 mM phosphate buffer (pH 8.0), 350 mM Na
3Citrate (Sigma, St. Louis, MI), 0.1 mg/mL K23Q human recombinant αSyn (Impact Biologicals, Pennsylvania, USA) and 10 µM ThT (Sigma, St. Louis, MI). Recombinant αSyn was thawed from -80°C storage and filtered through a 100 kDa Amicon ultra-0.5 centrifugal filter unit (Sigma, St. Louis, MI) for 20 minutes, 4°C at 10,000
g. 40 µL of the reaction mixture and 10 µL of the FFPE-extracted protein (5 µg of total protein from PBS-soluble fraction) was loaded into each well of a 384-well plate with a clear bottom (Thermo Scientific, Waltham, MA, USA). For serial dilution experiments, 1.25, 2.5, 5 and 10 µg of total protein were used as seeds for the SAA reaction. For every plate, a positive control (1 µg of pre-formed αSyn fibrils) and a negative control (deionized water) was included. The plate was sealed with DNase-/RNase-free clear polyolefin sealing tape (Thermo Scientific, Waltham, MA, USA).
To evaluate the differential seeding behavior between LBD and MSA subjects, two SAA protocols were performed[
20,
21]. In the first protocol, an LBD-favoring SAA, the plate was incubated at 42°C with cycles of 1-minute shaking and 1-minute rest. In the second protocol, an MSA-favoring SAA, the plate was incubated at 37°C in a BMG FLUOstar Omega plate reader with cycles of 1-minute shaking (400 rpm double orbital) and 14-minute rest. In both protocols, ThT fluorescence measurements (450 ± 10 nm excitation and 480 ± 10 nm emission, bottom read) were taken every 15 minutes for a period of 90 hours.
2.8. Transmission Electron Microscopy
10 μL of αSyn SAA end-products were loaded onto freshly glow-discharged 400 mesh carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA, USA) and adsorbed for 1 min. Once dry, the grids were visualized using a Talos L120C transmission electron microscope (Thermo Scientific, Waltham, MA, USA) using an acceleration voltage of 200 kV. Electron micrographs were recorded using an Eagle 4kx4k CETA CMOS camera (Thermo Scientific, Waltham, MA, USA).
2.9. Statistical Analysis
All statistical analyses were performed using Prism (v9, GraphPad Software, San Diego, CA). Kinetic curves were plotted and area under the curve (AUC) was obtained using Prism. Kinetic curves were compared using unpaired two-tailed parametric t-test with significance declared at p < 0.05.
3. Results
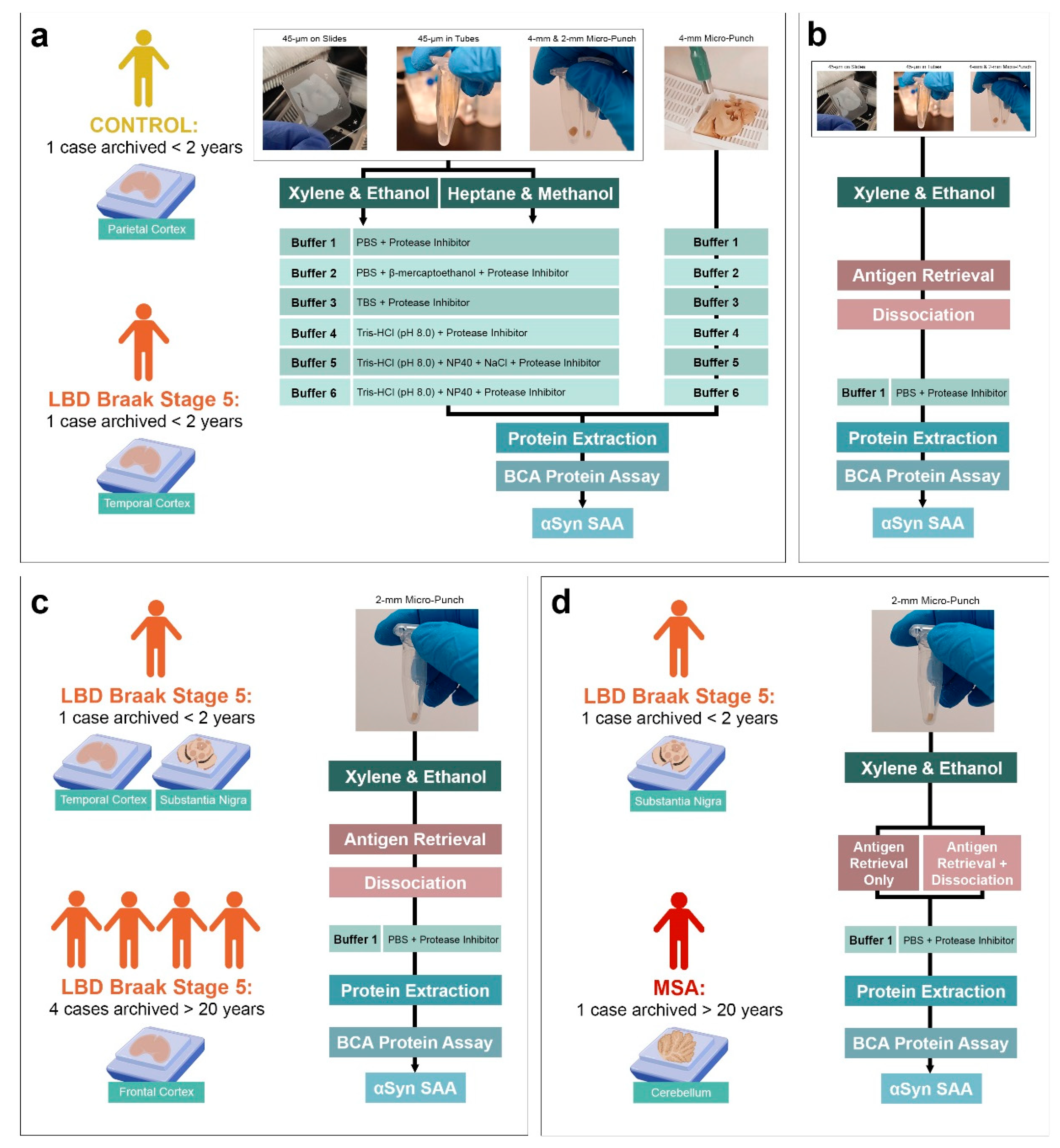
To evaluate whether αSyn seeding is preserved in FFPE human brain tissue, we tested different tissue preparations, deparaffinization methods and extraction buffers that have been previously reported (
Figure 1a). However, we observed minimal seeding activity with these methods. In order to determine the most efficient protein extraction method that yields the highest protein concentration while preserving the disease-specific αSyn seeding behavior, we then tested a novel approach using a heat-induced antigen retrieval and dissociation process using a commercially available kit (
Figure 1b). Using this novel approach, we observed an improvement in the protein extraction efficiency compared to conventional extraction protocols and an increase in αSyn seeding. We validated our optimized protocol using FFPE brain tissue from 5 LBD (4 archived more than 20 years) and 2 MSA subjects (1 archived more than 20 years) (
Figure 1c).
3.1. Protein Extraction and SAA from FFPE Human Brain Tissue
3.1.1. Comparison of protein extraction efficiency using different tissue preparation, deparaffinization and extraction buffers
As previous FFPE protein extraction protocols have used 5- or 6-µm-thick FFPE sections either mounted on glass slides or scrolls in tubes, we tested the protein extraction efficiency using a total of ten 4.5-µm-thick FFPE temporal cortex sections deparaffinized on positively charged glass slides or directly placed into low protein binding tubes. Alternatively, to conserve the FFPE tissues as much as possible while maximizing the amount of tissue used, we also tested the protein extraction efficiency from a 4-mm micro-punched FFPE tissue. With slight modifications to previously reported protein extraction buffers[
37], we compared the protein extraction efficiency of six different extraction buffers made in-house. The composition of different buffers is outlined in
Table 2.
