1. Introduction
At 662 billion tonnes of carbon, marine dissolved
organic matter (DOM) holds > 200 times as much organic matter (OM) as the
living marine biomass and around 100 times more than the dead particulate
organic carbon (1) (Hansell et al., 2009). Marine (DOM) contains as much carbon
as the Earth’s atmosphere, and represents a critical component of the global
carbon cycle (2) (McCarren et al. 2010). In the ocean, this DOM is primarily
produced by eukaryotic and prokaryotic phytoplankton, with some macroalgae,
that reduce CO2 to OM, using sunlight as energy source. The molecular
characteristics of the proteins produced are controlled by the organisms'
genes. Further proteins, and other OM, are then manufactured using enzymes,
which also are proteins. This OM consists of particulate matter (POM), mainly
the solid parts plankton organisms, and DOM that is secreted by phytoplankton
and lost from cells during lysis. This paper is not concerned with the POM,
treating only the DOM as well as mucus consisting of exopolymeric substances
(EPS). Ocean DOM can be usefully characterized in two ways.
The first way to categorize DOM is by chemical
composition. The most abundant primary component of DOM is sugars, followed in
decreasing order by amino acids, fatty acids and nucleic acid bases, which
correspond for EPS to polysaccharides, proteins, lipids and nucleic acids (DNA
and RNA). Much of the EPS consists of polymer molecules bearing a variety of
functional groups, which determine their physicochemical properties and roles
in the ecosystem.
The first way to categorize DOM is by age. The
largest fraction of DOM is weeks to hundreds or thousands of years old and has
the highest proportion of DOM recalcitrant to biological breakdown (1) (3)
(Hansell et al., 2009; Jiao et al., 2010), as well as showing little surfactant
or rheological activity (4) (Jenkinson et al., 2015, JPR). The second, smaller,
fraction of DOM is seconds to days or weeks old. It is thus biologically and
chemically labile. Some of it is highly surface-active (hydrophilic,
amphiphilic or hydrophobic) and some of it adds viscoelastic properties to the
water, thus thickening it (4) (Jenkinson et al., 2015 JPR). This category of
DOM is relatively the most abundant in the photic zone, particularly in certain
harmful algae blooms and mucus events (4) (Jenkinson, 2015 JPR). It also
includes most of the signalling, pheromone and toxin molecules (5) (Brown et
al., 2019), that may modulate ecosystem structure (6) (Yamasaki et al., 2009)
and bidirectional vertical fluxes, of OM within the water column (7) (Mari et
al., 2017) and of matter and energy across the ocean-atmosphere interface (8)
(9) (Wurl et al., 2017; Jenkinson et al., 2021).
Given that the allochthonous ocean OM is produced
by marine organisms' genes, and that some of this OM changes the physical
properties of bulk ocean water, as well as its interfaces and fluxes across
them, it follows that these properties and fluxes are partly under genetic
control, and thus subject to natural selection and evolution (10) (11) (Darwin,
2003; Dawkins, 2016).
Rapid progress is being made cataloguing the genes
of the pelagic ecosystem, as well as discovering both their roles in producing
proteins, and ultimately, via enzymes, of polysaccharides and lipids. Progress
is also being made in categorizing the rheological properties of ocean waters
and soft polymeric structures, as well as how these properties and structures
modulate processes and fluxes. The terrestrial/atmospheric and submarine
climates are currently changing (12) (IPCC, 2021), which is stressing and
altering the biota, including humans. The first aim of this paper, therefore,
is to suggest some of the possible roles of genomes in promoting biorheological
changes that influence biogeochemical processes. The second aim is to promote
bridging of the gap between ocean science and rheology, and to suggest how
collaborative research programmes including genomics, ocean rheology, ocean
ecology and flux studies can be set up to understand how genes control internal
ocean processes, and their interaction with the atmosphere. As an added bonus
such studies are likely to throw up commercially exploitable new natural
bioactive products.
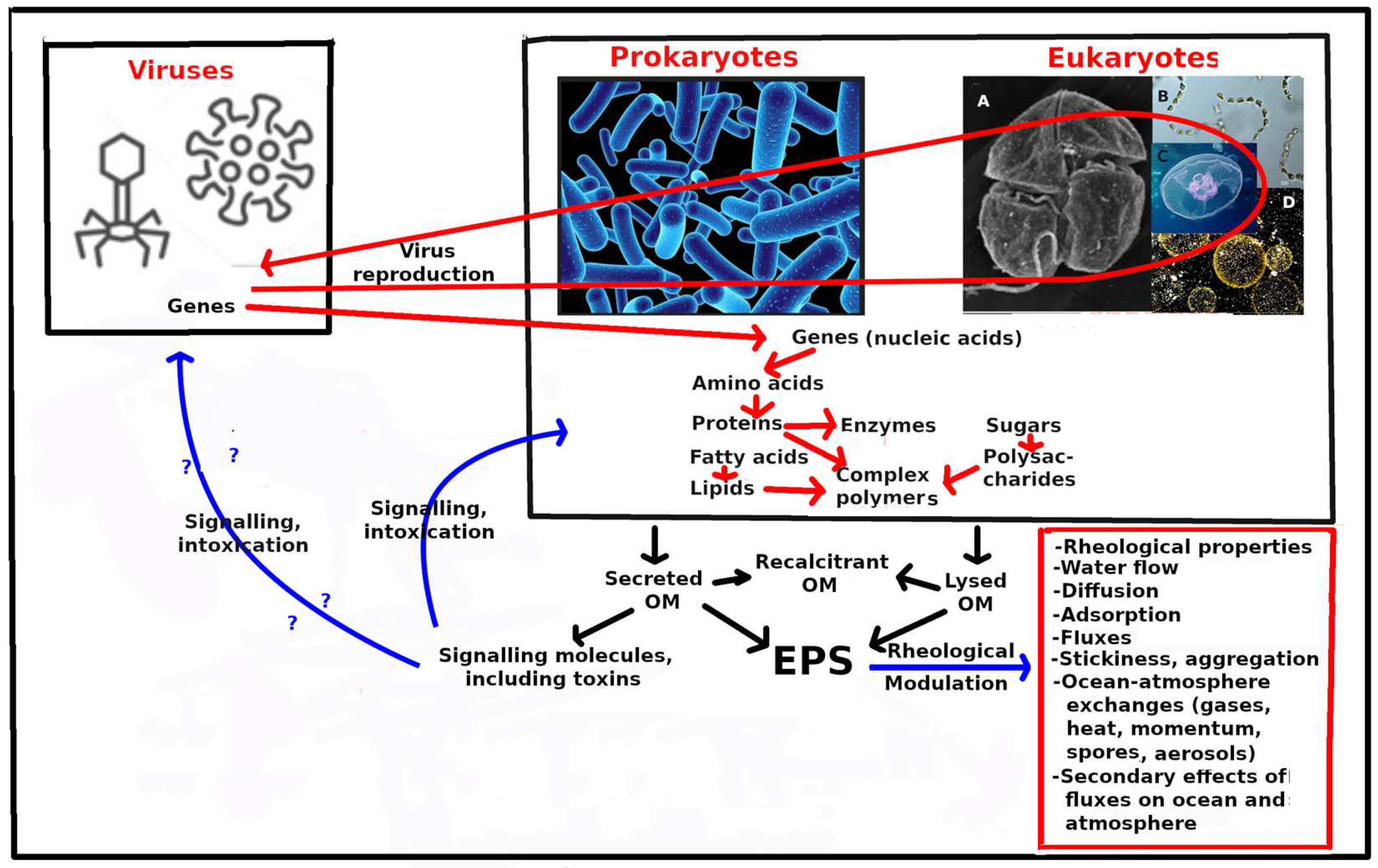
A sketch of these ideas is shown in Figure 1.
Figure 1.
Three categories of gene-bearing ("living") organisms are presented, viruses, prokaryotes and eukaryotes. Prokaryotes and eukaryotes are shown in the same compartment, as generally they both have an independent machinery to form amino acids and proteins (including enzymes) from "instructions" in their genes. Enzymes modulate (catalyse) intracellular molecular transformations (modulation pathways not shown). Viruses are placed in a separate box as they need to introduce their genes into prokaryotic or eukaryotic cells, taking some control of these cells in order to reproduce their genes and outer casing. Red arrows represent the pathways of viral reproduction, and transfer of viral genes to replace or modulate prokaryotic or eukaryotic genes (13) (Rosenwasser et al., 2016). Red arrows are used also to represent transfers between the major types of organic molecules inside cells. Black arrows represent transformations between the major suggested extracellular pools of organic matter (OM). Blue arrows represent modulation by extracellular polymeric substances (EPS) on physical and physicochemical processes in the ocean and at the ocean-atmosphere interface, as well as secondary effects in the ocean and the atmosphere (inside red box). Blue arrows also represent signalling and intoxication pathways by signalling molecules and toxins, respectively. Eukaryotic plankton is represented by: A - a dinoflagellate; B - a diatom; C - a jellyfish medusa; D - a raphidophyte. This is not a sketch of trophic or energy pathways.
Figure 1.
Three categories of gene-bearing ("living") organisms are presented, viruses, prokaryotes and eukaryotes. Prokaryotes and eukaryotes are shown in the same compartment, as generally they both have an independent machinery to form amino acids and proteins (including enzymes) from "instructions" in their genes. Enzymes modulate (catalyse) intracellular molecular transformations (modulation pathways not shown). Viruses are placed in a separate box as they need to introduce their genes into prokaryotic or eukaryotic cells, taking some control of these cells in order to reproduce their genes and outer casing. Red arrows represent the pathways of viral reproduction, and transfer of viral genes to replace or modulate prokaryotic or eukaryotic genes (13) (Rosenwasser et al., 2016). Red arrows are used also to represent transfers between the major types of organic molecules inside cells. Black arrows represent transformations between the major suggested extracellular pools of organic matter (OM). Blue arrows represent modulation by extracellular polymeric substances (EPS) on physical and physicochemical processes in the ocean and at the ocean-atmosphere interface, as well as secondary effects in the ocean and the atmosphere (inside red box). Blue arrows also represent signalling and intoxication pathways by signalling molecules and toxins, respectively. Eukaryotic plankton is represented by: A - a dinoflagellate; B - a diatom; C - a jellyfish medusa; D - a raphidophyte. This is not a sketch of trophic or energy pathways.
2. Inorganic matter (IM) and organic matter (OM) in the oceans
2.1. Sources of available N and Fe
Estimated annual sources and sinks of organic
nitrogen (ON), fixed inorganic nitrogen (IN) and Fe for the global coastal
ocean (depth ≤200 m) the offshore ocean (>200 m) and the whole ocean (14)
(Liu et al., 2021) are summarized in Table 1.
Table 1.
Global coastal ocean nitrogen, N, and iron, Fe, budget terms (Tg y-1) (estimated annual means).
Table 1.
Global coastal ocean nitrogen, N, and iron, Fe, budget terms (Tg y-1) (estimated annual means).
| |
Coastal Ocean (1) (14) |
Whole ocean (15) (16) (2,3) |
Open ocean (16) (3) |
| Inorganic N (IN) |
|
|
|
| Atmospheric deposition (DIN) |
+ 4.5 |
|
|
| River input (DIN) |
+20.4 |
+23 (16) (3) |
+17(a)(16) (3) |
| Denitrification (water + sediments) (DIN) |
-51.9 |
|
|
| Coastward net influx from offshore (DIN) |
+47.4 |
|
|
| Total Δ TIN |
+20.4 |
|
|
| |
|
|
|
| Organic N (ON) |
|
|
|
| Sedimentary burial (TON) |
-12.3 |
|
|
| River input (TON) |
+ 27.1 |
|
|
| River input (DON) |
|
+11(a) (16) (3) |
>0 to <11(a) (16) (3) |
| N2 fixation (TON) |
+15.4 |
164 (16) (3) |
0 (16) (3) |
| Oceanward net outflux to offshore (TON)
|
-50.2 |
|
|
| Total Δ TON |
-20.0 |
|
|
| Discrepancy (ΔTIN – ΔTON) |
+0.4 |
|
|
|
Net community production (DIN+TON)
|
35.5 |
|
|
| Atmospheric N deposition |
|
39(b) (16) (3) |
>30(b) (16) (3) |
| Atmosphere-ocean Fe budget |
|
|
|
| Fe emissions from fires (<20 µm) |
|
1.1 (15) (2) |
|
| Soluble Fe flux to the ocean (from dust) |
|
0.19 to 0.28 (15) (2) |
|
| Soluble Fe flux to the ocean (from fires) |
|
0.035 to 0.063 (15) (2) |
|
| Soluble Fe flux to the ocean (anthropogenic) |
|
0.016 to 0.034 (15) (2) |
|
| Soluble Fe flux to the ocean (Total) |
|
0.24 to 0.38 (15) (2) |
|
| Soluble P flux to the ocean (from dust) |
|
0.031 to 0.094 (15) (2) |
|
| Soluble P flux to the ocean (from fires) |
|
0.005 (15) (2) |
|
| Soluble P flux to the ocean (anthropogenic) |
|
0.0094 to 0.11(15) (2) |
|
| Soluble P flux to the ocean (Total) |
|
0.045 to 0.21 (15) (2) |
|
1 – Ref. (14) Liu et al. (2021); 2 – Ref. (15) Hamilton et al. (2022); 3 - Ref. (16) Jickells et al. (2017).
a – 75% of riverine input escapes beyond the shelf break; b – 75% of atmospheric input deposited outside the shelf break. |
2.2. Origins and classes of allochthonous IM and OM
The main origins of dissolved IM (DIM) are soluble
salts associated with: riverine and terrestrial diffuse sources. They include
nutrient salts, notably nitrate, nitrite, ammonia and urea, dissolved inorganic
phosphate and dissolved silicate. Anthropogenic sources have increased, not
only of nitrogenous nutrients and phosphate, but also of silicate due to
disruption of soils and coastal development. The current estimated annual
global budget in for coastal waters (depth ≤200 m) (14) (Liu et al., 2021) is shown
in Table 1 for marine reactive N.
Important sources and sinks of OC and reactive N in
the ocean are atmospheric deposition, sedimentary burial (15) (Hamilton et al.,
2022).
2.3. Origins and classes of autochthonous OM
Most OM in the ocean is produced autochthonously.
Ocean Phytoplankton and bacteria sensu lato produce a wide variety of
organic molecules. They result from direct extracellular secretion by living
cells, as well as leakage from lysed and predated cells as dissolved OM (DOM),
normally defined as that passing through a 0.2-µm filter, as well as
particulate OM (POM), which is retained by a 0.5µm filter (17) (Shen and
Benner, 2019). Between these extremes lies colloidal OM (COM). In order of
abundance the DOM and COM comprise sugars, amino-acids, fatty acids and nucleic
acid bases, polymerized to varying degrees into carbohydrates, proteins, lipids
and nucleic acids, respectively, along with complex molecules bearing different
radical groups.
2.4. Autochthonous particulate organic matter (POM)
POM produced within the pelagic ocean ecosystem
consists principally of living cells and dead remains of cells. These particles
act as mechanically solid surfaces allowing colonization by bacteria and
protists such as ciliates. These particles also become included in marine
aggregates, together with living bacteria and protists, within the more-or-less
gluey polymeric matrix of COM. POM is believed to be negatively buoyant in
general, and to act, together with autochthonous and allochthonous PIM to
ballast marine organic aggregates, thus increasing downward organic flux (7)
(Mari et al., 2017). More work is required on the functional density and
sinking behaviour of marine POM.