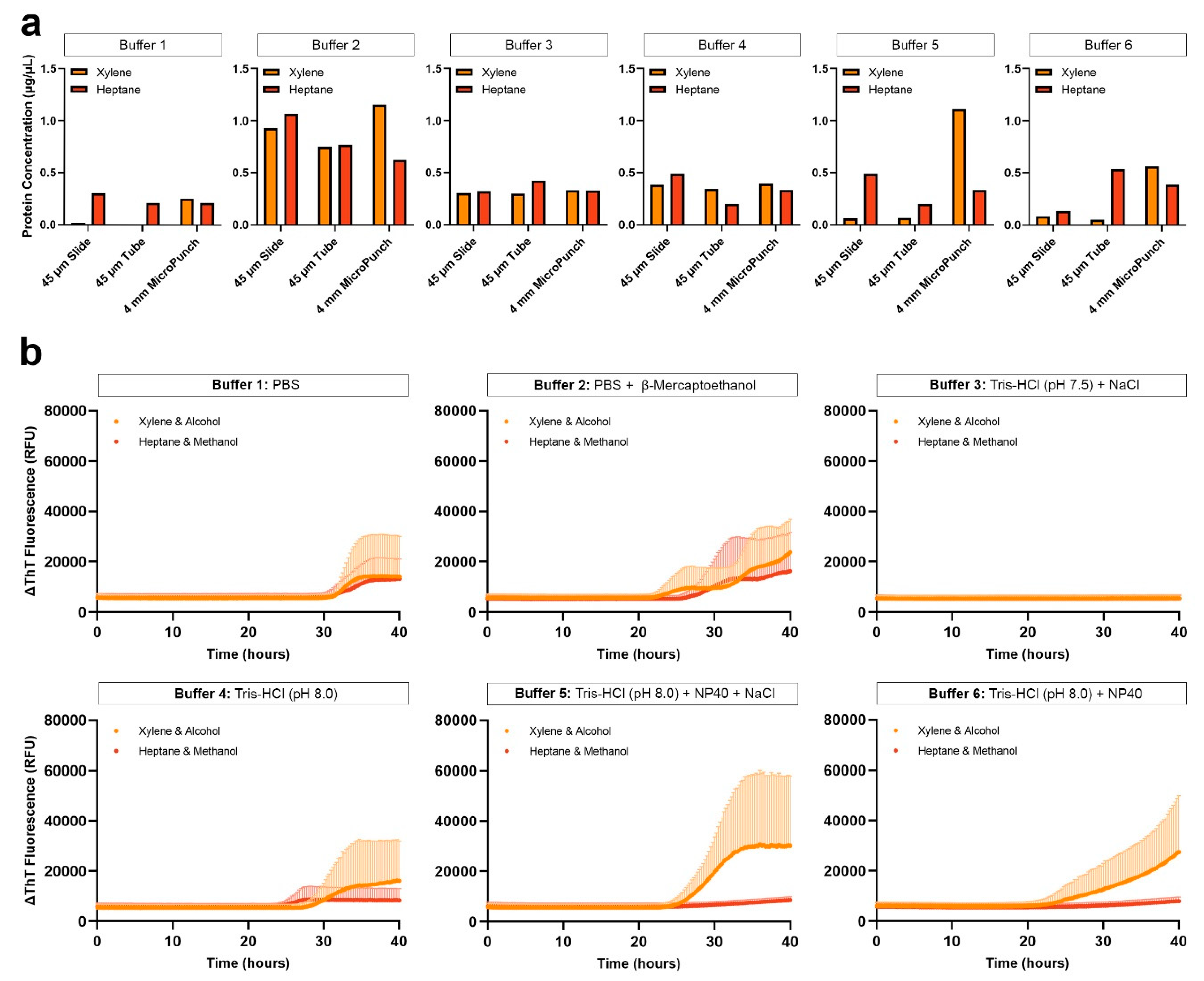
Using the xylene-ethanol deparaffinization, we found that protein extraction with buffer 1 yielded the lowest amount of protein across all tissue preparation types and at the other extreme, BCA showed the highest protein measurement with buffer 2 (
Figure 2a). When buffer 1, 2, 5 or 6 was used, the protein concentrations of FFPE micro-punch were higher than those from FFPE sections. On the other hand, FFPE micro-punch had similar protein concentration to those from FFPE sections when buffers 3 or 4 were used with xylene-ethanol deparaffinization. Between FFPE sections on slides and in tubes, protein concentrations had subtle differences across the extraction buffers, except buffer 2 where FFPE sections deparaffinized on slides had higher concentration than those in tubes.
Based on previous studies highlighting the importance of complete paraffin removal without using toxic solvents like xylene that can impeded protein extraction[
45], we tested the protein extraction efficiency using an alternative deparaffinization and rehydration method consisting of heptane-methanol. All extraction buffers, with the exception of buffer 4, yielded higher protein concentrations in FFPE sections when deparaffinized with heptane-methanol, compared to those deparaffinized with xylene-ethanol. When buffer 4 was used with heptane-methanol deparaffinization, FFPE sections on slides had higher protein concentrations while FFPE sections in tubes had lower concentrations compared to those deparaffinized with xylene-ethanol. For proteins extracted from 4-mm FFPE micro-punch, concentrations were lower in all extraction buffers with heptane-methanol deparaffinization, except buffer 3, compared to those deparaffinized with xylene-ethanol. When buffer 3 was used, protein concentrations were similar between those deparaffinized with xylene-ethanol or heptane-methanol. However, with heptane-methanol deparaffinization, we observed a thick layer of paraffin residue on top of the buffers at the end of the protein extraction, regardless of the yield of protein extracted.
3.1.2. Seeding of αSyn extracted using different tissue preparation, deparaffinization and extraction buffers
The heptane-methanol deparaffinization yielded varying protein concentrations across all extraction buffers but we observed consistent improvement in extraction using 4-mm FFPE micro-punch across all extraction buffers using xylene-ethanol deparaffinization. Therefore, we used the 4-mm FFPE micro-punch of the test LBD subject to compare the seeding of αSyn extracted using different deparaffinization and extraction buffers. αSyn from tissues deparaffinized with heptane-methanol showed seeding at around 30 hours with buffer 1, inconsistent seeding at around 28 hours with buffer 2 and no seeding with buffers 3, 4, 5 or 6 (
Figure 2b). We suspect that this lack of seeding may be due to the insufficient removal of paraffin using the heptane-methanol deparaffinization. For tissues deparaffinized with xylene-ethanol, misfolded αSyn extracted with buffer 1 showed seeding at 30 hours, inconsistent seeding at 22 hours with extraction buffer 2 and no seeding with extraction buffer 3. Tris-containing extraction buffers 4, 5 and 6, but not buffer 3, showed αSyn seeding. When proteins were extracted with only tris-HCl at pH 8.0 (buffer 4), we observed αSyn seeding activity at 30 hours with the lowest AUC compared to buffers 5 and 6, while those extracted with NaCl and NP40 (buffer 5) had higher AUC compared to buffer 4 and those extracted with NP40 (buffer 6) had higher AUC than both tris-containing buffers 4 and 5. Altogether, we observed minimal seeding activity in tissues deparaffinized with xylene-ethanol but delayed or negative αSyn seeding in tissues deparaffinized with heptane-methanol with visibly incomplete deparaffinization. Therefore, we resorted to using xylene-ethanol henceforward.
3.2. Protein Extraction and SAA from Formaldehyde-Fixed Human Brain Tissue
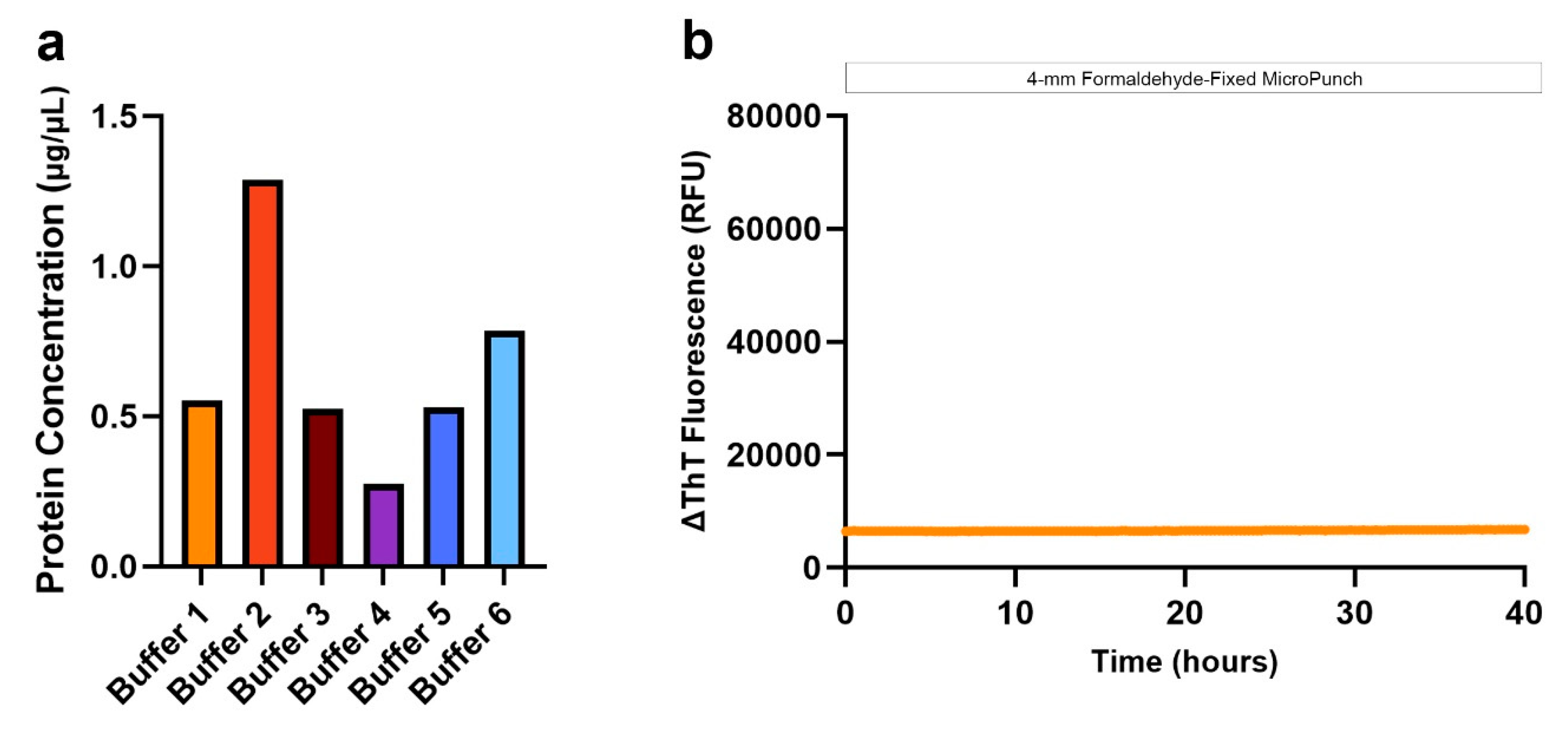
Based on previous SAA studies and our observation that paraffin residues contribute to the low protein extraction efficiency, we compared the protein concentrations obtained from formaldehyde-fixed and FFPE temporal cortex. As expected, BCA measurements showed higher protein concentration from a 4-mm micro-punch of formaldehyde-fixed tissue than from a 4-mm micro-punch of FFPE tissue, when extracted with buffers 1, 2, 3 or 6 with xylene-ethanol deparaffinization, and buffers 1, 2, 3, 5, 6 with heptane-methanol deparaffinization (
Figure 3a). To avoid buffer carry-over, we evaluated the seeding of αSyn extracted using buffer 1 and although the protein concentration was the highest with formaldehyde-fixed brain tissue, we did not observe seeding (
Figure 3b). Because hospitals and brain banks rarely archive formaldehyde-fixed human brain tissue on a large scale for future research, and because our results were negative, we eliminated this tissue type from our study. Further investigation and additional optimization are needed to reverse the crosslinks and extract proteins with seeding potential from 2-week formaldehyde-fixed human brain tissues.