The relationships between composition, measured
density and sinking/rising rates of both non-living and living POM has subject
to much research (18) (19) (20) (7) (Bienfang et al., 1977; Bienfang, 1980;
Wakeham et al., 1984; Mari et al., 2017), this has given rise to varied and
confusing results. Largely this may arise from the Derjaguin-Landau-Verwey-Overbeek
assumption (DLVO), taught almost universally in engineering textbooks during
the 20th century, of the quasi-universal non-stick non-slip interface between
water and solid surfaces. Recent research on fluid dynamics at sub-mm and particularly
sub-µm length scales, has shown very considerable departure from DLVO between
even Newtonian liquids, such as water, and solid surfaces of different
qualities, such as rough, smooth, hydrophobic or hydrophilic (21) (22) (23)
(Rothstein, 2010; Conlisk, 2013; Jenkinson, 2014). Moreover, the surfaces of
POM and even PIM adsorb a covering of OM, which may show varied and complex
surface properties. Furthermore, living cells, including those in aggregates
and biofilms, manage their surface properties through electric fields, partly
by means of glycocalyxes.
2.5. Autochthonous dissolved organic matter (DOM)
Old DOM is mostly refractory (rDOM), while DOM
newly produced by phytoplankton (pDOM) tends to be highly reactive and labile
Most DOM in surface, mesopelagic and deep waters is
old rDOM. In laboratory studies, Shen and Benner(17) (2019) found that, over a
time scale of 180 days, about 6-7% of DOM in surface water (50 and 100 m), 1-3%
in mesopelagic water (300 m and 750 m) and 0% in deep pelagic water (1500 m)
was removed by microbial degradation. The authors found that the amount of
degradation depended on the depth origin of the OM, not on that of the
microbial community.
pDOM was sampled by filtering (0.7 µm) an in-situ
bloom was found to be utilized 50-75% within 7 d, and 76-94% within 180 d. By
contrast, microbial utilization of DOM from 1500 m was not measurable even
after 180 d: it consisted all of rDOM. Spectrophotometry at wavelengths from
250 to 750 nm of both pDOM and rDOM revealed that pDOM showed two absorbance
shoulders, at 250-265 nm and at 300-350 nm due to chromophore molecules or
groups, but rDOM did not. These two wavelength ranges correspond to labile
compounds, including amino-acids and mycosporine-like amino-acids, respectively.
Furthermore, elemental analysis revealed that while pDOM had a C/N value, 6.2,
similar to the Redfield ration, the corresponding value for rDOM was 36.2,
confirming that microbes utilized N-rich DOM referentially, and thus caused the
remaining DOM to become depleted in N (17) (Shen and Benner, 2019), and thus
probably in protein.
3. DOM with signalling and allelopathic functions
Many algal and protist species produce allelopathic
compounds. The raphidophyte, Heterosigma akashiwo, produces high-molecular mass
(>1 MDa) allelopathic polysaccharide-protein complexes that inhibit its
competitor, the diatom Skeletonema costatum (now S. marinoi), by binding to the
latter's cell surface. The authors suggested that viscoelastic, colloidal
and/or selectively adhesive of these and other allelopathic
polysaccharide-protein complexes (APPCs). The Skeletonema in turn were found to
produce several fractions (separated by solid phase extraction) that
allelopathically inhibited the Heterosigma in a dose-related manner (6)
(Yamasaki et al., 2009).
At bloom concentrations the diatoms, Skeletonema
costatum, Chaetoceros danicus and Thalassiosira decipiens, all slowed the
swimming of the dinoflagellate, Cochlodinium (now Margalefidinium) polykrikoides(24)
(Lim, A.S. et al., 2014), and the authors suggested that this might have
impaired vertical migration of the latter and hence its ability to form blooms.
The nature of the allelopathic agent(s), however, was not determined.
Axenically cultured Margalefidinium polykrikoides
was found to produce copious mucus, giving average yields of crude
polysaccharide of 26 mg/L of culture medium., which on hydrolysis yielded
mannose, galactose, glucose and uronic acid together with sulphate groups (7-8%
w/w S). The purified polysaccharide inhibited 11 virus strains out of 15 tested
at concentrations of 0.8 to 25 mg/L. This abundant occurrence of sulphur-rich
molecules is compatible with mucous gel formation by inter-group disulphide
links (25) (Hasui et al., 1995).
The first diatom pheromone was identified by
Gillard et al. (26) (2012), Pheromones are defined as molecules involved in
intraspecific signalling, notably facilitating sexual encounter, while those
used for interspecific communication are allelochemicals(27) (Frenket al.2014).
Many algal pheromones are organic acids or
alcohols, while some are also proteins or glycoproteins (27) (Frenket al.,
2014). For example, in the freshwater colonial heterothallic alga, Volvox
carteri f. nagariensis, that survives drying out as dormant zygotes, the end of
drought induces male colonies to secrete a pheromone inducer that is a
large-molecular-mass glycoprotein, and that affects both male and female
colonies. This pheromone inducer acts at a concentration of only10-16
M. It is produced by somatic cells, but initiates gametogenesis by male and
female cells. The gene that encodes the pheromone has been discovered (28)
(Tschochner et al., 1987) and the corresponding protein contains 208 amino
acids with a molecular mass of 22 kDa (29) (27) (Mages et al., 1988; Frenkel et
al., 2014).
Much laboratory work has been carried out on the
influence of bacteria on phytoplankton biosynthesis of natural products (5)
(Brown et al., 2019). The results obtained may give general clues to the
production and effects of signalling compounds in the ocean, as well as the
roles of these compounds in structuring ecosystems.
Particularly since the 2010s, the emphasis of work
has evolved from allelopathic interactions to intraspecific signalling (30)
(Schwartz et al., 2016). Recent work has also targeted phytoplankton chemical
defences, particularly where the molecules involved, e.g. paralytic shellfish
toxins (PSTs) or amnesiac shellfish toxins (ASTs), can impact human health (17)
(Shen and Benner , 2019). The use of –omics (metabolomics, proteomics and transcriptomics)
are revealing mechanisms of biological response to many chemical signals and
cues. Outside the area of compounds showing potential for medical or commercial
innovation, characterization of the multitude of often unstable and very dilute
compounds responsible for chemical mediation of pelagic interactions may remain
impracticable (5) (Brown et al., 2019). Studies of molecules that modify
turbulence, diffusion and binding processes, notably those produced in harmful
algal blooms (31) (32) (Jenkinson and Sun, 2010; Gobler et al, 2017), in
organic aggregates (33) (Karlusich et al., 2022), in biofilms and interaction
of such molecules with pollutants (34) (Santschi et al., 2021) may also benefit
from investigation using–omics. (See section 17.)
A study by Poulson-Ellestad et al. (35) (2014a-JPR)
of the allelopathic effects of the toxic dinoflagellate, Karenia breve, on 9
species of planktonic diatoms typically present in the areas where K. breve
blooms in the Gulf of Mexico, showed only weak stimulatory or inhibitory
effects on the diatoms, leading the authors to suggest that allelopathic
effects could not have been useful to K. breve at the start of the bloom, but
could be useful in maintaining blooms once they were established. A second
study by Poulson-Ellestad et al. (36) (2014b-PNAS) on the allelopathic effects
of K. breve on two different diatoms, one of the species, Thalassiosira
pseudonana, turned out to be the more susceptible. That K. breve affected
nutrient limitation of T. pseudonana was considered unlikely as concentrations
were non-limiting. Metabolomic and proteomic investigation using gene ontology
categories for a long suite of proteins associated with metabolic pathways and
functions in T. pseudonana revealed that many processes, including those
involved in cellular carbohydrate metabolism, were strongly stimulated when
exposed to K. breve, while others, including photosynthesis and chromatin
assembly, were strongly inhibited. That T. pseudonana was susceptible to K.
breve may be associated with the finding that the two species do not co-occur
in nature. Although K. breve produces the toxin, brevetoxin, it is not known if
this was an allelopathic agent acting against T. pseudonana.
In a study from the Baltic, Hakenen et al. (37)
(2014) tested 10-µm filtrate of cultures of 10 local strains of a recurring
dinoflagellate, Alexandrium ostenfeldii on different flagellates. At
characteristic bloom concentrations, all the strains caused allelopathic
effects on the cryptophyte, Rhodomonas salina and the dinoflagellates,
Kryptoperidinium foliaceum, Levanderina fissa and Heterocapsa triquetra. All
the strains of A. ostenfeldii showed allelopathic effects on all the target
species. K. foliaceum reacted by encysting, but excysted again within 24 h, a proportion
of the L. fissa cells lysed, and H. triquetra shed their thecae and became
immotile, but within 24 hours they had mostly recovered. The chemical nature of
the allelopathic agent was not investigated.
4. Molecules in intraspecific and interspecific signalling
Pheromones, secreted by copepods such as Temora
longicornis and Eurytemora affinis, change the swimming behaviour of
conspecifics of the opposite sex (both males and females). This is consistent
with evolved use of pheromones to optimize encounters which may lead to mating
(38) (Seuront and Stanley, 2014).
Another use of intraspecific signalling is for
communication in biofilms. Bacteria, Vibrio cholerae, cooperate to protect
themselves and each other against predatory amoebae, Acanthamoeba casalanii,
using vibriopolysaccharide, an extracellular matrix of proteins, nucleic acids,
and sugars (39) (Yildiz et al., 2014), which are in part controlled by the
quorum sensing (QS) regulator, HapR (40) (Sun et al., 2013). The genes, vpsR
and vpsT, were shown to confer enhanced resistance to amoeboid grazing, since
grazing was enhanced in knockdown mutants. Further reduction in grazing
resistance occurred when QS was interrupted by knocking out the hapR
regulator(40) (Sun et al., 2013).
As well as signalling by DMS (See section 5),
selection of bacterial prey by the ascidian tunicate, Microcosmus exasperatus,
may depend on surface molecules of the bacteria more than on their shape or
size. Marine picocyanobacteria, notably Synecococcus and Prochorococcus, have
sticky, hydrophobic coatings, and are retained with relatively high efficiency
by the feeding nets of the ascidian. In contrast, Pelagibacter ubique, and
other species of the SAR11 guild have a non-sticky, non-hydrophobic coatings,
which allows them to slip through the mucous feeding nets and thus show low
retention by this tunicate. While such a coating may reduce adhesion to
nutrient-rich organic particles, it may confer resistance to grazing by
mucous-net suspension feeders(41) (Dadon-Pilosof et al., 2017).
Rosa et al.(42) (2017) carried out a study on
feeding selection by two lamellibranch molluscs, Mytilus edulis and Crassostrea
virginica, of 10 species of nanoflagellates. The selection and sorting
structures in these molluscs are complex and dynamic, but like in ascidians,
mucus is heavily involved. Amongst the nanoflagellates studied, only Pavlova
lutheri had a wettable, hydrophilic surface, and its retention by the molluscs
was also the least. The wettability (hydrophilicity) and surface charge of
surface of many cells is largely modulated by lectins in the glycolcalyx.
Lectins are widely distributed glycoproteins, with important functions in
molecular recognition.
5. Dimethylsulphide (DMS) in signalling and structuring consortia
DMS is responsible for the characteristic smell of
algae culture rooms. It is secreted by most dinoflagellates, including the
hosts of the parasitic dinoflagellate, Parvilucifera sinerae. Garcés et al.
(43) (2013) found that concentrations of 270-300 nM DMS end dormancy in P.
sinerae. Correspondingly, all dinoflagellate species that produce DMS in
sufficient concentration cause P. sinerae to wake up, but only some of these
species get parasitized. Garcés et al. (43) (2013) suggested that this was a
rare demonstrated example of the now classical idea of “watery arms race[s]” in
the ocean(44) (Smetacek, 2001). Are watery arms races so rare, however?
Schwartz et al. (30) (2016) review many examples of models as well as
laboratory and field studies supporting complex dynamics between competing
species, where one or more species deploys allelopathic compounds to inhibit or
even lethally poison competitors. (See Sections
3, 4 and 7.) Outcomes can be the elimination of one competitor or else
co-existence, depending on environmental conditions such as nutrient
concentrations or even allelopathically influenced nutrient uptake dynamics.
Signalling molecules are not all aggressive, but
may be mutually helpful. Studies of mutualism in the sea have long been
concentrated on bitrophic interactions and it was widely assumed that more
complex mutualistic systems in the ocean were rare (30) (Schwartz et al.,
2016). Recently, however, Savoca and Nevitt (45) (2014) produced evidence for
tritrophic mutualism in the Southern Ocean, where phytoplankton are grazed by
crustaceans. This phytoplankton releases copious DMS, which in turn attracts
procelliiform seabirds that feed on these primary consumers, thereby reducing
grazing pressure on the phytoplankton. In a similar vein, Amo et al. (46) (2013)
found experimentally that the nestlings of the krill-eating chinstrap penguin,
Pygoscelis antarctica, are attracted to the scent of DMS. Thus it is likely
that DMS released by zooplankton grazing acts as a signalling molecule via the
penguins within a consortium at length scales at least up to 100s of m. In this
consortium cohesion was achieved by the penguins’ behavioural attraction to
DMS.
6. Consortia structured by rheological properties, including stickiness, of polymers
In many other consortia, such as biofilms (47) (48)
(49) (Kerfahi et al., 2022; Imai et al., 2021; Karn et al., 2020), lake and
marine organic aggregates(50) (51) (Qin et al., 2021; McManus et al., 2021),
however, cohesion is achieved by physical processes such as stickiness (52) (Santschi
et al., 2020), gelling (53) (Duan et al., 2022) or increased viscosity (54)
(Guadayol et al., 2020) mediated by exopolymeric substances (EPS). Schwartz et
al. (30) (2016) suggested that consortia with more elements than 3 are likely
to exist, but that the number of degrees of freedom would make their existence
difficult to prove.
Aron et al. (55) (2020) have introduced Global
Natural Product Social Molecular Networking (GNPS) as a tool for analysing the
infrastructure of molecular datasets. and curating the data in a public
database in the style of GenBase. GNPS may have the potential for
investigating diverse marine consortia and other biotopes, and ultimately the
whole ocean. It may desirable to accompany such a molecular database with
another on associated measured rheological properties in the ocean environment
at different scales.
7. Prey-capture and predator-avoidance
Chemical cues exuded by prey or occurring on their
surface can be used by predators to locate and choose their prey. The copepod, Temora
longicornis, feeds on sinking marine snow (MDS) particles, which leave a
chemical trail behind them (56) (Lombard et al., 2012). The detection of
tunicate exoskeletons (as an experimental proxy for MS) produced doubling of
swimming velocity towards the falling particle; the authors suggested that
detection must have been chemical, as distances were too great for
hydromechanical methods. They also drew parallels with how pheromones secreted
by the copepods, T. longicornis and Eurytemora affinis, change the swimming
behaviour of the opposite sex (38) (Seuront & Stanley, 2014). (See Section 4.)
8. Predator-prey interactions
Schwartz et al. (30) (2016) reviewed the effects of
chemical signalling in protist-protist and copepod-protist pairs. Many different
reactions have been reported, both on predator-prey reactions and on the
swimming behaviour of both prey and predators. Brown et al. (5) (2019) also
reviewed the effects of signalling molecules on predator-prey pairs,
particularly invoking the effects of phytoplankton toxins, acting either to
harm potential predators or as aids in capturing prey.
Ianora and Miralto(57) (2009) review the
short-chain polyunsaturated aldehydes (PUAs), secreted when copepods such as
Calanus finmarchicus, or Temora longicornis graze on diatoms such as
Thalassiosira rotula, T. pseudonana, Phaeodactylum tricornutum, Skeletonema
marinoi, Chaetoceros affinis, C. decipiens or C. socialis, and as a result
produce deformed and thus unviable larvae. Some species of the planktonic diatom
genus, Pseudo-nitzschia, such as P. seriata, internally produce and partially
release a nerve toxin that produce Amnesic Shellfish Poisoning (ASP) in humans,
as well as behavioural changes in marine vertebrates and some invertebrates.
Tammilehto et al. (58) (2015) found that P. seriata releases the secondary
metabolite, domoic acid (DA), when damaged through grazing by copepods, such as
C. hyperboreus and C. finmarchicus. The same copepods, however, were highly
resistant to the DA (59) (Harðardóttir et al., 2015), which suggested to Brown
et al. (5) (2019) that some populations of copepods may have evolved resistance
to DA.
Prince et al. (60) (2013) showed that, as well as
acting as a neurotoxin, DA, produced by Pseudo-nitzschia delicatissima,
inhibits growth of another diatom, S. marinoi, only slightly under low (perhaps
limiting) concentrations of Fe, (0.18 µmol L-1), but much more
strongly when Fe was replete (~18 µmol L-1) for diatom growth. DA
also inhibited growth of S. marinoi, while slightly stimulating growth by P.
delicatissima. In the experimental work of Prince et al. (60) (2013), it was
not clear whether P. delicatissima actually produced DA, like its congener, P.
seriata. If it does, the DA might then play two roles: firstly, favouring diatoms
that produce it; secondly, inhibiting competing phytoplankton possibly by
scavenging Fe, and by poisoning potential predatory zooplankton.
9. Mucus trophic structures ("mucus traps").
Rather like terrestrial web-weaving spiders, many
marine organisms feed using structures of polymeric mucus that entrap passing
prey by more or less sticky polymers that in some cases are toxic as well,
killing, or just immobilizing the prey.
Some multi-cellular zooplankters release mucus to
the environment, and appear to provide manure and "garden" their prey
before eating it. The harpacticoid copepod, Diarthrodes nobilis,
secretes mucus fibres through vents in its carapace. It then weaves these
fibres to produce an enmeshing capsule, to which adds its own faeces. This allows
prokaryotes to multiply on the capsules, which are then ingested by the copepod
(61) (Hicks & Grahame, 1979). While it is unclear how much of the mucus
remains in the environment, the authors suggest this "gardening", on
"mucus-traps" is analogous to procedures previously described in
marine nematodes (62) (Riemann & Schrage, 1978).
Mucus traps produced by protists are structured in
different ways. For example, the dinoflagellate genus, Dinophysis,
produces various toxins, including okadaic acid, pectenotoxin (PTX2) and
dinophysistoxin 1b (DTX1b) but from calculations of amount secreted
extracellularly, Nielsen et al. (63) (2013) calculated that field
concentrations would have been too low to support the idea that these toxins
act as allelopathic agents. Instead, the dinoflagellate, Dinophysis
acuminata, uses "mucus threads" to trap its prey, the ciliate, Mesodinium
rubrum, which is an obligate part of its mixotrophic life cycle (64) (65)
(Ojamäe et al., 2016; Jiang et al., 2018).
Ostreopsis cf. ovata cells produce
collective benthic webs of sticky mucous fibrils associated with the toxins,
ovatoxin-a, -b, -c, -d/e and putative palytoxin(66) (Honsell et al., 2013).
Organisms that get stuck or entangled on the webs are then attacked, often by
several cells collectively, and devoured.
The planktonic dinoflagellate, Alexandrium
pseudogonyaulax, secretes transparent spheroidal mucous traps, which are
sticky and entrap small flagellates. The trap often remains attached to the
dinoflagellate by its trailing flagellum, then the dinoflagellate engulfs these
prey individually, finally abandoning the spent mucus trap (67) (Blossom et
al., 2012).
In gradients of viscosity significant at the length
scale of cell size, motile cells are expected to be slowed more on their side
in the more viscous water, thus being turned to swim into the more viscous region.
Zones of high viscosity may this act as traps for swimming cells. Such
"viscous traps" are sometimes used by heterotrophic and mixotrophic
protists to catch prey, and in some cases the protists even lace the
high-viscosity zones with lytic toxins (68) (Blossom & Hansen, 2020). Stehnach
et al. (69) (2021), however, observed that in gradients of viscosity the
chlorophyte, Chlamydomonas, uses viscophobic turning to actually steer
their swimming away from zones of higher viscosity. This behaviour would allow
these flagellates to avoid such viscous traps. It also implies that they are
able to sense viscosity and the direction of viscosity gradients, a capability
reminiscent of that of diatoms that detect and react to turbulence fields (70)
(Falciatore et al., 2000). (See section 13.)
10. Mucus as a retention tool
At the ecosystem scale, endosymbiotic algae in the
coral polyps, the zooxanthellae, account for most of the reef's primary
production (PP). In the Great Barrier Reef, for example, half of this PP is
exuded by the polyps as mucus. This mucus traps organic matter from the water
column, settles and carries energy and organic matter to the reef sediments.
Dissolved mucus (~50-80% of the total mucus) is filtered through the lagoon
sands, where it is quickly (~7%/h) degraded. Undissolved mucus aggregates trap
particles, increasing their organic C and organic N by 2 orders of magnitude
within 2 h. Currents concentrate these aggregates into the lagoon. Coral mucus
thus provides light energy, harvested by the zooxanthellae and trapped
particles to the heterotrophic benthic community of the reef (71) (Wild et al.,
2004). Filtration of DOM and POM by reef sponges may significantly add to the
reef-scale trapping of OM (72) (de Goeij et al., 2013). This constitutes a
recycling loop that retains energy and nutrients within reef ecosystems, known
for their outstandingly high biodiversity and productivity. Evolutionary
processes and structures within the community-scale genome of coral reefs
deserve investigation.
11. The roles of cross-linked gels, rheological changes and reactive oxygen species in toxicity to fish
The sulphated amino acid, cysteine and its
derivative acetyl-N-L-cysteine (NAC) are mucolytic and antioxidant. In
human medicine, the mucolytic effect of NAC is based on the presence of the
free sulphydryl group (—SH), that opens up disulphide bonds (S—S) of the
high-molecular-weight glycoproteins of human mucus, thus reducing the viscosity
and elasticity of the mucus. NAC can also lyse DNA in sputum. NAC is also a
direct and indirect antioxidant. The direct effect is produced by the free
sulphydryl group, which is a source of electron donors that inactivate (i.e.
scavenge) reactive oxygen species (ROS). NAC scavenges •NO2,
CO3•-, and thiol radicals quickly, but O2•-,
H2O2 and peroxynitrite only slowly and O2 or
NO not at all (73) (74) (Samuni et al., 2013; Calzetta et al., 2018).
Yang and Albright (75) (1994) sought treatment for
coho salmon, Oncorhynchus kisutch, killed by blooms of the diatom, Chaetoceros
concavicornis. This species bears sharp, pointed spines that are believed
to irritate the gills and induce them to produce excess mucus. This mucus is a
proteinaceous material which consists mainly of mucopolysaccharides, with the
long, interconnected, fibrous molecules occurring within a gel. The physical
properties of mucous secretions are largely determined by the high molecular
weight glycoproteins which consist of a protein backbone with many
oligosaccharide side chains, often called mucin. The peptide chain of mucin
contains some non-glycosylated regions, which contain many cysteine residues.
Many mucous glycoproteins are composed of polymerized glycoprotein subunits
through the formation of disulphide bonds in the non-glycosylated region of
each protein core, probably involving interaction between adjacent cysteine
residues which results in a network of matted molecules. Yang and Albright
found that salmon administered L-cysteine ethyl ester (LCEE) in their feed
showed increased survival. Since cysteine and its derivatives, NAC and cysteine
L-cysteine ethyl ester (LCEE) can break S=S bonds and thus fluidify mucus,
including mucus secreted by gills (76) (Powell et al., 2007), Yang and Albright
concluded that this effect was responsible for reducing mortality. NAC, also
included in feed, was additionally found to reduce the toxic effect to fish of
cylindrospermopsin, a toxin produced by several harmful cyanobacteria (77)
(Gutiérrez-Praena et al., 2014).
After finding that gas bubbles were preventing from
rising in a bloom of the fish- and invertebrate-killing dinoflagellate, Karenia
mikimotoi (also then known as "Gyrodinium aureolum" or
Gymnodinium nagasakiense) (78) (Jenkinson & Connors,1980), and
measuring the viscoelasticity of cultures of the different species of
phytoplankton (79) (Jenkinson, 1986), Jenkinson(80) (81) (1989) modelled that K.
mikimotoi could slow flow and thus reduce O2 supply, thereby
suffocating the fish when present in sufficient concentration. Jenkinson &
Arzul (82) (80) (1998, 2002)) found that found that cultures of K. mikimotoi,
and the fish-killing raphidophyte, Heterosigma akashiwo, flowed
through fish gills more slowly than culture of the harmless haptophyte, Pavlova
lutheri, which itself did not slow flow relative to that of pure culture
medium. H. akashiwo showed more variable results than either K.
mikimotoi or P. lutheri, suggesting that the EPS it produced was
more heterogeneous. The relationship between flow rate and hydrostatic pressure
difference over the gills suggested that K. mikimotoi and H. akashiwo
added significant amounts of gel-like EPS to their ambient milieu, but that
P. lutheri did not. In addition, however, K. mikimotoi was found to
produce reactive oxygen species (ROS) (82) (80) that contribute haemolytic
toxicity(83) (Gentien et al., 2007), that is assayed by measuring the toxin's
lysis of red blood cells. K. mikimotoi isolated from European waters
(France and Ireland) may be more active rheologically than that from East Asian
waters. The term, "rheotoxity" was used by Jenkinson & Arzul (82)
(2002) to mean "toxicity" (i.e. harm) done to organisms by increased
viscoelasticity. An allied meaning is local increase the concentration of
chemical toxins again by increases in viscoelasticity thus reducing dispersal
(79).
Harmful algae other than Karenia mikimotoi
that produce conspicuous amounts of observed mucus, measured increases in
viscosity and/or foam include blooms of Karenia species, mainly K.
selliformis (84) (Orlova et al., 2022), the dinoflagellates, Margalefidinium
(= Cochlodinium) polykrikoides (85) (Kim & Oda, 2010) Ostreopsis
cf. ovata (86) (Berdalet et al., 2017) and the haptophytes, Phaeocystis
globosa (87) (88) (Seuront & Vincent, 2008; Kang et al., 2020), P.
pouchetii (89) (Balkis-Özdelice et al., 2021) and P. antarctica
(90) (Seuront et al., 2010).
In July 1985, at Caño Island, close to the Pacific
coast of Costa Rica, massive mortality of corals occurred, along with that of many
species of fish (including scarids, acanthurids, pomacentrids, tetradontids and
balistids), crabs and gastropods (91) (Guzman et al., 1990). The plankton
contained 8.3 × 105 live cells and >3 × 106 cells in
total. The dinoflagellates, Margalefidinium catenatum comprised
97% and Gonyaulax monilata (=Alexandrium monilatum) 1%. In
October and November 1985 at Uva Island, close to the Pacific coast of Panama,
about 300 km SE of Caño Island, a red-brown bloom of dinoflagellates lasted
several days. Dinoflagellates and viscous foam co-occurred, suggesting to the
authors that the former had produced the latter. Pocilliporid corals were found
bleached. The authors concluded that the mortality of the reef organisms at
Caño and Uva Islands was most likely caused by adhering of the mucus and, in
the case of polyps, interference with their expansion, although chemical
toxicity and oxygen depletion may also have contributed (91) (Guzman et al.,
1990).
Kim et al. (92) (2002) investigated toxicity in M.
polykrikoides and in the raphidophyte, Chattonella marina, using
human epithelial carcinoma (HeLa) cells as target. In culture, growth of both
species was at first exponential, reaching a plateau phase. After 12 days,
cultures of M. polykrikoides and C. marina the polysaccharide
contents increased to reach respective concentrations of 47 and only 4 µg/ml
glucose equivalent. The M. polykrikoides cultures became noticeably more
viscous, but the C. marina cultures did not. Nevertheless, as antiviral
activity had been previously reported in M. polykrikoides mucus (25)
(Hasui et al., 1995). Kim t al. (92) ((2002) proposed that cytotoxic agents may
have contributed to ichthyotoxicity by M. polykrikoides.
During extensive blooms of M. polykrikoides
around Oman and Muscat, associated with the deaths of hundreds of tons of fish
and shellfish, Al Gheilani et al. (93) (2012) reported that while strong odours
occurred, thought to be caused by methyl sulphide, no toxicity was detected in
mouse tests. Scanning electron microscopy, however, showed mucus proliferation,
which might have clogged gills, and fish gills also appeared damaged.
Working on M. polykrikoides from South
Korea, Lee et al.(94) (1996) conversely showed haemolytic activity in both the
water-soluble and chloroform-soluble fractions isolated from methanol extracts
of M. polykrikoides. Yet C.S. Kim et al.(95) (1999) found that M.
polykrikoides, also isolated from South Korea, produced high quantities of
reactive oxygen species (ROS), which they suggested was the primary cause of
fish mortality, through damage to gills. Kim and Oda (85) (2010) investigated
the fish-killing mechanisms of Chattonella marina and M.
polykrikoides from the Yatsusiro Sea, Japan. Their results suggested that C.
marina has an NADPH-dependent superoxide generation system in its
glycocalyx. Their results also suggested that C. marina continuously
releases H2O2 into the medium during culture, whereas M.
polykrikoides may not release H2O2, at least under
normal physiological conditions. Their results suggest that continuous
accumulation of discharged glycocalyx on the gill surface occurs during C.
marina exposure, which may be responsible for the ROS-mediated severe gill
tissue damage leading to fish death. Compared to C. marina, the levels
of O2– and H2O2 detected in Margalefidinium
polykrikoides were only trace amounts. Both lectins and mucus prepared from
fish skin and gills of yellowtail from C. marina, but not from M.
polykrikoides, when administered separately, produced markedly
increased levels of O2- in C. marina, but
not in M. polykrikoides. Further results suggested that the O2-
generation system of C. marina is located on the cell surface, whereas
only slight evidence of cell-surface generation was shown in M.
polykrikoides. Evidence was shown, however, for the production of H2O2
in both species. Cell-free aqueous solutions prepared from both C. marina
and M. polykrikoides were tested on HeLa cells as target. After 24h
treatment with 10% final concentration of each extract in α-minimal essential
medium containing 10% fetal bovine serum, cytological changes took place on the
HeLa cells and colony formation was reduced, whereas corresponding extract from
C. marina produced almost no effect. Kim & Oda (85) (2010) suggested
that the difference between Lee et al.’s (94) (1996) findings and their own
could have reflected differences in strain characteristics.
Flores-Leñero et al. (96) (2022), studying
ichthyotoxicity induced by the raphidophyte, Heterosigma akashiwo, in
Patagonian fords, found that ROS and polyunsaturated fatty acid (PUFA) was too
weak to explain the fish kills that occurred, and the authors suggested that
further studies should explore other fish-killing mechanisms, such as the
production o mucus or extracellular polymeric substances (EPS). These results
may reflect the conclusions of Yamasaki et al. (97) (2010) that the role of
PUFAs and ROS in H. akashiwo blooms may be rather part of a suite of
non-lethal signalling molecules controlling marine microbial community
structure and function. (See sections 1 and 3).
Blooms with the presence of either of the
dinoflagellates, Gonyaulax fragilis or G. hyalina, have been
associated with viscous/slimy water and foam, frequently associated with mass
mortality of marine organisms. Such phenomena have been recorded from: Sea of
Marmara, Turkey (G. fragilis - foamy mucilage) (89) (Balkis-Özdelice, et
al., 2021); Tasman Bay, New Zealand (98) (MacKenzie et al., 2002); Northern
Adriatic (G. fragilis - mucilaginous masses) (99) (100) (Honsell et al.,
1992; Pompei et al., 2003). G. fragilis and G. hyalina may have
been confused by some authors, as they are similar, but separate species (101)
(Carbonell-Moore & Mertens, 2019). Both produce copious mucus from their
apical pore, resulting in noticeable mucilage in the field even at cell
concentrations as low as several thousand cells per litre.