3.3. Optimization of FFPE Protein Extraction and αSyn SAA
3.3.1. FFPE protein extraction with heat-induced antigen retrieval and dissociation
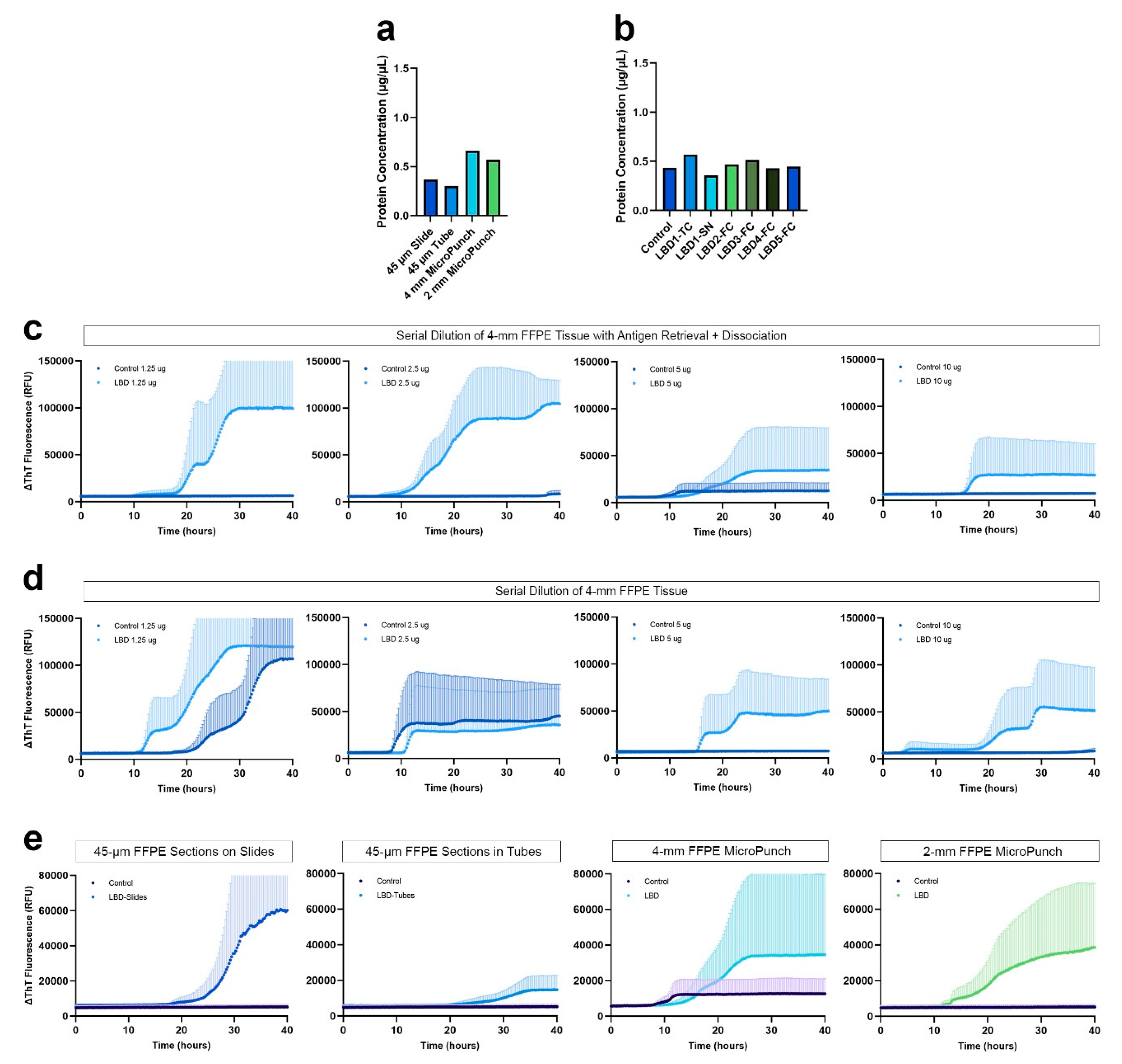
Despite the effort, conventional protein extraction from FFPE tissue was inadequate to detect αSyn seeding. Thus, based on previous literature emphasizing the importance of heat exposure for crosslink reversal, we incorporated a heat-induced antigen retrieval together with dissociation prior to protein extraction using a commercially available kit, and in addition, resorted to a simple PBS extraction buffer. Using xylene-ethanol deparaffinization and subsequent antigen retrieval and dissociation, we observed more than 2-fold increase in the yield of proteins extracted with buffer 1, even with a 2-mm micro-punch (
Figure 4a). Specifically, protein concentrations from highest to lowest were as follows: 4-mm FFPE micro-punch > 2-mm FFPE micro-punch > 45-µm FFPE sections deparaffinized on slide > 45-µm FFPE sections deparaffinized in tubes. We also compared the protein extraction efficiency with FFPE tissue that had been archived for more than 20 years. The amount of protein extracted from a 2-mm micro-punch in the frontal cortex of 4 LBD subjects archived for more than 20 years was similar to that of the temporal cortex and substantia nigra of LBD1 that have been archived for less than 2 years (
Figure 4b).
3.3.2. Optimization of αSyn SAA using proteins extracted from FFPE
We compared the αSyn seeding from 4-mm FFPE micro-punch using buffer 1, with and without the additional antigen retrieval and dissociation prior to protein extraction. When we evaluated the seeding of serially diluted proteins extracted with antigen retrieval and dissociation, we observed consistent αSyn seeding across the 4 LBD replicates with 5 and 10 µg of protein (
Figure 4c). The control case was negative for all 4 dilutions. However, with proteins extracted without the antigen retrieval and dissociation, we observed that the seeding of αSyn was inconsistent across the 4 replicates for all 4 dilutions, with the control case also showing positive curves with 1.25 and 1.50 µg of protein (
Figure 4d). Without the antigen retrieval and dissociation prior to protein extraction, the distinction between LBD and control was unclear and even a 4-mm micro-punch was not enough to consistently seed misfolded αSyn in FFPE LBD tissue. In contrast, 5 µg of proteins extracted with antigen retrieval and dissociation was sufficient to generate consistent, positive αSyn seeding in FFPE LBD subjects, while showing negative curves for controls. Therefore, we used 5 µg of total protein in all of our SAAs.
3.3.3. αSyn seeding of proteins extracted from FFPE tissue archived for more than 20 years
To test whether seeding is preserved in FFPE human brain tissue that were archived for more than 20 years, we performed the same seeding assay with the frontal cortex of four additional LBD subjects. Not only did we observe αSyn seeding, but also intra-subject variability in seeding activity (
Figure S2). Comparing frontal cortex of the 4 additional LBD subjects (LBD 2-5), each subject had different lag phases and AUCs.