Riccardi et al. (102) (2010) studied the role of G.
fragilis in producing mucilage events in the N. Adriatic, as well as
sterols as potential lipid biomarkers associated with this mucus. They also
extracted DNA from G. fragilis, and using PCR to characterize it,
successfully developed a species-specific DNA probe. In culture,
moreover, G. fragilis was associated with the 4α-methylsterols. That
association between G. fragilis and specific sterols was found not to be
clear in N. Adriatic mucilage-rich field samples was ascribed to rapid decay or
transformation of the dinoflagellate cells and of the sterols. However, the
authors suggested that in future, rapid genomic detection coupled with
identification of phytoplankton cells in the field could be used to investigate
their association with lipid biomarkers, such as sterols.
The dinoflagellate, Karlodinium arminger produces
karlotoxins 1, 2, 8 and 9, that have all been implicated in fish kills (5)
(Brown et al., 2019). This toxin lysed trout gill cells with a LC50
of 125 nM, and it showed a somewhat higher LC50 of 400 nM for the
copepod Acartia tonsa, a potential predator. Huge numbers of algal toxins,
particularly those harmful to humans, have been revealed in the last 20-30
years (reviewed by (30) (5) Schwartz et al., 2016; Brown et al., 2019). While
further details are outside the present paper’s scope, evaluating the genes
associated with these toxins is should be given priority.
Species of the dinoflagellate genus, Dinophysis,
produce various toxins, including pectenotoxin 2 (PTX2) and dinophysistoxin 1b
(DTX1b) (63) (Nielsen et al., 2013). According to Schwartz (30) (2016),
however, calculated field concentrations would have been too low to allow to
support the idea these toxins act as allelopathic agents.
12. Mechanisms of killing microbes
Direct killing may be the strongest signal. Some
bacteria produce toxins that are lethally toxic to microalgae. Hu et al. (107)
(2019) found that algicidal bacterium, CZBC1, is lethal to the cyanobacteria, Oscillatoria
chlorina, O. tenuis and O. planctonica, to the extent that the authors have
patented culturing these bacteria to control cyanobacteria in aquaculture
facilities. Hu et al. (107) (2019) also reviewed the effects of other algicidal
bacteria on microalgae, including cyanobacteria. Bacteria of the genera, Cytophaga
and Saprospura, contact and lyse dinoflagellates and diatoms. A strain of Pseudomonas
putida kills the diatom, Stephanopyxis, by direct alginolysis, but most
algicidal bacteria act indirectly by secreting algicidal compounds. Bacillus strain
LZH-5 from Lake Taihu, China, acts strongly on Microcystis aeruginosa.
Concerning the size of the signalling (toxic)
molecules, the marine bacterium, Bacillus cereus Strain CZBC1, produces
an alginolytic compound retained by a 10 kDa filter (107) (Hu et al., 2019). On
the other hand, Lee et al. (108) (2000) found that Pseudoalteromonas Strain
A28 produces a serine protease activity responsible for algal lysis, but
they also had DNase activity; the supernatant from Strain A28, which passed
through a 10-kDa filter could kill the diatom, Skeletonema costatum. In
addition, Hu et al. (107) (2019) showed that that Strain CZBC1 lysed O.
chlorina and O. tenuis by direct alginolysis, while its extracellular products
lysed O. planctonica. Various authors cited by Hu et al.(107) (2019) showed
that algolytic effects of bacteria are concentration-dependent.
13. Quorum sensing
Quorum sensing (QS) is a density-dependent communicating
mechanism that allows organisms to regulate a wide range of important processes
and can be inhibited by quorum quenching (QQ), for example in marine organic
aggregates (MOAs) (109) (Su et al., 2021) and probably also at larger scales.
Falciatore et al. (70) (2000) may have weakened Svedrup et al.’s (110) (1942)
old paradigm that plankton are passive and incapable of resisting physical
forces at any scale. Reviewing post-Sverdrup findings that "[some]
plankton control buoyancy, local fluid viscosity and life cycles",
Falciatore et al. (70) (2006) showed experimentally that diatoms detect and
respond to physicochemical changes in their environment using sophisticated
perception systems based on changes in cytoplasm concentrations of Ca2+
as a second messenger (111) (Endo, 2006).
Microbes communicate with each other using
diffusable molecules such s N-acetylhomosereine lactones (AHL). Communication
of the information in a signal requires sensing of the signal and it is not
clear whether the different types of sensing use secondary messengers sensu
Endo (111) (2006). Nevertheless, these different signals are likely
physiological and behavioural cell-density-dependent gene regulators (112)
(Ianora et al., 2006), involving quorum sensing and controlling microbial
processes.
Whether in bacteria, algae or metazoans, tighter
spatial association will increase intensity of interactions, as well as
reducing the distances and, mostly, the times of signal transmission (113)
(Jenkinson and Wyatt, 1992), whether by diffusion or by radiation, of
information agents such as pheromones, light, sound or other electrical or
mechanical signals. Association may be effected by attractive behaviour or by
rheological means, such as increasing the viscosity or the yield stress of the
ambient medium. When yield stress is larger than the shear stresses tending to
deform the ambient medium, the spatial distribution of particles, such as
organisms, in the medium is gelled. Even when the yield stress is less than the
ambient mechanical stresses, the increases viscosity will slow the medium's
deformation and hence dispersion of the particles and molecules.
Modification of the mechanical (rheological)
properties, such as viscosity (31) (54) (114) (88) (Jenkinson & Sun, 2010;
Guadayol et al., 2020; Seuront et al., 2007; Kang at al., 2020), of the
transmitting medium will also tend to reduce the intensity of signal
transmission. Such modification is largely carried out by secreted EPS,
particularly that bearing saccharide groups. Such rheological modification by
EPS will therefore also modify the signals that signalling molecules, including
toxins, transmit. Such EPS may thus be considered to include "auxiliary
signalling molecules", which participate in niche engineering(115) (116)
(117) (109) (110) (Hastings, 2007; Reddington et al., 2020), formerly called
"physical environmental management"(118) (Jenkinson and Wyatt).
MOAs may be considered as 3D equivalents of marine
biofilms (MBs) (119) (120) (8) (Camacho-Chab et al., 2016; Sretenovic et al.,
2017; Wurl et al., 2017). In MOA- and BF-associated prokaryotes, including the
Gram-negative alphabacterium, Paracoccus carotinifaciens, and the
gammaproteobacterium, Pantoea ananatis, resistance to viral infection and to
protozoan predation is achieved by secretion of various homosereine lactones
and ammonium, respectively(121) (122) (109) (Jatt et al., 2014; Decho &
Guttieriez, 2017; Su et al., 2021). In the bacterium, Vibrio cholerae, living
in biofilms, resistance to protozoan grazing is effected by secretion of the
metabolite at concentrations of up to 3.5 mM, which was found to reduce
concentrations of the protistan grazer, Rhynchomonas nasuta, by >80% (123)
(Sun et al., 2015).
By contrast, other bacteria, including the fish
pathogen, Vibrio anguillarum, defend against viral (phage) infection by
increasing expression of the ompK gene, which correlates with the degree of
cell aggregation, being low in free-living variants (124) (Tan et al.,
2015).The gene, ompK, produces N-acylhomoserine lactone (AHL) a molecule with
QS signalling and many metabolic functions, and there may thus be a link among ompK,
AHL and aggregation. So far, however, no causal mechanism among ompK, AHL and
aggregation has been demonstrated. . Indeed, investigation of which genes are
involved in producing enzymes involved in producing sugars and assembling them
into polysaccharides such as EPS, as well as in EPS destruction, either in pro-
or eukaryotic aquatic, single-celled organisms, appears to have started only
recently (109) (Su et al., 2021).
AHLs are probably the most intensively studied
class of mediators in cell-density-dependent gene regulation (112) (125)
(Ianora et al., 2006; Pappas et al., 2004), and have been found in bacterial
biofilms and marine snow, in which it is believed that bacteria, interacting
with ambient pressures and constraints, control its form and phenotypic traits
(126) (127) (Gram et al., 2002; Parsek & Fuqua, 2004). In biofouling,
surface sensing via AHLs of bacterial biofilms is the initial step in the
settling of the macroalga, Ulva (128) (129) (Tait et al., 2005; Wheeler et al.,
2006).
Outside of biofilms and MOAs, microalgae, too, may
alter behaviour in multi-celled organisms. Increases in water viscosity due to
secretion of polymers by the haptophyte, Phaeocystis globosa, were observed to
make swimming patterns in the copepod, Temora longicornis, more compact (87)
(Seuront & Vincent, 2008), while the same species reduced measured feeding
rates and filtering rates in Temora stylifera (130) (Li et al., 2021).
In bacterially-dominated biofilms, reviewed by Karn
et al. (49) (2020) for their role in promotion or inhibition of corrosion,
carbohydrates are generally the most abundant constituents, accounting for
40-95% by mass, while proteins typically contribute 1-60%, lipids 1-40%and
nucleic acids 1-10%. The biofilm matrix acts as a recycling centre, by
preventing the molecular products of live cells from dispersing and becoming
lost to the consortium (131) (122) (Flemming and Wingender, 2010). These
products include DNA, which could represent a reservoir of genes for horizontal
gene transfer over small distances. Proteins, along with humic substances,
might play a role as electron donors or acceptors by forming bacterial pili and
nanowires. Modulation of rheological properties in biofilms, such as binding
and stabilization, which affect retention of enzymes, may take place mainly by
interactions with polysaccharides and proteins in the biofilm in reaction to
mechanical forces, including those caused by deformation in the surrounding
milieu (132) (131) (Hohne et al., 2009; Flemming and Wingender, 2010).
In a study related to photo-aggregation related to
reactive oxygen species (ROS), Sun et al.(133) (2019) found that MOA size was
positively related to protein/carbohydrate (P/C) ratio. The authors measured
MOA size after ultrafiltration through 0.2-µm polycarbonate filters, which are
hydrophilic, and allowing the polymer particles to re-form spontaneously in the
filtrate, indicating that the inter-protein bonds were stronger than
inter-polysaccharide bonds. In this respect, microrheological measurement
carried out near phytoplankton cells (54) (Guadayol et al., 2020) found that
viscosity increased by up to a factor of 2 to 5 at distances of 2 to 5 µm from
the cell, typically declining to a factor of 1.2 at 10 £m from the cell.
Furthermore, Stehnach et al. (69) (2021) report viscophobic turning in the
flagellate, Chlamydomonas reinhardtii. Such behaviour might be critical
to avoid being slowed down or trapped in mucus-reinforced zones of high
viscosity, such as mucous traps (67) (96) (Blossom et al., 2012).
Like marine organic aggregates (MOAs), sewage
sludge organic aggregates (SOAs) consist mainly of bacteria and diverse debris
held together with loosely-bound EPS, in a slimy matrix of closely-bound or
unbound EPS. The more concentrated nature of SOAs sewage sludge compared with
the ocean, together the need to dewater it for economical transport and
disposal, drives lively research activity on the rheology and surface science
of SOAs (134) (Zhang et al., 2018), as well as on ecological chemistry of SOAs
and biofilms (135) (49) (Lear, 2016; Karn et al., 2020). This activity provides
results and expertise with a strong potential to inspire and guide research on
MOAs.
14. Scales (granulometry) of toxicity
The term, toxicity, is usually used in the sense of
causing harm by toxic molecules to living organisms. This implies one length
scale of the chemical action (nm) and another at that of a cell (1-100 µm) or
even of multicellular organisms. Toxic effects of molecules produced genomes
may act at an environmental scale of km to 1000s of km and be more difficult to
identify. In both spontaneously aggregated OM (136) (137) (Verdugo, 2012,
2021), or in biologically produced mucus, fluid flow and often molecular
diffusion are reduced, while physical density may be increased or decreased
(138) (139) (Drost-Hansen, 2006; Mari et al., 2008).
Much research is currently under way on the
allelopathic effects of polyunsaturated aldehydes (PUAs), which act as
signalling compounds (5) (Brown et al., 2019), which some diatoms release
amongst the copious DOM, particularly towards the end of their blooms. Bartual
et al. (140) (2017), working with laboratory blooms of the diatom, Thalassiosira
rotula, studied the effect of adding PUA (2.5 µM mixture of three PUAs, 2E,
4E-heptadienal; 2E,4E-octadienal; 2E,4E-decadienal) on the aggregation of
diatom-secreted TEP into OAs. They found that when the presence of PUA resulted
in larger OAs and they suggested that PUA acts as glue, consolidating the OAs.
Since larger OAs sinks faster as marine snow, the diatom-produced PUA might be
contributing to increased vertical organic flux. The physico-chemical
mechanisms of how these PUAs stick within TEP, as well as the genes responsible
for PUA production require investigation.
15. Hydrophobicity, organic aggregate size and rheology
The polymeric secretions of algae and bacteria
include polysaccharides, proteins, lipids and nucleic acids. Proteins are
amphiphilic: they bear both hydrophilic (polar, wettable) and hydrophobic
(non-polar) domains and they are considered to contribute most of the
hydrophobicity of EPS. In contrast, polysaccharides are mostly hydrophilic
through their polar oxygen groups. An increase in the degree of internal
hydrogen bonding, however, can increase their relative hydrophobicity (52)
(Santschi et al., 2020). Klun et al. (141) (2022) investigated colloidal OM
(COM) secreted in culture by a chlorophyte nanoflagellate,
Tetraselmis
sp., a diatom,
Chaetoceros socialis and a dinoflagellate,
Prorocentrum minimum, all isolated from the Gulf of Trieste. Table 2 summarizes their results. The OM had
been 0.45 µm e-filtered. It was then ultrafiltered through membranes with
nominal molecular-weight cut-off of 5 kDa. The polysaccharide fraction was the
highest in the retentate of
Tetraselmis sp. (61%), while lipids and
proteins each accounted for 19%. In the permeate, protein represented the
highest portion (41%). The
C. socialis retentate and permeate contained
the highest polysaccharide levels (63% and 46%, respectively), followed by proteins
(22% and 36%) and lipids (14% and 16%).
P. minimum retentate and
permeate showed very different compositions of secreted OM, with polysaccharide
proportions of only 32 % and 25%, respectively, compared with high proportions
of proteins (46% and 57%, respectively) and with intermediate proportions of
lipids (22% and 17%, respectively). For all three taxa, the proportion of
polysaccharides was thus higher in the retentate than in the permeate, while
the proportion of proteins was higher in the permeate, while for lipids the
corresponding situation varied. The overall proportion of lipids found in both
the retentates and the permeates was surprisingly high, from 14% to 36%.
Table 2.
Distribution of the integrated main groups of proton resonances (lipids, proteins and carboxyl-rich alicyclic molecules (CRAM), polysaccharides and formate) in 1H MNR spectra (6/ppm), concentrations of Corg. (µmol L-1) in retentates and permeates and % COC from exudates of cultured phytoplankters. Protons that resonated in certain chemical shift range of integrated main groups are in bold (second row). Summarized from ref (141) (Klun et al., 2022).
Table 2.