3.3.4. αSyn seeding of proteins extracted from FFPE tissue with different tissue preparations
Comparing the seeding of αSyn between different types of tissue preparations, both 4-mm and 2-mm micro-punch of FFPE temporal cortex from LBD subject showed positive curves. Therefore, we determined that 2-mm micro-punch was sufficient for future experiments. Interestingly, proteins extracted from 45-µm FFPE sections on slides had greater AUC compared to proteins extracted from 45-µm FFPE sections in tubes (
Figure 4e). During the deparaffinization and rehydration solution changes, we unexpectedly observed tissue loss due to breakage of thin sections and subsequent removal by micro-pipettes. This could not be resolved by centrifuging the tubes. Consequently, seeding of αSyn extracted from FFPE sections deparaffinized in tubes was significantly lower with delayed seeding and low maximum ThT levels compared to those deparaffinized on slides (p < 0.0001) (
Figure 4e). Therefore, if FFPE tissues are deparaffinized and rehydrated on slides prior to protein extraction, tissue loss can be avoided, resulting in increased protein amount during extraction and more reliable αSyn seeding activity.
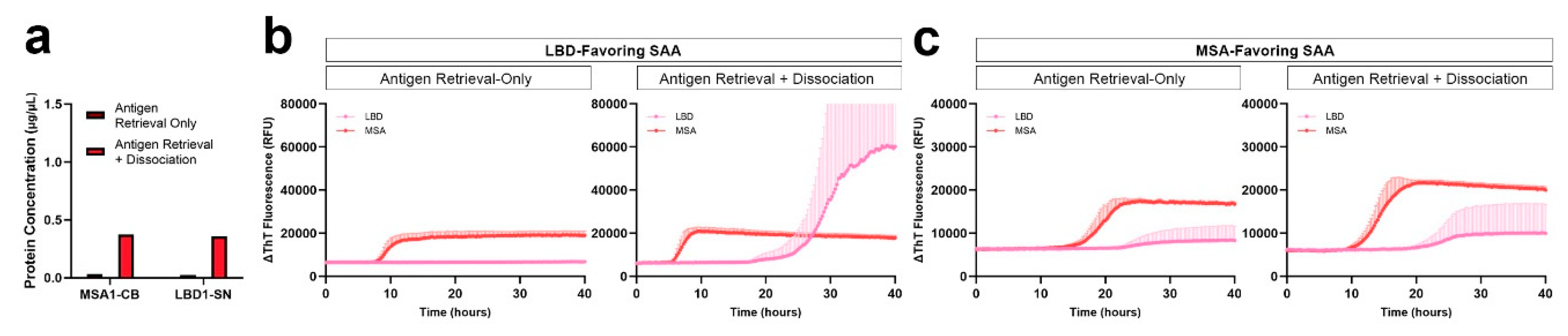
3.4. Comparison of Protein Extraction Efficiency and αSyn Seeding Activity with and without Dissociation
Taking into consideration the vigorous tissue treatment in the dissociation step that may lead to loss of proteins during the process, we tested whether the dissociation step could be eliminated. We used a 2-mm FFPE micro-punch of the substantia nigra from the test LBD subject and the cerebellum from the test MSA subject. In order to compare both LBD and MSA, we used both LBD-favoring and MSA-favoring SAA protocol previously established in our group. In both FFPE tissue of LBD and MSA subjects, the yield of protein extracted from tissue with only antigen retrieval was significantly lower compared to those with both antigen retrieval and dissociation prior to protein extraction (
Figure 5a). Consistent with our extraction results, proteins extracted from LBD and MSA FFPE tissue with only the antigen retrieval showed longer lag phase and lower AUC compared to those that were extracted after both the antigen retrieval and dissociation. The LBD-favoring SAA amplifies the seeding of misfolded αSyn in LBD more than MSA. αSyn seeding of LBD was negative when proteins were extracted with antigen retrieval-only while seeding was observed with both antigen retrieval and dissociation, demonstrating a significant difference in seeding between LBD tissues with and without dissociation (
p < 0.0001) (
Figure 5b). In addition, the MSA FFPE tissue also showed lower AUC when extracted with antigen retrieval-only, compared to those extracted with both antigen retrieval and dissociation, exhibiting a significant difference between seeding of MSA tissues with and without dissociation (
p = 0.0103). On the other hand, the MSA-favoring SAA amplifies the seeding of misfolded αSyn in MSA more than LBD. Using the MSA-favoring protocol, αSyn seeding of LBD had lower AUC when extracted using antigen retrieval-only compared to those extracted with both antigen retrieval and dissociation (
Figure 5c). This shows a significant difference between αSyn seeding of LBD tissues with and without dissociation (p < 0.0001). Lastly, when proteins were extracted with antigen retrieval-only, αSyn seeding of MSA showed lower AUC compared to those extracted with both the antigen retrieval and dissociation, demonstrating a significant difference in seeding between MSA tissues with and without dissociation (p < 0.0001).
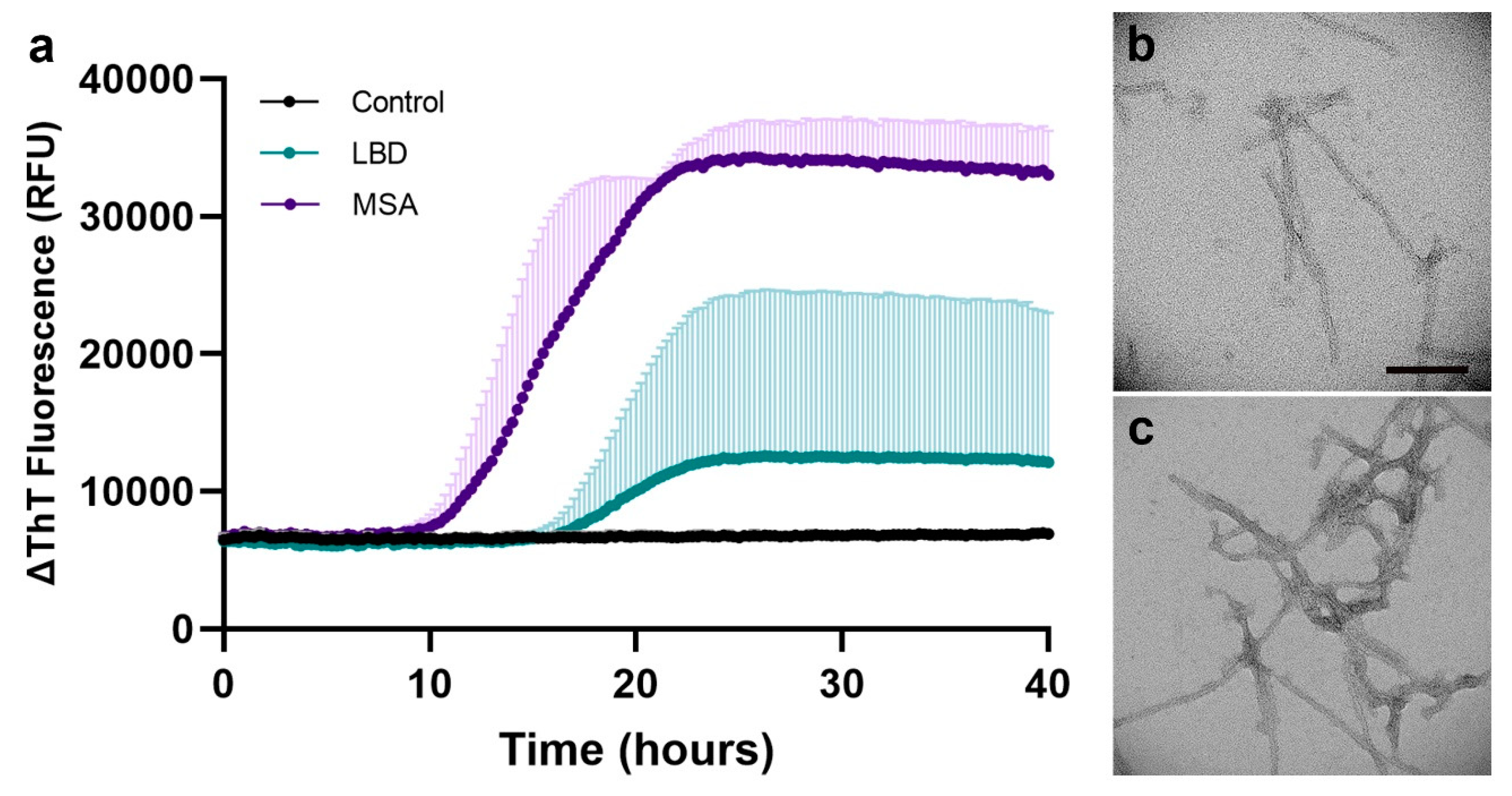
3.5. Replication of Disease-Specific Seeding Patterns in FFPE Human Brain Tissue
To further validate our optimized protein extraction and SAA results, we compared the seeding of additional LBD and MSA subjects using 5 µg of extracted proteins from 2-mm FFPE micro-punch, deparaffinized with xylene-ethanol, and processed with antigen retrieval and dissociation prior to protein extraction using buffer 1. With the MSA-favoring SAA, we observed higher AUC in MSA compared to LBD while misfolded αSyn from LBD showed positive seeding but with lower AUC compared to MSA (
Figure 6a). We did not observe seeding in the control subject. This is consistent with our previous findings in the post-mortem frozen human brain tissue[
20,
21].