Distribution of the integrated main groups of proton resonances (lipids, proteins and carboxyl-rich alicyclic molecules (CRAM), polysaccharides and formate) in 1H MNR spectra (6/ppm), concentrations of Corg. (µmol L-1) in retentates and permeates and % COC from exudates of cultured phytoplankters. Protons that resonated in certain chemical shift range of integrated main groups are in bold (second row). Summarized from ref (141) (Klun et al., 2022).
| |
Lipids |
Proteins and CRAM |
Polysaccharides |
Formate |
Corg.
|
% COC * |
| |
HCH2-CH2- |
HC-HCOR |
HC-OH HC-O-C |
HCOO |
|
|
| 6 **/ppm |
0-1.8 |
1.8-3.0 |
3.0-4.6 |
8.0-9.0 |
µmol L-1 |
% |
| |
|
|
Tetraselmis sp. |
|
|
|
| 0.2 µm filtrate |
|
|
|
|
915.1 |
|
| Retentate |
19.4 |
18.8 |
61.4 |
0.4 |
364 |
39.8 |
| Permeate |
35.7 |
40.9 |
20.8 |
2.6 |
507 |
|
| |
|
|
Chaetoceros socialis |
|
|
|
| 0.2 µm filtrate |
|
|
|
|
2285 |
|
| Retentate |
14.4 |
21.9 |
62.9 |
1.1 |
526 |
23.0 |
| Permeate |
16.4 |
36.4 |
45.5 |
1.7 |
1765 |
|
| |
|
Prorocentrum minimum |
|
|
|
| 0.2 µm filtrate |
|
|
|
|
439.3 |
|
| Retentate |
21.8 |
45.6 |
31.9 |
0.8 |
154 |
35.1 |
| Permeate |
17.4 |
56.7 |
24.6 |
1.2 |
418 |
|
16. Molecules, produced by other organisms and associated bacteria, that are toxic and allelopathic to phytoplankton
Vidal-Melgosa et al.(142) (2021) reported a polysaccharide of still unknown structure, called fucose-containing sulphated polysaccharide (FCSP) that appears to be directly secreted by diatoms. Detected by monoclonal antibody technique, it was found abundantly distributed on cell surfaces and spines of the diatom, Chaetoceros socialis, in spring blooms in the Helgoland Bight. It appears to have a complicated structure, and unlike other polysaccharides present, it lasted »10 days in laboratory culture. This suggests that, over a certain concentration, it might be very important in aggregating to particulate organic matter (POM), and thus mediating vertical flux (143) (Denis et al., 2022). This FCSP is strongly negatively charged, and thus form part of the complex of acid polysaccharides secreted by phytoplankton as diverse as dinoflagellates(66) (Honsell et al., 2013) and cyanobacteria(144) (Liu et al., 2015).
Some seaweeds and seagrasses also exert allelopathic action on harmful algae. Laabir et al. (145) (2013) showed that the methanolic and aqueous extracts of the seagrasses, Zostera marina and Z. noltii, inhibited the harmful dinoflagellate, Alexandrium catenella. These extracts contained flavinoids and phenolic acids, which were themselves toxic to A. catenella, The authors suggested that phenolic acids were the likely candidates for allelopathic action, and that increases in A. catenella blooms in lagoons and other French waters may have been partly caused by declines in seagrass beds.
Subsequently, Onishi et al. (146) (2014) showed that only whole-plant extracts of seagrasses, and not exudates, were algicidal. Two strains of Flavobacteriaceae isolated from biofilms on the seagrass leaves show algicidal activity on Alexandrium tamarense. This suggests that the above mentioned findings of Laabir et al.(145) (2013) might have been due to bacteria epiphytic on seagrass, leaves rather than to the seagrasses themselves, particularly as Flavobacteriaceae have been shown be algicidal towards several fish-killing rhaphidophytes, dinoflagellates and diatoms. Similarly in the freshwater Lake Biwa, Japan, the bacterium, Agrobacterium vitis, which colonises surfaces of leaves of the water plant, Egeria densa, is strongly allelopathic to the harmful cyanobacterium, Microcystis aeruginosa, and an increase over several years of E. densa has coincided with a reduction in blooms of M. aeruginosa (147) (Imai et al., 2013).
Bacteria in the mucus phycosphere of the diatom, Skeletonema costatum, and of the dinoflagellate, Scrippsiella trochoidea, were found by Yang et al.(148) (2013) to be able to lyse their host cells, leading to mass deaths of the hosts. The mechanisms for this lysis were not elucidated.
Research on metabolomics in consortia (see section 6), particularly in biofilms, is intense in the fields of biocorrosion (49) (Karn et al., 2020), medicine (149) Qian et al., (2020) and sewage sludge (150) (Kotay & Das, 2010). It appears, however, still too rare in aquatic plankton ecosystem (116) (117) (Reddington et al., 2020). In such biofilm consortia, progress is being made in semi-automated data mining of intra- and extra- cellular DNA to identify gene sets involved in microbial metabolism and production of different molecules. The software is inspired by homologous data mining of texts on the internet. Thakur et al. (151) (2023) have made a promising start on bacterial consortia in biofilms associated with biocorrosion. The technique may be capable of development to mine pro- and eu-karyotic gene sets from ecosystems such as the world ocean, stored in GenBase. Singh(152) (2021) pointed out that in human bodies, as in the ocean, glycoproteins play crucial roles in biological processes like cell signalling, host-pathogen interaction and disease. Glycoproteomics aims to determine the positions and identities of the complete repertoire of glycans and glycosylated proteins in a given cell or tissue. The roles of glycoproteomics in ocean consortia may be analogous, and the links with genes, as least for key processes, should be established. While the main drivers of Thakur et al.'s (151) study may have been the need to understand and control biocorrosion of steel by sulphur-reducing bacteria, and those of the studies cited in Singh’s mini-review are improvement in human health, the purposes of mining ocean-derived data on gene sets might be to understand and control bio-geo-physico-chemical fluxes of matter and energy in the oceans, as well as cell-cell signalling and other interactions and their roles in ecological control (6) Yamasaki et al. 2009).
17. Organic polymers and Gaia
Some molecules polymerize, and the resultant biopolymers strengthen organic aggregates, biofilms and volumes of water with increased viscosity (135) (153) (4) (Lear, 2016; Alldredge et al., 1993; Jenkinson et al., 2015). Biopolymers tend to concentrate at surfaces, particularly in the surface microlayer (SML). Here they will thus tend to change properties including surface tension and 2D viscoelasticity (154) (Williams et al., 1986), as well as 3D viscosity(155) (156) (Carlson, 1987; Zhang, et al., 2003), thus modulating ocean-atmosphere fluxes of various types of matter and energy (157) (Jenkinson et al., 2018). Since organisms are genetically controlled and they participate in the production, metamorphoses and breakdown of these biomolecules, their genomes thus influence oceanic and atmospheric physico-chemical conditions. Where they are harmful, this may be considered toxicity at the scale of the Planetary ecosystem, consistent with evolution of Gaia (158) (159) (Lovelock, 198(158)8; Lenton, 1998) by natural selection of its genes (11) (Dawkins, 2016). An example of such Planetary scale toxicity, at least from an anthropic point of view, is the current increase in atmospheric and oceanic CO2.
18. Polymer modulation of fluxes: discovering the genomes involved.
Fluxes of matter and energy between the ocean and the atmosphere are functions of physical, chemical and biological parameters. In particular, viruses, bacteria sensu lato and eukaryotic protists are expelled towards the atmosphere by bursting bubbles, and may then be carried by updrafts and winds from a few metres(160) (Thornton, 1999) to thousands of km (161) (Hamilton & Lenton 1998). The surface charge and hydrophobicity of cells and non-living matter strongly influences tendency to be expelled(9) (Jenkinson et al., 2021). For species in which such expulsion leads to dispersion and greater Darwinian fitness, it is may represent a pre-adaptation leading to further evolution of hydrophobic/hydrophilic control of ocean-atmosphere flux. Irrespective of the length, time or hyperspace scales at which such natural selection is driven, it will have effects also over other biological and chemical species (collateral effects) in driving ocean-atmosphere fluxes, which are important for climate control (157) (9) (12) (Jenkinson et al., 2018; 2021; IPCC, 2021).
Notably in the SML and biofoam, both important to modulating ocean-atmosphere fluxes of matter and energy, the EPS present is shows different characteristics. The different species and their expressed genes that produce the EPS thus determine the different ways in which EPS modulates these fluxes.
19. Vertical organic flux of OM.
The use of metabolomics and proteomics has already illuminated the mechanisms of biological response to many chemical cues and may be helpful in determining their molecular targets. Overall, the ocean represents a vast source of novel interactions and well as new molecules (30) (Schwartz, 2016). As Schwartz (30) (2016) pointed out, both models and laboratory experiments are needed to evaluate the roles of toxic, allelopathic and signalling interactions in the pelagic. To these interactions might be added those involving modulation of fluxes, diffusion and fluid deformation.
Vidal-Melgosa et al.(142) (2021) showed the importance of fucose-containing sulphated polysaccharide (FCSP), which is secreted by diatoms, particularly Chaetoceros socialis, which produces mucilaginous colonies and which dominated the North Sea blooms they worked on. FCSP was far more resistant than the non-sulphated polysaccharide, laminarin, to degradation by Bacteroidea and Gammaproteobacteria. These organisms encode enzymes for laminarin degradation, and they are key degraders of laminarin in the field (162) (Teeling et al., 2012). Because FCSP is degraded far more slowly than laminarin, Vidal-Melgosa et al. (142) (2021) suggested that C. socialis and other diatoms that secrete large amounts of FCSP, are likely among the key species that modulate vertical carbon flux in aggregates, thus contributing to the sequester of CO2 from the atmosphere.
20. Ocean foam
As well as sequestration of CO2, ocean foams, such as whitecaps, help to keep the Earth cool (9) (Jenkinson et al., 2021). The fraction of incident solar radiant energy that is reflected is known as albedo. Foam-free ocean surface has a typical albedo of only ~0.05, but most foam is white and its albedo is ~0.5. Therefore, when foam is more stabilized by algal DOM percentage foam cover increases and the oceans reflect more solar energy back into space, reducing solar heating of the Earth (163) (164) (Stabeno and Monahan, 1986; Evans et al., 2010). The decay of these foams proceeds by the coalescence of its constituent bubbles as the inter-bubble water drains, allowing the surfaces of adjoining bubbles to touch and burst (165) (Cantat et al., 2013). Draining is retarded and foam lifetime becomes longer if the liquid is a surfactant that binds to bubble surfaces or if it is more viscous. Relative to their duration in waters of low phytoplankton biomass (PB) or low primary production (PP), ocean foam duration is increased in waters of high PP or high PB (166) (Callaghan et al., 2012), particularly in the presence of certain specific taxa. Blooms that produce a lot of foam are dominated by dinoflagellates on North Atlantic and Asian coasts (167) (Pierce et al., 2003), including Margalefidinium polykrikoides (168) (Vargas-Montero et al., 2006), Karenia spp. blooms dominated by K. selliformis in Russian Pacific waters (84) (Orlova et al., 2022) as well as the haptophyte, Phaeocystis globosa, in the North Sea (169) (Kesaulya et al., 2008). Blooms of the fish-killing dinoflagellate, Alexandrium monilatum, on the Pacific coast of Costa Rica have been associated with cream- or beige-coloured foam, with the water producing violent itching. The species also produces goniodomines (170) (171) (Calvo et al., 2005; Lassus et al., 2016). The primary chemistry of the EPS and associated genomes of these organisms, which also frequently increase viscosity and stabilize foam, requires further investigation.
21. Conclusions
Ocean ecosystems that need data mining of pro- and eu-karyotic genomes may include: biofilms on marine plastic debris, macroalgae and seagress, sea-ice structures, marine organic aggregates, the sea-surface microlayer and ultimately the whole ocean. Boundaries of consortia, at all granularities, need to be investigated, particularly the ocean-atmosphere boundary. Because the physical properties of polymers, both proteins and polysaccharides control water shearing and molecular diffusion, such studies should be combined with rheological studies at appropriate scales.
Fluxes of many types of matter and energy in the oceans are modulated, not only by proteins and lipids, but more especially by different dissolved and particulate polysaccharides. An important key to understanding, and perhaps ultimately controlling, these oceanic fluxes thus lies in exploring associations between these polymers and the genes present and expressed in the planktonic microbial community. Polysaccharides, as well as glycoproteins, glycolipids and some proteins are assembled by enzymes, which are themselves proteins. Different polysaccharides are therefore unlikely to be "mirrored" directly by corresponding genomes. This difficulty has slowed progress in ocean glycomics. Recently, however, enzymes responsible for selective cleavage of specific links in different marine microbial polymers have been linked to their roles in bacteria and diatoms in facilitating or preventing aggregation and de-aggregation of OM and adhering microbes(142) (Vidal-Melgosa et al., 2021). This exciting avenue needs to be accelerated and combined with quantitative multi-scale sampling and analysis of biomolecules as well as matching sampling of genes (already in GenBase), organisms, with the addition of a new database of measured rheological properties in the same environment. Intelligent data-mining of these database may take advantage of GNPS (55) (Aron et al., 2020) and techniques already developed for bacterial biofilms involved in corrosion (151) (Thakur et al 2023) (see sections 6 and 18). Expansion of such studies to ocean exploration is likely to vastly improve understanding of ecological and metabolic interactions, as well as fluxes, in the ocean. As a bonus they are almost certain to reveal commercially exploitable natural products.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
This review is not associated with any data.
Acknowledgments
This review has enormously benefited from discussions and exchanges with members of the International Working Group on Rheology, nano- and micro-Fluidics, bioFouling and bioFoam in the Oceans and other natural waters (RHEFFFO), as well as with Linda Medlin and Beatriz Reguera.
Conflicts of Interest
The author declares no conflict of interest.
References
- Hansell, D.A.; Carlson, C.A.; Repeta, D.J.; Schlitzer, R. Dissolved organic matter in the ocean : a controversy stimulates new insights Oceanography. 2009, 22, 202–211. [CrossRef]
- McCarren, J.; Becker, J.W.; Repeta, D.J.; Shi, Y.; Young, C.R.; Malmstrom, R.R.; Chisholm, S.W.; DeLong, E.F. Microbial community transcriptomes reveal microbes and metabolic pathways associated with dissolved organic matter turnover in the sea Proceedings of the National Academy of Sciences. 2010, 107, 16420–16427. [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F.; Azam, F. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean Nature Reviews of Microbiology. 2010, 8, 393–399. [CrossRef]
- Jenkinson, I.R.; Sun, X.X.; Seuront, L. Thalassorheology, organic matter and plankton: towards a more viscous approach in plankton ecology Journal of Plankton Research. 2015, 37, 1100–1109. [CrossRef]
- Brown, E.R.; Cepeda, M.R.; Mascuch, S.J.; Poulson-Ellestad, K.L.; Kubanek, J. Chemical ecology of the marine plankton Natural Product Reports. 2019, 36, 1093–1116. [CrossRef]
- Yamasaki, Y.; Shikata, T.; Nukata, A.; Ichiki, S.; Nagasoe, S.; Matsubara, T.; Shimasaki, Y.; Nakao, M.; Yamaguchi, K.; Oshima, Y.; Oda, T.; Ito, M.; Jenkinson, I.R.; Asakawa, M.; Honjo, T. Extracellular polysaccharide protein complexes of a harmful alga mediate the allelopathic control within the phytoplankton community Int Soc Microbiol Ecol J. 2009, 3, 808–817. [CrossRef]
- Mari, X.; Passow, U.; Migon, C.; Burd, A.B.; Legendre, L. Transparent exopolymer particles: Effects on carbon cycling in the ocean Progress in Oceanography. 2017, 151, 13–37. [CrossRef]
- Wurl, O.; Ekau, W.; Landing, W.M.; Zappa, C.J. Sea surface microlayer in a changing ocean - a perspective Elem Sci Anth. 2017, 5, 31. [CrossRef]
- Jenkinson, I.R.; Berdalet, E.; Chin, W.-C.; Denis, M.; Ding, H.; Duan, J.; Elias, F.; Emri, I.; Karn, S.K.; Li, Z.; Malej, A.; Mari, X.; Seuront, L.; Sun, J.; Wyatt, T.; Zhang, W.; Wurl, O. The rôles of plankton and neuston microbial organic matter in climate regulation Journal of Plankton Research. 2021, 43, 801–821. [CrossRef]
- Darwin, C. ; On the origin of Species. Harvard University Press, Cambridge, Mass., USA, 2003.