Altogether, we have confirmed that FFPE human brain tissue preserves seeding potential in short and long-term archived materials, and most importantly shows disease-specific seeding between LBD and MSA subjects. Our optimized protocol is outlined in
Table S1.
3.6. Structural Validation of Disease-Specific FFPE-Derived αSyn
To confirm the structural differences between LBD and MSA as reported in the literature, we evaluated the fibrils generated during the seeding assays under TEM. Since proteins extracted from FFPE human brain tissue are much lower in quantity compared to those extracted from post-mortem frozen brain, we used our optimized protein extraction protocol to extract protein from a whole FFPE block of an additional LBD and MSA subject, archived for less than 2 years. We confirmed the disease-specific, ultra-structural differences between the αSyn fibrils in LBD and MSA, where fibrils generated from LBD-seeded reaction had long and thin fibrils with occasional twists (
Figure 6b) compared to those generated from MSA-seeded reaction that had thinner fibrils with intertwined and frequently twisted strands (
Figure 6c).
4. Discussion
In this study we have demonstrated that disease-specific seeding of misfolded αSyn, including ultra-structural differences of the generated fibrils, is preserved in FFPE human brain tissue (
Figure 5). Extraction of sufficient amount of high-quality proteins from FFPE tissue has consistently been reported to be challenging, particularly in seeding studies where the detection of misfolded αSyn was relatively low (75-76% sensitivity)[
28,
29]. Many studies have investigated different deparaffinization methods and extraction buffers, including commercially available FFPE protein extraction kits, to achieve a higher protein yield from FFPE tissue[
46,
47,
48,
49]. Although commercially available protein extraction kits can be effective for proteomic analysis, the extraction leaves the proteins suspended in extraction buffers that are composed of proprietary reagents, which can alter the performance of SAAs. To overcome these limitations, our study has established an optimized protein extraction protocol that uses antigen retrieval and dissociation to remove paraffin residues and reverse crosslinks, leaving a pellet of dissociated tissue that can be used for protein extraction with a simple PBS buffer commonly used to homogenize post-mortem frozen brain tissue for SAAs. Our protocol also circumvents buffer carry-over into SAAs, unlike FFPE extraction kits and other extraction methods, preserving the original SAA buffer composition. Furthermore, the tissue pellet can be resuspended in any buffer of choice, providing wide applicability to other SAA protocols or biochemical analyses.
Previous SAA studies have reported the detection of misfolded αSyn in formaldehyde-fixed human tissue of PD, incidental LBD and MSA subjects and more recently, Hepker
et al. developed a kinetic assay seeding ability recovery (KASAR) protocol using FFPE core of globus pallidus with great sensitivity and specificity[
50,
51,
52]. However, despite the successful findings, brain tissues were fixed for a relatively short amount of time (18 hours) or in a less concentrated formaldehyde solution (4%) or fixed in paraformaldehyde that does not penetrate the tissue as rapidly. This does not reflect the tissue processing procedures used in most hospitals and brain banks. Short fixation time or fixation in diluted concentrations can lead to similar seeding potential seen with αSyn extracted from frozen brain homogenates. In this study, all our brain tissue has been processed in 10% neutral buffered formaldehyde for 2 to 3 weeks prior to systemic tissue processing and paraffin-embedding, highlighting the applicability of our findings to archived FFPE human brain tissues across many hospitals, brain banks and research laboratories. In addition, we have obtained high protein yield and observed αSyn seeding in FFPE human brain tissues that were fixed and embedded in paraffin more than 20 years ago. Most importantly, to preserve the disease-specific αSyn seeding, we avoided long exposure to high temperatures for crosslink reversal, as it is unclear whether prolonged exposure to heat alters the original fibril structure or results in a partial loss of proteins.
Furthermore, most studies utilizing proteins extracted from FFPE only use a small number of FFPE sections (5-6 µm), leading to low protein concentrations[
28,
29]. However, with our streamlined protocol, including antigen retrieval and dissociation, the proteins extracted from a 2-mm FFPE micro-punch were sufficient to generate a positive seeding activity. We have also demonstrated that disease-specific seeding activity is preserved in FFPE brain tissue. Supporting the notion that different polymorphs of αSyn determine the cell-to-cell spreading and the existence of distinct cell-type specific polymorphs[
53], indeed, we found that MSA subjects consistently showed higher seeding activity, with shorter lag phases compared to LBD subjects in both conditions that favor either LBD or MSA seeding. The optimized method also offers to capture the ultra-structural differences between LBD and MSA fibrils, when αSyn fibrils generated from our FFPE SAA are evaluated under TEM. This finding is consistent with the structural variability reported in the literature, where thin αSyn fibril formation in LBD subjects may be due to the single protofilament structure observed with cryo-electron microscopy and thick intertwined fibril formation in MSA subjects can be due to fibril structure made of two different protofilaments[
10,
11].
A variety of αSyn polymorphs may exist in different subcellular locations in a single human brain tissue. It is unknown whether a subset of proteins (i.e., membrane or cytoplasmic) are preferentially extracted during the FFPE protein extraction process, or whether certain processes in the protein extraction step induce a partial removal of either soluble or insoluble proteins, emphasizing the need for cautious interpretation. Despite this limitation, we observed clear differences between LBD and MSA subjects using our optimized FFPE protein extraction and SAA method. With frozen tissue remaining as the gold standard for protein analysis and seeding assays, our streamlined protein extraction protocol can reveal the disease-specific seeding activity preserved in FFPE human brain tissue. This will allow the re-evaluation of large collections of archived FFPE tissues, paving the path for future protein-based studies.
5. Conclusions
To the best of our knowledge, this study is the first to recapitulate disease-specific differences between LBD and MSA in post-mortem FFPE human brain tissue. We have developed a streamlined protein extraction protocol that extends the utility of FFPE tissue beyond immunohistochemical purposes. Furthermore, our study allows the expansion of research on distinct αSyn polymorphs and further subclassification of disease based on seeding differences, with the use of largely available and highly characterized FFPE human brain tissue as an alternative to frozen tissue.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Immunohistochemistry of disease-specific αSyn detected in FFPE subjects; Figure S2: Intra-subject heterogeneity of αSyn seeding activity in FFPE LBD human brain tissue archived for < 2 years and > 20 years; Table S1: Summary of optimized protein extraction protocol for FFPE brain tissue.
Author Contributions
Conceptualization, A.K., I.M.V., A.E.L. and G.G.K.; methodology, A.K., I.M.V. and G.G.K.; validation, A.K., I.M.V. and G.G.K.; formal analysis, A.K. and G.G.K.; investigation, A.K., I.M.V., J.L., and G.G.K.; resources, A.E.L. and G.G.K.; writing – original draft preparation, A.K. and G.G.K.; writing – review & editing, A.K., I.M.V., J.L., A.E.L. and G.G.K.; visualization, A.K. and G.G.K.; supervision, G.G.K.; project administration, A.K., I.M.V. and G.G.K.; funding acquisition, A.E.L. and G.G.K.
Funding
This study was supported by the Edmond J Safra Philanthropic Foundation, the Krembil Foundation, the Maybank Foundation, and the Rossy Foundation. The funding bodies did not take part in design of the study, in collection, analysis, or interpretation of data, or in writing the manuscript.
Institutional Review Board Statement
This study was approved by the University Health Network Research Ethics Board (Nr. 20-5258).
Informed Consent Statement
Autopsy tissues from human brains were collected with informed consent of patients or their relatives and approval from local institutional review boards.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors particularly acknowledge the patients and their families for their donation. We would like to acknowledge Lindsey Fiddes and the Microscopy Imaging Lab at the Temerty Faculty of Medicine, University of Toronto for their support with the electron microscopy. We also would like to acknowledge Ali Karakani for the technical support and Dr. Joel Watts for equipment use. Figure 1 was designed with Biorender.com.
Conflicts of Interest
G.G.K. holds shared patent for the 5G4 antibody. Other authors declare no competing interests.
References
- Maroteaux, L.; Campanelli, J.; Scheller, R. Synuclein: A Neuron-Specific Protein Localized to the Nucleus and Presynaptic Nerve Terminal. J. Neurosci. 1988, 8, 2804–2815. [Google Scholar] [CrossRef]
- Fujiwara, H.; Hasegawa, M.; Dohmae, N.; Kawashima, A.; Masliah, E.; Goldberg, M.S.; Shen, J.; Takio, K.; Iwatsubo, T. α-Synuclein Is Phosphorylated in Synucleinopathy Lesions. Nat. Cell Biol. 2002, 4, 160–164. [Google Scholar] [CrossRef]
- Vilar, M.; Chou, H.T.; Lührs, T.; Maji, S.K.; Riek-Loher, D.; Verel, R.; Manning, G.; Stahlberg, H.; Riek, R. The Fold of α-Synuclein Fibrils. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 8637–8642. [Google Scholar] [CrossRef]
- Kovacs, G.G. Molecular Pathology of Neurodegenerative Diseases: Principles and Practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.-Y.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. α-Synuclein in Lewy Bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Papp, M.I.; Kahn, J.E.; Lantos, P.L. Glial Cytoplasmic Inclusions in the CNS of Patients with Multiple System Atrophy (Striatonigral Degeneration, Olivopontocerebellar Atrophy and Shy-Drager Syndrome). J. Neurol. Sci. 1989, 94, 79–100. [Google Scholar] [CrossRef]
- Gilman, S.; Wenning, G.K.; Low, P.A.; Brooks, D.J.; Mathias, C.J.; Trojanowski, J.Q.; Wood, N.W.; Colosimo, C.; Durr, A.; Fowler, C.J.; et al. Second Consensus Statement on the Diagnosis of Multiple System Atrophy. Neurology 2008, 71, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Bousset, L.; Pieri, L.; Ruiz-Arlandis, G.; Gath, J.; Jensen, P.H.; Habenstein, B.; Madiona, K.; Olieric, V.; Böckmann, A.; Meier, B.H.; et al. Structural and Functional Characterization of Two Alpha-Synuclein Strains. Nat. Commun. 2013, 4, 2575. [Google Scholar] [CrossRef]
- Van der Perren, A.; Gelders, G.; Fenyi, A.; Bousset, L.; Brito, F.; Peelaerts, W.; Van den Haute, C.; Gentleman, S.; Melki, R.; Baekelandt, V. The Structural Differences between Patient-Derived α-Synuclein Strains Dictate Characteristics of Parkinson’s Disease, Multiple System Atrophy and Dementia with Lewy Bodies. Acta Neuropathol. 2020, 139, 977–1000. [Google Scholar] [CrossRef]
- Schweighauser, M.; Shi, Y.; Tarutani, A.; Kametani, F.; Murzin, A.G.; Ghetti, B.; Matsubara, T.; Tomita, T.; Ando, T.; Hasegawa, K.; et al. Structures of α-Synuclein Filaments from Multiple System Atrophy. Nature 2020, 585, 464–469. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Schweighauser, M.; Zhang, X.; Kotecha, A.; Murzin, A.G.; Garringer, H.J.; Cullinane, P.W.; Saito, Y.; Foroud, T.; et al. Structures of α-Synuclein Filaments from Human Brains with Lewy Pathology. Nature 2022, 610, 791–795. [Google Scholar] [CrossRef]
- Lau, A.; So, R.W.L.; Lau, H.H.C.; Sang, J.C.; Ruiz-Riquelme, A.; Fleck, S.C.; Stuart, E.; Menon, S.; Visanji, N.P.; Meisl, G.; et al. α-Synuclein Strains Target Distinct Brain Regions and Cell Types. Nat. Neurosci. 2020, 23, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Telling, G.C.; Parchi, P.; DeArmond, S.J.; Cortelli, P.; Montagna, P.; Gabizon, R.; Mastrianni, J.; Lugaresi, E.; Gambetti, P.; Prusiner, S.B. Evidence for the Conformation of the Pathologic Isoform of the Prion Protein Enciphering and Propagating Prion Diversity. Science (80-. ). 1996, 274, 2079–2082. [Google Scholar] [CrossRef]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and Ultra-Sensitive Quantitation of Disease-Associated α-Synuclein Seeds in Brain and Cerebrospinal Fluid by ASyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Becker, K.; Donadio, V.; Siedlak, S.; Yuan, J.; Rezaee, M.; Incensi, A.; Kuzkina, A.; Orrú, C.D.; Tatsuoka, C.; et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2021, 78, 30. [Google Scholar] [CrossRef] [PubMed]
- Biancalana, M.; Koide, S. Molecular Mechanism of Thioflavin-T Binding to Amyloid Fibrils. Biochim. Biophys. Acta - Proteins Proteomics 2010, 1804, 1405–1412. [Google Scholar] [CrossRef]
- Buell, A.K.; Galvagnion, C.; Gaspar, R.; Sparr, E.; Vendruscolo, M.; Knowles, T.P.J.; Linse, S.; Dobson, C.M. Solution Conditions Determine the Relative Importance of Nucleation and Growth Processes in α-Synuclein Aggregation. Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 7671–7676. [Google Scholar] [CrossRef]
- Ramis, R.; Ortega-Castro, J.; Vilanova, B.; Adrover, M.; Frau, J. Unraveling the NaCl Concentration Effect on the First Stages of α-Synuclein Aggregation. Biomacromolecules 2020, 21, 5200–5212. [Google Scholar] [CrossRef] [PubMed]
- Metrick, M.A.; do Carmo Ferreira, N.; Saijo, E.; Hughson, A.G.; Kraus, A.; Orrú, C.; Miller, M.W.; Zanusso, G.; Ghetti, B.; Vendruscolo, M.; et al. Million-Fold Sensitivity Enhancement in Proteopathic Seed Amplification Assays for Biospecimens by Hofmeister Ion Comparisons. Proc. Natl. Acad. Sci. U. S. A. 2019, 116, 23029–23039. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Visanji, N.P.; Kim, A.; Lau, H.H.C.; So, R.W.L.; Alshimemeri, S.; Gao, A.; Seidman, M.A.; Luquin, M.R.; Watts, J.C.; et al. Alpha-Synuclein Seeding Shows a Wide Heterogeneity in Multiple System Atrophy. Transl. Neurodegener. 2022, 11, 1–14. [Google Scholar] [CrossRef]
- Martinez-Valbuena, I.; Swinkin, E.; Santamaria, E.; Fernandez-Irigoyen, J.; Sackmann, V.; Kim, A.; Li, J.; Gonzalez-Latapi, P.; Kuhlman, G.; Bhowmick, S.S.; et al. α-Synuclein Molecular Behavior and Nigral Proteomic Profiling Distinguish Subtypes of Lewy Body Disorders. Acta Neuropathol. 2022, 144, 167–185. [Google Scholar] [CrossRef]
- Sutherland, B.W.; Toews, J.; Kast, J. Utility of Formaldehyde Cross-Linking and Mass Spectrometry in the Study of Protein–Protein Interactions. J. Mass Spectrom. 2008, 43, 699–715. [Google Scholar] [CrossRef]
- Loiacono, C.M.; Beckwith, N.; Kunkle, R.A.; Orcutt, D.; Hall, S.M. Detection of PrP Sc in Formalin-Fixed, Paraffin-Embedded Tissue by Western Blot Differentiates Classical Scrapie, Nor98 Scrapie, and Bovine Spongiform Encephalopathy. J. Vet. Diagnostic Investig. 2010, 22, 684–689. [Google Scholar] [CrossRef]
- Priola, S.A.; Ward, A.E.; McCall, S.A.; Trifilo, M.; Choi, Y.P.; Solforosi, L.; Williamson, R.A.; Cruite, J.T.; Oldstone, M.B.A. Lack of Prion Infectivity in Fixed Heart Tissue from Patients with Creutzfeldt-Jakob Disease or Amyloid Heart Disease. J. Virol. 2013, 87, 9501–9510. [Google Scholar] [CrossRef] [PubMed]
- Hoover, C.E.; Davenport, K.A.; Henderson, D.M.; Pulscher, L.A.; Mathiason, C.K.; Zabel, M.D.; Hoover, E.A. Detection and Quantification of CWD Prions in Fixed Paraffin Embedded Tissues by Real-Time Quaking-Induced Conversion. Sci. Rep. 2016, 6, 25098. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.-T.-T.; Akagi, A.; Nonaka, T.; Nakagaki, T.; Mihara, B.; Takao, M.; Iwasaki, Y.; Nishida, N.; Satoh, K. Formalin RT-QuIC Assay Detects Prion-Seeding Activity in Formalin-Fixed Brain Samples from Sporadic Creutzfeldt–Jakob Disease Patients. Neurobiol. Dis. 2021, 159, 105504. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.C. Calling α-Synuclein a Prion Is Scientifically Justifiable. Acta Neuropathol. 2019, 138, 505–508. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Serrano, G.E.; Anantharam, V.; Kanthasamy, A.; Adler, C.H.; Beach, T.G.; Kanthasamy, A.G. Blinded RT-QuIC Analysis of α-Synuclein Biomarker in Skin Tissue From Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 2230–2239. [Google Scholar] [CrossRef]