- Dawkins, R. ; The Extended Selfish Gene. Oxford University Press, Oxford, UK, 2016.
- IPCC Summary for policy makers. In: Masson-Delmotte V.; Zhai P.; Pirani A.; Connors S. L.; Péan C.; Berger S.; Caud N.; Chen Y.; Goldfarb L.; Gomis M. I.; Huang M.; Leitzell K.; Lonnoy E.; Matthews J.; Maycock T. K.; Waterfield T.; Yelekçi O.; Yu R., Zhou B. (Eds), Climate Change 2021, The Physical Science Basis.Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press, Cambridge, UK, 2021.
- Rosenwasser, S.; Ziv, C.; van Creveld, S.G.; Vardi, A. Virocell Metabolism: Metabolic Innovations During Host–Virus Interactions in the Ocean Trends in Microbiology. 2016, 24, 821–832. [CrossRef]
- Liu, X.; Stock, C.A.; Dunne, J.P.; Lee, M.; Shevliakova, E.; Malyshev, S.; Milly, P.C.D. Simulated Global Coastal Ecosystem Responses to a Half-Century Increase in River Nitrogen Loads Geophysical Research Letters. 2021, 48. [CrossRef]
- Hamilton, D.S.; Perron, M.M.; Bond, T.C.; Bowie, A.R.; Buchholz, R.R.; Guieu, C.; Ito, A.; Maenhaut, W.; Myriokefalitakis, S.; Olgun, N.; Rathod, S.D.; Schepanski, K.; Tagliabue, A.; Wagner, R.; Mahowald, N.M. Earth, Wind, Fire, and Pollution: Aerosol Nutrient Sources and Impacts on Ocean Biogeochemistry Annual Review of Marine Science. 2022, 14, 303–330. [CrossRef]
- Jickells, T.D.; Buitenhuis, E.; Altieri, K.; Baker, A.R.; Capone, D.; Duce, R.A.; Dentener, F.; Fennel, K.; Kanakidou, M.; LaRoche, J.; Lee, K.; Liss, P.; Middelburg, J.J.; Moore, J.K.; Okin, G.; Oschlies, A.; Sarin, M.; Seitzinger, S.; Sharples, J.; Singh, A.; Suntharalingam, P.; Uematsu, M.; Zamora, L.M. A reevaluation of the magnitude and impacts of anthropogenic atmospheric nitrogen inputs on the ocean Global Biogeochemical Cycles. 2017. [CrossRef]
- Shen, Y.; Benner, R. Molecular properties are a primary control on the microbial utilization of dissolved organic matter in the ocean Limnology and Oceanography. 2019, 65, 1061–1071. [CrossRef]
- Bienfang, P.; Laws, E.; Johnson, W. Phytoplankton sinking rate determination: technical and theoretical aspects, an improved methodology. J exp mar Biol Ecol. 1977, 30, 283–300. [CrossRef]
- Bienfang, P. Phytoplankton sinking rates in oligotrophic waters off Hawaii Marine Biology. 1980, 61, 66–77.
- Wakeham, S.G.; Lee, C.; Farrington, J.W.; Gagosian, R.B. Biogeochemistry of particulate organic matter in the oceans: results from sediment trap experiments Deep-Sea Research. 1984, 11, 509–528.
- Rothstein, J.P. Slip on Superhydrophobic Surfaces Ann Rev Fluid Mech. 2010, 42, 89–209. [CrossRef]
- Conlisk, A.T. Essentials of Micro- and Nano-Fluidics. Cambridge University Press, Cambridge, UK, 2013.
- Jenkinson, I.R.; Sun, J. Drag increase and drag reduction found in phytoplankton and bacterial cultures in laminar flow: Are cell surfaces and EPS\ producing rheological thickening and a Lotus-leaf Effect? Deep Sea Research Part II: Topical Studies in Oceanography. 2014, 101, 216–230. [CrossRef]
- Lim, A.S.; Jeong, H.J.; Jang, T.Y.; Jang, S.H.; Franks, P.J. Inhibition of growth rate and swimming speed of the harmful dinoflagellate Cochlodinium polykrikoides by diatoms: Implications for red tide formation Harmful Algae. 2014, 37, 53–61. [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses International Journal of Biological Macromolecules. 1995, 17, 293–297. [CrossRef]
- Gillard, J.; Frenkel, J.; Devos, V.; Sabbe, K.; Paul, C.; Rempt, M.; Inzé, D.; Pohnert, G.; Vuylsteke, M.; Vyverman, W. Metabolomics Enables the Structure Elucidation of a Diatom Sex Pheromone Angewandte Chemie International Edition. 2012, 52, 854–857. [CrossRef]
- Frenkel, J.; Vyverman, W.; Pohnert, G. Pheromone signaling during sexual reproduction in algae The Plant Journal. 2014, 79, 632–644. [CrossRef]
- H. Tschochner, F.Lottspeichl, M.Sumper The sexual inducer of Volvox carteri: purification, chemical characterization and identification of its gene EMBO Journal. 1987, 6, 2203–2207.
- Mages, H.-W.; Tschochner, H.; Sumper, M. The sexual inducer of Volvox carteri: Primary structure deduced from cDNA sequence FEBS letters. 1988, 234, 407–410.
- Schwartz, E.R.; Poulin, R.X.; Mojib, N.; Kubanek, J. Chemical ecology of marine plankton Natural Product Reports. 2016, 33, 843–860. [CrossRef]
- Jenkinson, I.R.; Sun, J. Rheological properties of natural waters with regard to plankton thin layers. A short review Journal of Marine Systems. 2010, 83, 287–297. [CrossRef]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehmann, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans Proceedings of the National Academy of Sciences. 2017, 114, 201619575. [CrossRef]
- Karlusich, J.J.P.; Pelletier, E.; Zinger, L.; Lombard, F.; Zingone, A.; Colin, S.; Gasol, J.M.; Dorrell, R.G.; Henry, N.; Scalco, E.; Acinas, S.G.; Wincker, P.; Vargas, C.; Bowler, C. A robust approach to estimate relative phytoplankton cell abundances from metagenomes Molecular Ecology Resources. 2022. [CrossRef]
- Santschi, P.H.; Chin, W.-C.; Quigg, A.; Xu, C.; Kamalanathan, M.; Lin, P.; Shiu, R.-F. Marine Gel Interactions with Hydrophilic and Hydrophobic Pollutants Gels. 2021, 7, 83. 7. [CrossRef]
- Poulson-Ellestad, K.; Mcmillan, E.; Montoya, J.P.; Kubanek, J. Are offshore phytoplankton susceptible to Karenia brevis allelopathy? Journal of Plankton Research. 2014, 36, 1344–1356. [CrossRef]
- Poulson-Ellestad, K.L.; Jones, C.M.; Roy, J.; Viant, M.R.; Fernández, F.M.; Kubanek, J.; Nunn, B.L. Metabolomics and proteomics reveal impacts of chemically mediated competition on marine plankton Proceedings of the National Academy of Sciences. 2014, 111, 9009–9014. [CrossRef]
- Hakanen, P.; Suikkanen, S.; Kremp, A. Allelopathic activity of the toxic dinoflagellate Alexandrium ostenfeldii: Intra-population variability and response of co-occurring dinoflagellates Harmful Algae. 2014, 39, 287–294.
- Seuront, L.; Stanley, H.E. Anomalous diffusion and multifractality enhance mating encounters in the ocean Proceedings of the National Academy of Sciences. 2014, 111, 2206–2211. [CrossRef]
- Yildiz, F.; Fong, J.; Sadovskaya, I.; Grard, T.; Vinogradov, E. Structural Characterization of the Extracellular Polysaccharide from Vibrio cholerae O1 El-Tor {PLoS} {ONE}. 2014, 9, e86751. [CrossRef]
- Sun, S.; Kjelleberg, S.; McDougald, D. Relative Contributions of Vibrio Polysaccharide and Quorum Sensing to the Resistance of Vibrio cholerae to Predation by Heterotrophic Protists {PLoS} {ONE}. 2013, 8, e56338. [CrossRef]
- Dadon-Pilosof, A.; Conley, K.R.; Jacobi, Y.; Haber, M.; Lombard, F.; Sutherland, K.R.; Steindler, L.; Tikochinski, Y.; Richter, M.; Glöckner, F.O.; Suzuki, M.T.; West, N.J.; Genin, A.; Yahel, G. Surface properties of SAR11 bacteria facilitate grazing avoidance Nature Microbiology. 2017, 2, 1608–1615. [CrossRef]
- Rosa, M.; Ward, J.E.; Holohan, B.A.; Shumway, S.E.; Wikfors, G.H. Physicochemical surface properties of microalgae and their combined effects on particle selection by suspension-feeding bivalve molluscs JEMBE. 2017, 486, 48–59. [CrossRef]
- Garcés, E.; Alacid, E.; Reñé, A.; Petrou, K.; Simó, R. Host-released dimethylsulphide activates the dinoflagellate parasitoid Parvilucifera sinerae The {ISME} Journal. 2013, 7, 1065–1068. [CrossRef]
- Smetacek, V. A watery arms race Nature. 2001, 411, 745.
- Savoca, M.S.; Nevitt, G.A. Evidence that dimethyl sulfide facilitates a tritrophic mutualism between marine primary producers and top predators Proceedings of the National Academy of Sciences. 2014, 111, 4157–4161. [CrossRef]
- Amo, L.; Rodrguez-Gironés, M.; Barbosa, A. Olfactory detection of dimethyl sulphide in a krill-eating Antarctic penguin Marine Ecology Progress Series. 2013, 474, 277–285. [CrossRef]
- Kerfahi, D.; Harvey, B.P.; Kim, H.; Yang, Y.; Adams, J.M.; Hall-Spencer, J.M. Whole community and functional gene changes of biofilms on marine plastic debris in response to ocean acidification Microbial Ecology. 2022. [CrossRef]
- Imai, I.; Inaba, N.; Yamamoto, K. Harmful algal blooms and environmentally friendly control strategies in Japan Fisheries Science. 2021, 87, 437–464. [CrossRef]
- Karn, S.K.; Bhambri, A.; Jenkinson, I.R.; Duan, J.; Kumar, A. The roles of biomolecules in corrosion inductionand inhibition of corrosion: a possible insight Corrosion Reviews. 2020, 38, 403–421. [CrossRef]
- Qin, B.; Deng, J.; Shi, K.; Wang, J.; Brookes, J.; Zhou, J.; Zhang, Y.; Zhu, G.; Paerl, H.W.; Wu, L. Extreme Climate Anomalies Enhancing Cyanobacterial Blooms in Eutrophic Lake Taihu, China Water Resources Research. 2021, 57. [CrossRef]
- McManus, M.; Greer, A.; Timmerman, A.; Sevadjian, J.; Woodson, C.; Cowen, R.; Fong, D.; Monismith, S.; Cheriton, O. Characterization of the biological, physical, and chemical properties of a toxic thin layer in a temperate marine system Marine Ecology Progress Series. 2021, 678, 17–35. [CrossRef]
- Santschi, P.H.; Xu, C.; Schwehr, K.A.; Lin, P.; Sun, L.; Chin, W.-C.; Kamalanathan, M.; Bacosa, H.P.; Quigg, A. Can the protein/carbohydrate (P/C) ratio of exopolymeric substances (EPS) be used as a proxy for their `stickiness' and aggregation propensity? Marine Chemistry. 2020, 218, 103734. [CrossRef]
- Duan, Z.; Tan, X.; Zeng, Q. Key physiological traits and chemical properties of extracellular polymeric substances determining colony formation in a cyanobacterium Journal of Oceanology and Limnology. 2022, 40, 1720–1731. [CrossRef]
- Guadayol, Ò.; Mendonca, T.; Segura-Noguera, M.; Wright, A.J.; Tassieri, M.; Humphries, S. Microrheology reveals microscale viscosity gradients in planktonic systems Proceedings of the National Academy of Sciences. 2020, 118. [CrossRef]
- Aron, A.T.; Gentry, E.C.; McPhail, K.L.; Nothias, L.-F.; Nothias-Esposito, M.; Bouslimani, A.; Petras, D.; Gauglitz, J.M.; Sikora, N.; Vargas, F.; van der Hooft, J.J.J.; Ernst, M.; Kang, K.B.; Aceves, C.M.; Caraballo-Rodrguez, A.M.; Koester, I.; Weldon, K.C.; Bertrand, S.; Roullier, C.; Sun, K.; Tehan, R.M.; Cristopher, A.; Boya, P.; Christian, M.H.; Gutiérrez, M.; Ulloa, A.M.; Mora, J.A.T.; Mojica-Flores, R.; Lakey-Beitia, J.; Vásquez-Chaves, V.; Zhang, Y.; Calderón, A.I.; Tayler, N.; Keyzers, R.A.; Tugizimana, F.; Ndlovu, N.; Aksenov, A.A.; Jarmusch, A.K.; Schmid, R.; Truman, A.W.; Bandeira, N.; Wang, M.; Dorrestein, P.C. Reproducible molecular networking of untargeted mass spectrometry data using GNPS Nature Protocols. 2020, 15, 1954–1991. [CrossRef]
- Lombard, F.; Koski, M.; Kiørboe, T. Copepods use chemical trails to find sinking marine snow aggregates Limnology and Oceanography. 2012, 58, 185–192. [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: a review Ecotoxicology. 2009, 19, 493–511. [CrossRef]
- Tammilehto, A.; Nielsen, T.G.; Krock, B.; Møller, E.F.; Lundholm, N. Induction of domoic acid production in the toxic diatom Pseudo-nitzschia seriata by calanoid copepods Aquatic Toxicology. 2015, 159, 52–61. [CrossRef]
- Harðardóttir, S.; Pančić, M.; Tammilehto, A.; Krock, B.; Møller, E.; Nielsen, T.; Lundholm, N. Dangerous Relations in the Arctic Marine Food Web: Interactions between Toxin Producing Pseudo-nitzschia Diatoms and Calanus Copepodites Marine Drugs. 2015, 13, 3809–3835. [CrossRef]
- Prince, E.; Irmer, F.; Pohnert, G. Domoic Acid Improves the Competitive Ability of Pseudo-nitzschia delicatissima against the Diatom Skeletonema marinoi Marine Drugs. 2013, 11, 2398–2412. [CrossRef]
- Hicks, G.R.F.; Grahame, J. Mucus production and its role in the feeding behaviour of Diarthrodes nobilis (Copepoda: Harpacticoida) Journal of the Marine Biological Association o fthe United Kingdom. 1979, 59, 321–330.
- Riemann, F.; Schrage, M. The mucus-trap hypothesis on feeding of aquatic nematodes and implications for biodegradation and sediment texture Oecologia. 1978, 34, 75–88. [CrossRef]
- Nielsen, L.T.; Krock, B.; Hansen, P.J. Production and excretion of okadaic acid, pectenotoxin-2 and a novel dinophysistoxin from the DSP-causing marine dinoflagellate Dinophysis acuta – Effects of light, food availability and growth phase Harmful Algae. 2013, 23, 34–45. [CrossRef]
- Ojamäe, K.; Hansen, P.J.; Lips, I. Mass entrapment and lysis of Mesodinium rubrum cells in mucus threads observed in cultures with Dinophysis Harmful Algae. 2016, 55, 77–84.
- Jiang, H.; Kulis, D.M.; Brosnahan, M.L.; Anderson, D.M. Behavioral and mechanistic characteristics of the predator-prey interaction between the dinoflagellate Dinophysis acuminata and the ciliate Mesodinium rubrum Harmful Algae. 2018, 77, 43–54. [CrossRef]
- Honsell, G.; Bonifacio, A.; Bortoli, M.D.; Penna, A.; Battocchi, C.; Ciminiello, P.; Dell'Aversano, C.; Fattorusso, E.; Sosa, S.; Yasumoto, T.; Tubaro, A. New Insights on Cytological and Metabolic Features of Ostreopsis cf. ovata Fukuyo (Dinophyceae): A Multidisciplinary Approach {PLoS} {ONE}. 2013, 8, e57291. [CrossRef]
- Blossom, H.E.; Daugbjerg, N.; Hansen, P.J. Toxic mucus traps: A novel mechanism that mediates prey uptake in the mixotrophic dinoflagellate Alexandrium pseudogonyaulax Harmful Algae. 2012, 17, 40–53. [CrossRef]
- Blossom, H.E.; Hansen, P.J. The loss of mixotrophy in Alexandrium pseudogonyaulax: Implications for trade-offs between toxicity, mucus trap production, and phagotrophy Limnology and Oceanography. 2020, 66, 528–542. [CrossRef]
- Stehnach, M.R.; Waisbord, N.; Walkama, D.M.; Guasto, J.S. Viscophobic turning dictates microalgae transport in viscosity gradients Nature Physics. 2021, 17, 926–930. [CrossRef]
- Falciatore, A.; Ribera d'Alcalà, M.; Croot, P.; Bowler, C. Perception of environmental signals by a marine diatom Science. 2000, 288, 2363–2366.
- Wild, C.; Huettel, M.; Klueter, A.; Kremb, S.G.; Rasheed, M.Y.; JÃÂârgense, B.B. Coral mucus functions as an energy carrier and particle trap in the reef ecosystem Nature. 2004, 428, 66–70.
- de Goeij, J.M.; Oevelen, D.; A.Vermeij, M.J.; Osinga, R.; J.Middelburg, J.; de Goeij, A.F.P.M.; Admiraal, W. Surviving in a Marine Desert: The Sponge Loop Retains Resources Within Coral Reefs Science. 2013, 342, 108–110. [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine Biochimica et Biophysica Acta ({BBA}) - General Subjects. 2013, 1830, 4117–4129. [CrossRef]
- Calzetta, L.; Matera, M.G.; Rogliani, P.; Cazzola, M. Multifaceted activity of N-acetyl-l-cysteine in chronic obstructive pulmonary disease Expert Review of Respiratory Medicine. 2018, 12, 693–708. [CrossRef]
- Yang, C.Z.; Albright, L.J. Anti-phytoplankton therapy of finfish: the mucolytic agent L-cysteine ethyl ester... Dis.Aquat.Org.. 1994, 20, 197–202. [CrossRef]
- Powell, M.D.; Ransome, J.; Barney, M.; Duijf, R.M.M.; Flik, G. Effect of Dietary Inclusion of N-Acetyl Cysteine on Mucus Viscosity and Susceptibility of Rainbow Trout, Oncorhynchus mykiss, and Atlantic Salmon, Salmo salar, to Amoebic Gill Disease Journal of the World Aquaculture Society. 2007, 38, 435–442.
- Gutiérrez-Praena, D.; Risalde, M.; Pichardo, S.; Jos, A.; Moyano, R.; Blanco, A.; Vasconcelos, V.; Cameán, A. Histopathological and immunohistochemical analysis of Tilapia (Oreochromis niloticus) exposed to cylindrospermopsin and the effectiveness of N-Acetylcysteine to prevent its toxic effects Toxicon. 2014, 78, 18–34. [CrossRef]
- Jenkinson, I.R.; Connors, P.P. The occurrence of the red-tide organism, Gyrodinium aureolum Hulburt (Dinophyceae) around the south and west of Ireland in August and September, 1979. Journal of Sherkin Island. 1980, 1, 127–146.
- Jenkinson, I.R. Oceanographic implications of non-newtonian properties found in phytoplankton cultures. Nature. 1986, 323, 435–437. [CrossRef]
- Jenkinson, I.R. , Arzul, G. Mitigation by cysteine compounds of rheotoxicity, cytotoxicity and fish mortality caused by the dinoflagellates, Gymnodinium mikimotoi and G.cf. maguelonnense. In: Hallegraeff, G.; Blackburn, S.; Bolch, C., Lewis, R. (Eds), Harmful Algal Blooms 2000., IOC of UNESCO, 2002, pp. 461–464.
- Jenkinson, I.R. Increases in viscosity may kill fish in some blooms.. In: Okaichi, T.; Anderson, D. M., Nemoto, T. (Eds), Red Tides., Elsevier, 1989, pp. 435–438.
- Jenkinson, I.R. , Arzul, G. Effect of the flagellates, Gymnodinium mikimotoi, Heterosigma akashiwo, and Pavlova lutheri, on flow through fish gills.. In: Reguera, B.; Blanco, J.; Fernandez, M., Wyatt, T. (Eds), Harmful Algae, Xunta de Galicia, Pontevedra & IOC of Unesco, Paris, 1998, pp. 425–428.
- Gentien, P.; Lunven, M.; Lazure, P.; Youenou, A.; Crassous, M.P. Motility and autotoxicity in Karenia mikimotoi (Dinophyceae) Philosophical Transactions of the Royal Society. 2007, 362, 1937–1946. [CrossRef]
- Orlova, T.Y.; Aleksanin, A.I.; Lepskaya, E.V.; Efimova, K.V.; Selina, M.S.; Morozova, T.V.; Stonik, I.V.; Kachur, V.A.; Karpenko, A.A.; Vinnikov, K.A.; Adrianov, A.V.; Iwataki, M. A massive bloom of Karenia species (Dinophyceae) off the Kamchatka coast, Russia, in the fall of 2020 Harmful Algae. 2022, 120, 102337. [CrossRef]
- Kim, D. , Oda, T. Possible Factors Responsible for the Fish-Killing Mechanisms of the Red Tide Phytoplankton, Chattonella marina and Cochlodinium polykrikoides. In: Ishimatsu, A., Lie, H.-J. (Eds), Coastal Environmental and Ecosystem Issues of the East China Sea,, Terrapub and Nagasaki University, 2010, pp. 245–268.
- Berdalet, E.; Tester, P.; Chinain, M.; Fraga, S.; Lemée, R.; Litaker, W.; Penna, A.; Usup, G.; Vila, M.; Zingone, A. Harmful Algal Blooms in Benthic Systems: Recent Progress and Future Research Oceanography. 2017, 30, 36–45. [CrossRef]
- Seuront, L.; Vincent, D. Increased seawater viscosity, Phaeocystis globosa spring bloom and Temora longicornis feeding and swimming behaviours Marine Ecology Progress Series. 2008, 363, 131–145. [CrossRef]
- Kang, Z.; Yang, B.; Lai, J.; Ning, Y.; Zhong, Q.; Lu, D.; Liao, R.; Wang, P.; Dan, S.F.; She, Z.; Jia, Z.; Lao, Y.; Li, N. Phaeocystis globosa Bloom Monitoring: Based on P. globosa Induced Seawater Viscosity Modification Adjacent to a Nuclear Power Plant in Qinzhou Bay, China Journal of Ocean University of China. 2020, 19, 1207–1220. [CrossRef]
- Balkis-Özdelice, N.; Durmuş, T.; Balci, M. A Preliminary Study on the Intense Pelagic and Benthic Mucilage Phenomenon Observed in the Sea of Marmara International Journal of Environment and Geoinformatics. 2021, 8, 414–422. [CrossRef]
- Seuront, L.; Leterme, S.C.; Seymour, J.R.; Mitchell, J.G.; Ashcroft, D.; Noble, W.; Thomson, P.G.; Davidson, A.T.; van den Enden, R.; Scott, F.J.; Wright, S.W.; Schapira, M.; Chapperon, C.; Cribb, N. Role of microbial and phytoplanktonic communities in the control of seawater viscosity off East Antarctica (30-80°E) Deep Sea Research Part {II}: Topical Studies in Oceanography. 2010, 57, 877–886. [CrossRef]
- Guzmán, H.M.; Cortés, J.; Glynn, P.W.; Richmond, R.H. Coral mortality associated with dinoflagellate blooms in the eastern Pacific Marine Ecology Progress Series. 1990, 60, 299–303.
- Kim, D.; Oda, T.; Muramatsu, T.; Kim, D.; Matsuyama, Y.; Honjo, T. Possible factors responsible for the toxicity of Cochlodinium polykrikoides, a red tide phytoplankton. Comparative Biochemistry and Physiology C Toxicology and Pharmacology. 2002, 132, 415–423. [CrossRef] [PubMed]
- Al Gheilani, H.; Al Azri, A.; Piontkovoski, S.; Debrotsov, S.; Al Amri, I.; Al Ambo Ali, I.; Al Jufaili, S.; Al Bousaidi, S.; Al Hajri, S.; Al Aisari, A.; Al Shaqsi, H.; Al Abri, N. , Al Hashmi, K. Blooms of Cochlodinium polykrikoides along the coast of Oman and their effect.. In: Pagou, P., Hallegraeff, G. (Eds), Proceedings of the 14 th International Conference on Harmful Algae, International Society for the Study of Harmful Algae and Intergovernmental Oceanographic Commission of UNESCO, 2013, pp. 129–131.
- Lee, J.-S. Bioactive components from red tide plankton, Cochlodinium polykrikoides Journal of the Korean Fisheries Society. 1996, 29, 165–173.
- Kim, C.; Lee, C.; Kim, H.; Jung, J. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides Journal of Plankton Research. 1999, 21, 2105–2115.
- Flores-Leñero, A.; Vargas-Torres, V.; Paredes-Mella, J.; Norambuena, L.; Fuenzalida, G.; Lee-Chang, K.; Mardones, J.I. Heterosigma akashiwo in Patagonian Fjords: Genetics, Growth, Pigment Signature and Role of PUFA and ROS in Ichthyotoxicity Toxins. 2022, 14, 577. [CrossRef]
- Yamasaki, Y.; Nagasoe, S.; Tameishi, M.; Shikata, T.; Zou, Y.; Jiang, Z.; Matsubara, T.; Shimasaki, Y.; Yamaguchi, K.; Oshima, Y.; Oda, T.; Honjo, T. The role of interactions between Prorocentrum minimum and Heterosigma akashiwo in bloom formation Hydrobiologia. 2010, 641, 33–44. [CrossRef]
- MacKenzie, L.; Sims, I.; Beuzenberg, V.; Gillespie, P. Mass accumulation of mucilage caused by dinoflagellate polysaccharide exudates in Tasman Bay, New Zealand Harmful Algae. 2002, 1, 69–83. [CrossRef]
- Honsell, G.; Cabrini, M.; Darin, M. Gonyaulax fragilis (Schütt) Kofoid: a dinoflagellate from gelatinous aggregates of the Northern Adriatic Sea Giornale botanico italiano. 1992, 126, 749–751. [CrossRef]
- Pompei, M.; Mazziotti, C.; Guerrini, F.; Cangini, M.; Pigozzi, S.; Benzi, M.; Palamidesi, S.; Boni, L.; Pistocchi, R. Correlation between the presence of Gonyaulax fragilis (Dinophyceae) and the mucilage phenomena of the Emilia-Romagna coast (northern Adriatic Sea) Harmful Algae. 2003, 2, 301–316. [CrossRef]
- Carbonell-Moore, M.C.; Mertens, K.N. Should Gonyaulax hyalina and Gonyaulax fragilis (Dinophyceae) remain two different taxa? Phycologia. 2019, 58, 685–689. [CrossRef]
- Riccardi, M.; Guerrini, F.; Serrazanetti, G.P.; Ventrella, V.; Pagliarani, A.; Pistocchi, R. Lipid and DNA features of Gonyaulax fragilis (Dinophyceae) as potential biomarkers in mucilage genesis Harmful Algae. 2010, 9, 359–366. [CrossRef]
- Zhang, W.; Zhang, X. Single molecule mechanochemistry of macromolecules Progress in Polymer Science. 2003, 28, 1271–1295. [CrossRef]
- Wiita, A.P.; Ainavarapu, S.R.K.; Huang, H.H.; Fernandez, J.M. Force-dependent chemical kinetics of disulfide bond reduction observed with single-molecule techniques Proceedings of the National Academy of Sciences. 2006, 103, 7222–7227. [CrossRef]
- Alting, A.C. ; Number of thiol groups rather than the size of the aggregates determines the hardness of cold set whey protein gels. Doctoral thesis, Wageningen University, NL, 2003.
- Quigg, A.; Santschi, P.H.; Burd, A.; Chin, W.-C.; Kamalanathan, M.; Xu, C.; Ziervogel, K. From Nano-Gels to Marine Snow: A Synthesis of Gel Formation Processes and Modeling Efforts Involved with Particle Flux in the Ocean Gels. 2021, 7, 114. [CrossRef]
- Hu, X.-J.; Xu, Y.; Su, H.-C.; Xu, W.-J.; Wang, L.-H.; Xu, Y.-N.; Li, Z.-J.; Cao, Y.-C.; Wen, G.-L. Algicidal bacterium CZBC1 inhibits the growth of Oscillatoria chlorina, Oscillatoria tenuis, and Oscillatoria planctonica {AMB} Express. 2019, 9. [CrossRef]
- Lee, S.-o., Kato, J.; Takiguchi, N.; Kuroda, A.; Ikeda, T.; Mitsutani, A.; Ohtake, H. Involvement of an Extracellular Protease in Algicidal Activity of the Marine Bacterium Pseudoalteromonas sp. Strain A28 Applied and Environmental Microbiology. 2000, 66, 4334–4339. [CrossRef] [PubMed]
- Su, Y.; Yang, Y.; Zhu, X.-Y.; Zhang, X.-H.; Yu, M. Metagenomic Insights Into the Microbial Assemblage Capable of Quorum Sensing and Quorum Quenching in Particulate Organic Matter in the Yellow Sea Frontiers in Microbiology. 2021, 11. [CrossRef]
- Svedrup, H.U.; Johnson, M.W. , Fleming, R.H.; The Oceans: Their Physics, Chemistry, and General Biology. First Ed.. Prentice Hall, NY, 1942.
- Endo, M. Calcium Ion as a Second Messenger With Special Reference to Excitation-Contraction Coupling Journal of Pharmacological Sciences. 2006, 100, 519–524. [CrossRef]
- Ianora, A.; Boersma, M.; Casotti, R.; Fontana, A.; Harder, J.; Hoffman, F.; Pavias, H.; Potin, P.; Poulet, S.; Toth, G. New Trends in Marine Chemical Ecology Estuaries and Coasts. 2006, 29, 531–551.
- Jenkinson, I.R.; Wyatt, T. Selection and control of Deborah numbers inplankton ecology. Journal of Plankton Research. 1992, 14, 1697–1721. [CrossRef]
- Seuront, L.; Lacheze, C.; Doubell, M.J.; Seymour, J.R.; Van Dongen-Vogels, V.; Newton, K.; Alderkamp, A.C.; Mitchell, J.G. The influence of Phaeocystis globosa on microscale spatial patterns of chlorophyll a and bulk-phase seawater viscosity Biogeochemistry. 2007, 83, 173–188. [CrossRef]
- Hastings, A.; Bryers, J.; Crooks, J.; Cuddington, K.; Jones, C.G.; Lambrinos, J.; Talley, T.; Wilson, W. Ecosystem engineering in space and time Ecology Letters. 2007, 10, 153–164.