- Manne, S.; Kondru, N.; Jin, H.; Anantharam, V.; Huang, X.; Kanthasamy, A.; Kanthasamy, A.G. A-Synuclein Real-Time Quaking-Induced Conversion in the Submandibular Glands of Parkinson’s Disease Patients. Mov. Disord. 2020, 35, 268–278. [Google Scholar] [CrossRef]
- Shin, C.; Han, J.Y.; Kim, S.I.; Park, S.H.; Yang, H.K.; Lee, H.J.; Kong, S.H.; Suh, Y.S.; Kim, H.J.; Choi, Y.P.; et al. In Vivo and Autopsy Validation of Alpha-Synuclein Seeding Activity Using RT-QuIC Assay in the Gastrointestinal Tract of Patients with Parkinson’s Disease. Park. Relat. Disord. 2022, 103, 23–28. [Google Scholar] [CrossRef]
- Fowler, C.B.; O’Leary, T.J.; Mason, J.T. Toward Improving the Proteomic Analysis of Formalin-Fixed, Paraffin-Embedded Tissue. Expert Rev. Proteomics 2013, 10, 389–400. [Google Scholar] [CrossRef]
- Hoffman, E.A.; Frey, B.L.; Smith, L.M.; Auble, D.T. Formaldehyde Crosslinking: A Tool for the Study of Chromatin Complexes. J. Biol. Chem. 2015, 290, 26404–26411. [Google Scholar] [CrossRef] [PubMed]
- Kamps, J.J.A.G.; Hopkinson, R.J.; Schofield, C.J.; Claridge, T.D.W. How Formaldehyde Reacts with Amino Acids. Commun. Chem. 2019, 2, 126. [Google Scholar] [CrossRef]
- Giusti, L.; Lucacchini, A. Proteomic Studies of Formalin-Fixed Paraffin-Embedded Tissues. Expert Rev. Proteomics 2013, 10, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, Y.; Kodera, Y.; Singh, A.; Matsumoto, M.; Matsumoto, H. Efficient Extraction of Proteins from Formalin-Fixed Paraffin-Embedded Tissues Requires Higher Concentration of Tris(Hydroxymethyl)Aminomethane. Clin. Proteomics 2014, 11, 4. [Google Scholar] [CrossRef]
- Shen, K.; Sun, J.; Cao, X.; Zhou, D.; Li, J. Comparison of Different Buffers for Protein Extraction from Formalin-Fixed and Paraffin-Embedded Tissue Specimens. PLoS One 2015, 10, e0142650. [Google Scholar] [CrossRef]
- O’Rourke, M.B.; Padula, M.P. Analysis of Formalin-Fixed, Paraffin-Embedded (FFPE) Tissue via Proteomic Techniques and Misconceptions of Antigen Retrieval. Biotechniques 2016, 60, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Thacker, J.S.; Andersen, D.; Liang, S.; Zieniewicz, N.; Trivino-Paredes, J.S.; Nahirney, P.C.; Christie, B.R. Unlocking the Brain: A New Method for Western Blot Protein Detection from Fixed Brain Tissue. J. Neurosci. Methods 2021, 348, 108995. [Google Scholar] [CrossRef] [PubMed]
- Addis, M.F.; Tanca, A.; Pagnozzi, D.; Crobu, S.; Fanciulli, G.; Cossu-Rocca, P.; Uzzau, S. Generation of High-Quality Protein Extracts from Formalin-Fixed, Paraffin-Embedded Tissues. Proteomics 2009, 9, 3815–3823. [Google Scholar] [CrossRef]
- Rodríguez-Rigueiro, T.; Valladares-Ayerbes, M.; Haz-Conde, M.; Blanco, M.; Aparicio, G.; Fernández-Puente, P.; Blanco, F.J.; Lorenzo, M.J.; Aparicio, L.A.; Figueroa, A. A Novel Procedure for Protein Extraction from Formalin-Fixed Paraffin-Embedded Tissues. Proteomics 2011, 11, 2555–2559. [Google Scholar] [CrossRef]
- Araújo, J.E.; Oliveira, E.; Otero-Glez, A.; Santos Nores, J.; Igrejas, G.; Lodeiro, C.; Capelo, J.L.; Santos, H.M. A Comprehensive Factorial Design Study of Variables Affecting Protein Extraction from Formalin-Fixed Kidney Tissue Samples. Talanta 2014, 119, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Dressler, F.F.; Schoenfeld, J.; Revyakina, O.; Vogele, D.; Kiefer, S.; Kirfel, J.; Gemoll, T.; Perner, S. Systematic Evaluation and Optimization of Protein Extraction Parameters in Diagnostic FFPE Specimens. Clin. Proteomics 2022, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Montine, T.J.; Phelps, C.H.; Beach, T.G.; Bigio, E.H.; Cairns, N.J.; Dickson, D.W.; Duyckaerts, C.; Frosch, M.P.; Masliah, E.; Mirra, S.S.; et al. National Institute on Aging–Alzheimer’s Association Guidelines for the Neuropathologic Assessment of Alzheimer’s Disease: A Practical Approach. Acta Neuropathol. 2012, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Wagner, U.; Dumont, B.; Pikkarainen, M.; Osman, A.A.; Streichenberger, N.; Leisser, I.; Verchère, J.; Baron, T.; Alafuzoff, I.; et al. An Antibody with High Reactivity for Disease-Associated α-Synuclein Reveals Extensive Brain Pathology. Acta Neuropathol. 2012, 124, 37–50. [Google Scholar] [CrossRef]
- Mansour, A.G.; Abou-Khalil, P.; Bejjani, N.; Chatila, R.; Dagher-Hamalian, C.; Faour, W.H. An Optimized Xylene-Free Protein Extraction Method Adapted to Formalin-Fixed Paraffin Embedded Tissue Sections for Western Blot Analysis. Histol. Histopathol. 2017, 32, 307–313. [Google Scholar] [CrossRef]
- Wolff, C.; Schott, C.; Porschewski, P.; Reischauer, B.; Becker, K.-F. Successful Protein Extraction from Over-Fixed and Long-Term Stored Formalin-Fixed Tissues. PLoS One 2011, 6, e16353. [Google Scholar] [CrossRef] [PubMed]
- Geoui, T.; Urlaub, H.; Plessmann, U.; Porschewski, P. Extraction of Proteins from Formalin-Fixed, Paraffin-Embedded Tissue Using the Qproteome Extraction Technique and Preparation of Tryptic Peptides for Liquid Chromatography/Mass Spectrometry Analysis. In Current Protocols in Molecular Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- Mansour, A.; Chatila, R.; Bejjani, N.; Dagher, C.; Faour, W.H. A Novel Xylene-Free Deparaffinization Method for the Extraction of Proteins from Human Derived Formalin-Fixed Paraffin Embedded (FFPE) Archival Tissue Blocks. MethodsX 2014, 1, 90–95. [Google Scholar] [CrossRef] [PubMed]
- García-Vence, M.; Chantada-Vazquez, M. del P.; Sosa-Fajardo, A.; Agra, R.; Barcia de la Iglesia, A.; Otero-Glez, A.; García-González, M.; Cameselle-Teijeiro, J.M.; Nuñez, C.; Bravo, J.J.; et al. Protein Extraction From FFPE Kidney Tissue Samples: A Review of the Literature and Characterization of Techniques. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Wang, X.; Vander Stel, K.; Chu, Y.; Kordower, J.; Ma, J. Detecting Alpha Synuclein Seeding Activity in Formaldehyde-Fixed MSA Patient Tissue by PMCA. Mol. Neurobiol. 2018, 55, 8728–8737. [Google Scholar] [CrossRef]
- Fenyi, A.; Duyckaerts, C.; Bousset, L.; Braak, H.; Del Tredici, K.; Melki, R. Seeding Propensity and Characteristics of Pathogenic ASyn Assemblies in Formalin-Fixed Human Tissue from the Enteric Nervous System, Olfactory Bulb, and Brainstem in Cases Staged for Parkinson’s Disease. Cells 2021, 10, 139. [Google Scholar] [CrossRef]
- Hepker, M.; Clabaugh, G.; Jin, H.; Kanthasamy, A.G. New Protocol for Kinetic Assay Seeding Ability Recovery “KASAR” from Formalin-Fixed Paraffin-Embedded Tissues. Front. Mol. Biosci. 2023, 10. [Google Scholar] [CrossRef] [PubMed]
- Peelaerts, W.; Bousset, L.; Van Der Perren, A.; Moskalyuk, A.; Pulizzi, R.; Giugliano, M.; Van Den Haute, C.; Melki, R.; Baekelandt, V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature 2015, 522, 340–344. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Optimization Schematic of FFPE Protein Extraction. (a) Testing protein extraction efficiency using FFPE and formaldehyde-fixed temporal cortex of test LBD subject with 3 different tissue preparations, 2 different deparaffinization and rehydration methods, and 6 different extraction buffers compared to a control subject (both archived for < 2 years); (b) Introduction of heat-induced antigen retrieval and dissociation to increase protein yield extracted with simple PBS buffer; (c) Application of optimized extraction protocol to evaluate protein extraction efficiency and αSyn seeding in different regions of FFPE tissues (archived > 20 years); (d) Evaluation of αSyn disease-specificity in LBD (archived < 2 years) and MSA (archived > 20 years) subjects and seeding differences between tissues processed with antigen retrieval only or both antigen retrieval and dissociation.