- Reddington, K.; Eccles, D.; O'Grady, J.; Drown, D.M.; Hansen, L.H.; Nielsen, T.K.; Ducluzeau, A.-L.; Leggett, R.M.; Heavens, D.; Peel, N.; Snutch, T.P.; Bayega, A.; Oikonomopoulos, S.; Ragoussis, J.; Barry, T.; van der Helm, E.; Jolic, D.; Richardson, H.; Jansen, H.; Tyson, J.R.; Jain, M.; Brown, B.L. Metagenomic analysis of planktonic riverine microbial consortia using nanopore sequencing reveals insight into river microbe taxonomy and function GigaScience. 2020, 9. [CrossRef]
- Reddington, K.; Eccles, D.; O'Grady, J.; Drown, D.M.; Hansen, L.H.; Nielsen, T.K.; Ducluzeau, A.-L.; Leggett, R.M.; Heavens, D.; Peel, N.; Snutch, T.P.; Bayega, A.; Oikonomopoulos, S.; Ragoussis, J.; Barry, T.; van der Helm, E.; Jolic, D.; Richardson, H.; Jansen, H.; Tyson, J.R.; Jain, M.; Brown, B.L. Corrigendum to: Metagenomic analysis of planktonic riverine microbial consortia using nanopore sequencing reveals insight into river microbe taxonomy and function {GigaScience}. 2020, 9. [CrossRef]
- Jenkinson, I.R. , Wyatt, T. Management by phytoplankton of physical oceanographic parameters.. In: Lassus, P.; Arzul, G.; Erard, E.; Gentien, P., Marcaillou, C. (Eds), Harmful Marine Algal Blooms, Lavoisier Intercept, Paris, 1995, pp. 603–607.
- Camacho-Chab, J.; Lango-Reynoso, F.; Castañeda-Chávez, M.; Galaviz-Villa, I.; Hinojosa-Garro, D.; Ortega-Morales, B. Implications of Extracellular Polymeric Substance Matrices of Microbial Habitats Associated with Coastal Aquaculture Systems Water. 2016, 8, 369. [CrossRef]
- Sretenovic, S.; Stojković, B.; Dogsa, I.; Kostanjšek, R.; Poberaj, I.; Stopar, D. An early mechanical coupling of planktonic bacteria in dilute suspensions Nature Communications. 2017, 8. [CrossRef]
- Jatt, A.N.; Tang, K.; Liu, J.; Zhang, Z.; Zhang, X.-H. Quorum sensing in marine snow and its possible influence on production of extracellular hydrolytic enzymes in marine snow bacterium Pantoea ananatis B9 FEMS Microbiology Ecology. 2014, 91, 1–13. [CrossRef]
- Decho, A.W.; Gutierrez, T. Microbial Extracellular Polymeric Substances (EPSs) in Ocean Systems Frontiers in Microbiology. 2017, 8. [CrossRef]
- Sun, S.; Tay, Q.X.M.; Kjelleberg, S.; Rice, S.A.; McDougald, D. Quorum sensing-regulated chitin metabolism provides grazing resistance to Vibrio cholerae biofilms The {ISME} Journal. 2015, 9, 1812–1820. [CrossRef]
- Tan, D.; Svenningsen, S.L.; Middelboe, M. Quorum Sensing Determines the Choice of Antiphage Defense Strategy in Vibrio anguillarum {mBio}. 2015, 6. [CrossRef]
- Pappas, K.M.; Weingart, C.L.; Winans, S.C. Chemical communication in proteobacteria: biochemical and structural studies of signal synthases and receptors required for intercellular signalling Molecular Microbiology. 2004, 53, 755–769. [CrossRef]
- Gram, L.; Grossart, H.-P.; Schlingloff, A.; Kiørboe, T. Possible Quorum Sensing in Marine Snow Bacteria: Production of Acylated Homoserine Lactones by Roseobacter Strains Isolated from Marine Snow Applied and Environmental Microbiology. 2002, 68, 4111–4116. [CrossRef]
- Parsek, M.R.; Fuqua, C. Biofilms 2003, Emerging Themes and Challenges in Studies of Surface-Associated Microbial Life Journal of Bacteriology. 2004, 186, 4427–4440. [CrossRef]
- Tait, K.; Joint, I.; Daykin, M.; Milton, D.L.; Williams, P.; Camara, M. Disruption of quorum sensing in seawater abolishes attraction of zoospores of the green alga Ulva to bacterial biofilms Environmental Microbiology. 2005, 7, 229–240. [CrossRef]
- Wheeler, G.L.; Tait, K.; Taylor, A.; Brownlee, C.; Joint, I. Acyl-homoserine lactones modulate the settlement rate of zoospores of the marine alga Ulva intestinalis via a novel chemokinetic mechanism Plant, Cell and Environment. 2006, 29, 608–618. [CrossRef]
- Li, J.; Lu, J.; Lan, C.; Lai, J.; Wang, J.; Wei, F. Inhibitory Effect of Increase in Water Viscosity of Phaeocystis globosa on Feeding of Temora stylifera (in Chinese, English abstract) Guangxi Science. 2021, 28, 56–64.
- Flemming, H.-C.; Wingender, J. The biofilm matrix Nature Reviews Microbiology. 2010, 8, 623–633. [CrossRef]
- Hohne, D.N.; Younger, J.G.; Solomon, M.J. Flexible Microfluidic Device for Mechanical Property Characterization of Soft Viscoelastic Solids Such as Bacterial Biofilms Langmuir. 2009, 25, 7743–7751. [CrossRef]
- Sun, L.; Chin, W.-C.; Chiu, M.-H.; Xu, C.; Lin, P.; Schwehr, K.A.; Quigg, A.; Santschi, P.H. Sunlight induced aggregation of dissolved organic matter: Role of proteins in linking organic carbon and nitrogen cycling in seawater Science of The Total Environment. 2019, 654, 872–877. [CrossRef]
- Zhang, J.; Li, N.; Dai, X.; Tao, W.; Jenkinson, I.R.; Li, Z. Enhanced dewaterability of sludge during anaerobic digestion with thermal hydrolysis pretreatment: New insights through structure evolution Water Research. 2018, 131, 177–185. [CrossRef]
- Lear, G. (Eds), Biofilms in Bioremediation: Current Research and Emerging Technologies. Caister Academic Press, 2016.
- Verdugo, P. Marine Microgels Ann Rev mar Sci. 2012, 4, 375–400. [CrossRef]
- Verdugo, P. Marine Biopolymer Dynamics, Gel Formation, and Carbon Cycling in the Ocean Gels. 2021, 7, 136. [CrossRef]
- Drost-Hansen, W. Vicinal hydration of biopolymers: Cell biological consequences. In: Pollack_et_al (Eds), Water and the Cell, Springer, Dordrecht, Netherlands, 2006, pp. 175–217.
- Mari, X. Does ocean acidification induce an upward flux of marine aggregates? Biogeosciences. 2008, 5, 1023–1031. [CrossRef]
- Bartual, A.; Vicente-Cera, I.; Flecha, S.; Prieto, L. Effect of dissolved polyunsaturated aldehydes on the size distribution of transparent exopolymeric particles in an experimental diatom bloom Marine Biology. 2017, 164. [CrossRef]
- Klun, K.; Šket, P.; Beran, A.; Falnoga, I.; Faganeli, J. Composition of Colloidal Organic Matter in Phytoplankton Exudates Water. 2022, 15, 111. [CrossRef]
- Vidal-Melgosa, S.; Sichert, A.; Francis, T.B.; Bartosik, D.; Niggemann, J.; Wichels, A.; Willats, W.G.T.; Fuchs, B.M.; Teeling, H.; Becher, D.; Schweder, T.; Amann, R.; Hehemann, J.-H. Diatom fucan polysaccharide precipitates carbon during algal blooms Nature Communications. 2021, 12. [CrossRef]
- Denis, M.; Lefevre, D.; Thysson, M.; Jenkinson, I.R.; Grégori, G. Pulsed export of carbon in the north-western MediterraneanSea Journal of Oceanology and Limnology. 2022. [CrossRef]
- Liu, L.; Qin, B.; Zhu, G.; Zhang, Y.; Gao, G.; Gong, Z.; Huang, Q. Distribution of dissolved acidic polysaccharides (dAPS) during cyanobacteria blooms in northern Lake Taihu Limnology. 2015, 16, 21–29. [CrossRef]
- Laabir, M.; Grignon-Dubois, M.; Masseret, E.; Rezzonico, B.; Soteras, G.; Rouquette, M.; Rieuvilleneuve, F.; Cecchi, P. Algicidal effects of Zostera marina L. and Zostera noltii Hornem. extracts on the neuro-toxic bloom-forming dinoflagellate Alexandrium catenella Aquatic Botany. 2013, 111, 16–25. [CrossRef]
- Onishi, Y.; Mohri, Y.; Tuji, A.; Ohgi, K.; Yamaguchi, A.; Imai, I. The seagrass Zostera marina harbors growth-inhibiting bacteria against the toxic dinoflagellate Alexandrium tamarense Fisheries Science. 2014, 80, 353–362. [CrossRef]
- Imai, I.; Kido, T.; Yoshinaga, I.; Ohgi, K. , Nagai, S. Isolation of Microcystis-killer bacterium Agrobacterium vitis from the biofilm onthe surface of the water plant Egeria densa. In: Pagou, K. A., Hallegraeff, G. M. (Eds), PROCEEDINGSOF THE 14TH INTERNATIONAL CONFERENCEON HARMFUL ALGAEHERSONISSOS-CRETE, GREECE,1-5 NOVEMBER 2010, ISSHA and IOC of UNESCO, 2013, pp. 150–152.
- Yang, Y.; Hu, X.; Zhang, J.; Gong, Y. Community level physiological study of algicidal bacteria in the phycospheres of Skeletonema costatum and Scrippsiella trochoidea Harmful Algae. 2013, 28, 88–96. [CrossRef]
- Qian, X.; Chen, L.; Sui, Y.; Chen, C.; Zhang, W.; Zhou, J.; Dong, W.; Jiang, M.; Xin, F.; Ochsenreither, K. Biotechnological potential and applications of microbial consortia Biotechnology Advances. 2020, 40, 107500. [CrossRef]
- Kotay, S.M.; Das, D. Microbial hydrogen production from sewage sludge bioaugmented with a constructed microbial consortium International Journal of Hydrogen Energy. 2010, 35, 10653–10659. [CrossRef]
- Thakur, P.; Alaba, M.O.; Rauniyar, S.; Singh, R.N.; Saxena, P.; Bomgni, A.; Gnimpieba, E.Z.; Lushbough, C.; Goh, K.M.; Sani, R.K. Text-Mining to Identify Gene Sets Involved in Biocorrosion by Sulfate-Reducing Bacteria: A Semi-Automated Workflow Microorganisms. 2023, 11, 119. [CrossRef]
- Singh, A. Glycoproteomics Nature Methods. 2021, 18, 28–28. [CrossRef]
- Alldredge, A.; Passow, U.; Logan, B. The abundance and significance of a class of large, transparent organic particles in the ocean. Deep-Sea Research. 1993, 40, 1131–1140. [CrossRef]
- Williams, P.; Carlucci, A.; Henrichs, S.; Van Vleet, E.; Horrigan, S.; Reid, F.; Robertson, K. Chemical and microbiological studies of sea-surface films in the Southern Gulf of California and off the West Coast of Baja California Marine Chemistry. 1986, 19, 17–98. [CrossRef]
- Carlson, D.J. Viscosity of sea-surface slicks Nature. 1987, 329, 823–825. [CrossRef]
- Zhang, Z.; Zhang, A.; Liu, L.; Liu, C.; Ren, C.; Xing, L. Viscosity of sea surface microlayer in Jiaozhou Bay and adjacent sea area Chinese Journal of Oceanology and Limnology. 2003, 21, 351–357. [CrossRef]
- Jenkinson, I.R.; Seuront, L.; Ding, H.; Elias, F. Biological modification of mechanical properties of the sea surface microlayer, influencing waves, ripples, foam and air-sea fluxes Elementa: Science of the Anthropocene. 2018, 6, 26. [CrossRef]
- Lovelock, J. ; The Ages of Gaia - A biography of our living Earth. Oxford University Press, Oxford, UK, 1988.
- Lenton, T.M. Gaia and natural selection Nature. 1998, 394, 439–447. [CrossRef]
- Thornton, D.C. Phytoplankton mucilage production in coastal waters: a dispersal mechanism in a front dominated system? Ethology Ecology \& Evolution. 1999, 11, 179–185. [CrossRef]
- Hamilton, W.; Lenton, T. Spora and Gaia: How microbes fly with their clouds Ethology, Ecology \& Evolution. 1998, 10, 1–16. [CrossRef]
- Teeling, H.; Fuchs, B.M.; Becher, D.; Klockow, C.; Gardebrecht, A.; Bennke, C.M.; Kassabgy, M.; Huang, S.; Mann, A.J.; Waldmann, J.; Weber, M.; Klindworth, A.; Otto, A.; Lange, J.; Bernhardt, J.; Reinsch, C.; Hecker, M.; Peplies, J.; Bockelmann, F.D.; Callies, U.; Gerdts, G.; Wichels, A.; Wiltshire, K.H.; Glöckner, F.O.; Schweder, T.; Amann, R. Substrate-Controlled Succession of Marine Bacterioplankton Populations Induced by a Phytoplankton Bloom Science. 2012, 336, 608–611. [CrossRef]
- Stabeno, P.J. , Monahan, E.C. The Influence of Whitecaps on the Albedo of the Sea Surface. In: Monahan, E. C., Mac Niocaill, G. (Eds), Oceanographic Sciences Library, Springer Netherlands, 1986, pp. 261–266.
- Evans, J.R.G.; Stride, E.P.J.; Edirisinghe, M.J.; Andrews, D.J.; Simon, R.R. Can oceanic foams limit global warming? Climate Research. 2010, 42, 155–160. [CrossRef]
- Cantat, I.; Cohen-Addad, S.; Elias, F.; Graner, F.; Höhler, R.; Pitois, O.; Rouyer, F. , Saint-Jalmes, A.Cox, S. (Eds), Foams. Oxford University Press, 2013.
- Callaghan, A.H.; Deane, G.B.; Stokes, M.D.; Ward, B. Observed variation in the decay time of oceanic whitecap foam Journal of Geophysical Research: Oceans. 2012, 117, C09015. [CrossRef]
- Pierce, R.H.; Henry, M.S.; Blum, P.C.; Lyons, J.; Cheng, Y.S.; Yazzie, D.; Zhou, Y. Brevetoxin Concentrations in Marine Aerosol: Human Exposure Levels During a Karenia brevis Harmful Algal Bloom Bulletin of Environmental Contamination and Toxicology. 2003, 70, 161–165. [CrossRef]
- Vargas-Montero, M.; Freer, E.; Jiménez-Montealegre, R.; Guzmán, J. Occurrence and predominance of the fish killer Cochlodinium polykrikoides on the Pacific coast of Costa Rica African Journal of Marine Sscience. 2006, 28, 215–217.
- Kesaulya, I.; Leterme, S.C.; Mitchell, J.G.; Seuront, L. The impact of turbulence and phytoplankton dynamics on foam formation, seawater viscosity and chlorophyll concentration in the eastern English Channel Oceanologia. 2008, 50, 167–182.
- Calvo, E.; Viquez, R.; Garcia, A. Alexandrium monilatum (Howell) Balech bloom in the Gulf od Nicoya, Puntarenas Harmful Algae News. 2005, 1-2.
- Lassus, P.; Chomérat, N.; Hess, P. , Nézan, E.; Toxic and Harmful Microalgae of the World Ocean. ISSHA and IOC of UNESCO, Copenhagen and Paris, 2016.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