Figure 1.
Optimization Schematic of FFPE Protein Extraction. (a) Testing protein extraction efficiency using FFPE and formaldehyde-fixed temporal cortex of test LBD subject with 3 different tissue preparations, 2 different deparaffinization and rehydration methods, and 6 different extraction buffers compared to a control subject (both archived for < 2 years); (b) Introduction of heat-induced antigen retrieval and dissociation to increase protein yield extracted with simple PBS buffer; (c) Application of optimized extraction protocol to evaluate protein extraction efficiency and αSyn seeding in different regions of FFPE tissues (archived > 20 years); (d) Evaluation of αSyn disease-specificity in LBD (archived < 2 years) and MSA (archived > 20 years) subjects and seeding differences between tissues processed with antigen retrieval only or both antigen retrieval and dissociation.
Figure 2.
Comparison of FFPE protein extraction efficiency and subsequent αSyn seeding using various tissue preparations, deparaffinization and extraction methods. (a) Protein yield obtained with either xylene-ethanol or heptane-methanol deparaffinization, extracted from 45-µm FFPE sections on slides, in tubes or 4-mm FFPE micro-punch of temporal cortex of test LBD subject, using 6 different extraction buffers; (b) Subsequent αSyn seeding potential of the extracted proteins.
Figure 2.
Comparison of FFPE protein extraction efficiency and subsequent αSyn seeding using various tissue preparations, deparaffinization and extraction methods. (a) Protein yield obtained with either xylene-ethanol or heptane-methanol deparaffinization, extracted from 45-µm FFPE sections on slides, in tubes or 4-mm FFPE micro-punch of temporal cortex of test LBD subject, using 6 different extraction buffers; (b) Subsequent αSyn seeding potential of the extracted proteins.
Figure 3.
Protein extraction efficiency of different extraction buffers using formaldehyde-fixed human brain tissue and subsequent αSyn seeding potential. (a) Protein yield obtained from 4-mm micro-punch of formaldehyde-fixed FFPE temporal cortex, extracted using the different buffers; (b) Negative αSyn seeding observed when proteins were extracted using buffer 1.
Figure 3.
Protein extraction efficiency of different extraction buffers using formaldehyde-fixed human brain tissue and subsequent αSyn seeding potential. (a) Protein yield obtained from 4-mm micro-punch of formaldehyde-fixed FFPE temporal cortex, extracted using the different buffers; (b) Negative αSyn seeding observed when proteins were extracted using buffer 1.
Figure 4.
Optimization of αSyn SAA using proteins extracted with antigen retrieval and dissociation. (a) Comparison of protein yields obtained with different tissue preparations; (b) Protein yields obtained from the FFPE temporal cortex of LBD subjects, that have been archived for < 2 years (LBD1) and > 20 years (LBD2-5); (c) αSyn seeding of serially diluted total protein from PBS-soluble fractions, extracted using antigen retrieval and dissociation prior to protein extraction; (d) αSyn seeding of serially diluted total protein from PBS-soluble fractions, extracted without antigen retrieval or dissociation; (e) Evaluation of αSyn seeding potential using 5 µg of total protein from PBS-soluble fractions, extracted from different tissue preparations using antigen retrieval and dissociation prior to protein extraction.
Figure 4.
Optimization of αSyn SAA using proteins extracted with antigen retrieval and dissociation. (a) Comparison of protein yields obtained with different tissue preparations; (b) Protein yields obtained from the FFPE temporal cortex of LBD subjects, that have been archived for < 2 years (LBD1) and > 20 years (LBD2-5); (c) αSyn seeding of serially diluted total protein from PBS-soluble fractions, extracted using antigen retrieval and dissociation prior to protein extraction; (d) αSyn seeding of serially diluted total protein from PBS-soluble fractions, extracted without antigen retrieval or dissociation; (e) Evaluation of αSyn seeding potential using 5 µg of total protein from PBS-soluble fractions, extracted from different tissue preparations using antigen retrieval and dissociation prior to protein extraction.
Figure 5.
Comparison of αSyn seeding activity between LBD and MSA FFPE brain tissue, extracted with or without dissociation. (a) Total protein yield obtained from the temporal cortex of test LBD subject and cerebellum of test MSA subject, extracted with antigen retrieval-only or both antigen retrieval and dissociation prior to protein extraction using PBS; (b) Evaluation of disease-specific αSyn seeding in LBD-favoring SAA and comparison of αSyn seeding activity of proteins extracted from dissociated or non-dissociated (antigen retrieval-only) tissues; (c) Evaluation of disease-specific αSyn seeding in MSA-favoring SAA and comparison of αSyn seeding of proteins extracted from dissociated or non-dissociated (antigen retrieval-only) tissues.
Figure 5.
Comparison of αSyn seeding activity between LBD and MSA FFPE brain tissue, extracted with or without dissociation. (a) Total protein yield obtained from the temporal cortex of test LBD subject and cerebellum of test MSA subject, extracted with antigen retrieval-only or both antigen retrieval and dissociation prior to protein extraction using PBS; (b) Evaluation of disease-specific αSyn seeding in LBD-favoring SAA and comparison of αSyn seeding activity of proteins extracted from dissociated or non-dissociated (antigen retrieval-only) tissues; (c) Evaluation of disease-specific αSyn seeding in MSA-favoring SAA and comparison of αSyn seeding of proteins extracted from dissociated or non-dissociated (antigen retrieval-only) tissues.
Figure 6.
Biochemical and ultra-structural validation of preserved αSyn disease-specificity in LBD and MSA FFPE brain tissue. (a) Disease-specific αSyn seeding differences observed in MSA-favoring SAA; (b) TEM confirmation of thin, occasional twists observed in SAA-generated αSyn fibrils using LBD FFPE temporal cortex; (c) TEM confirmation of thick, intertwined twists observed in SAA-generated αSyn fibrils using MSA FFPE cerebellum. Scale bar in b,c represents 100 nm.
Figure 6.
Biochemical and ultra-structural validation of preserved αSyn disease-specificity in LBD and MSA FFPE brain tissue. (a) Disease-specific αSyn seeding differences observed in MSA-favoring SAA; (b) TEM confirmation of thin, occasional twists observed in SAA-generated αSyn fibrils using LBD FFPE temporal cortex; (c) TEM confirmation of thick, intertwined twists observed in SAA-generated αSyn fibrils using MSA FFPE cerebellum. Scale bar in b,c represents 100 nm.
Table 1.
Demographics and neuropathological diagnosis
Table 1.
Demographics and neuropathological diagnosis
| Case |
Sex |
Age at Death |
ABC Score*
|
CAA |
Lewy Body Pathology |
Lewy Body Braak Stage |
LATE-NC Stage |
Other Neuropathological Diagnosis |
| Control 1 |
F |
52 |
None |
None |
None |
None |
None |
Early Fahr Disease |
| LBD 1 |
M |
74 |
A1B1C1 |
Aβ-positive Type 2 |
Neocortical |
Stage 5 |
None |
None |
| LBD 2 |
F |
73 |
A2B3C3 |
None |
Neocortical |
Stage 5 |
Stage 2 |
None |
| LBD 3 |
F |
73 |
A2B3C2 |
None |
Limbic |
Stage 4 |
Stage 2 |
None |
| LBD 4 |
F |
82 |
A2B2C2 |
None |
Neocortical |
Stage 5 |
None |
ARTAG Medial Temporal GM, WM, Brainstem |
| LBD 5 |
F |
62 |
A3B3C3 |
None |
Neocortical |
Stage 5 |
Stage 1 |
None |
| LBD 6 |
M |
78 |
A3B2C1 |
Aβ-positive Type 2 |
Neocortical |
Stage 5 |
Stage 2 |
Meningioma Right Frontal |
| MSA 1 |
F |
68 |
A1B1C0 |
Aβ-positive Type 2 |
None |
None |
None |
ARTAG Medial Temporal WM |
| MSA 2 |
M |
64 |
A0B1C0 |
None |
None |
None |
None |
PART (Braak stage II), AGD (Stage II) |
Table 2.
Protein extraction buffer compositions.
Table 2.
Protein extraction buffer compositions.
| Buffer |
Buffer Composition |
| Buffer 1 |
1X Phosphate-Buffered Saline (pH 7.4)
Protease inhibitor |
| Buffer 2 |
1X Phosphate-Buffered Saline (pH 7.4)
0.01% β-mercaptoethanol
Protease inhibitor |
| Buffer 3 |
1X Tris Buffered Saline
(50 mM Tris-HCl (pH 7.5)
150 mM NaCl)
Protease inhibitor |
| Buffer 4 |
50 mM Tris-HCl (pH 8.0)
Protease inhibitor |
| Buffer 5 |
50 mM Tris-HCl (pH 8.0)
1% Nonyl Phenoxypolyethoxylethanol-40
150 mM NaCl
Protease inhibitor |
| Buffer 6 |
50 mM Tris-HCl (pH 8.0)
1% Nonyl Phenoxypolyethoxylethanol-40
Protease inhibitor |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).