Introduction
Breast cancer (BC) is one of the most frequent cancer in women [
1]. Locally advanced breast cancer (LABC) occurs approximately in 30% of patients with breast malignancy at the time of diagnosis [
2].
Histological stratification represents the gold standard for the classification of BC, based primarily on the differentiation grade and the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) [
3]. Several studies demonstrated a large degree of inter- and intratumoral heterogeneity and distinct BC subtypes, each one corresponding to a differentiation state of mammary cells, representing a convergence point between normal mammary gland biology and breast cancer biology. One of the major hypothesis is that breast tumors consist of a mixture of inter-converting breast cancer subtypes that may affect the patient outcome [
4].
According to clinical guidelines [
5] neoadjuvant chemotherapy (NAC), followed by surgery and adjuvant systemic and local treatment, represents the current preferred treatment strategy. Indeed, it can be useful either in early-stage LABC patients to enable breast-conserving surgery (BCS), thus limiting diffusion of micrometastases, and to test potential drug resistance.
Positron emission tomography (PET) integrated with computed tomography (CT), is a multimodal examination providing volumetric distribution of positron-emitting radionuclides in the human body [
6]. [
18F]F--fluorodeoxyglucose (
18F-FDG) is the most used radiopharmaceutical in oncology and plays a role in the diagnostic workflow of BC, specifically as metabolic biomarker evaluating glycolytic activity in tumors [
7]. Evaluation of early response to therapy and chemosensitivity assessment are clinically relevant in NAC patients with impact on treatment management, preventing possible drug toxicity and influencing prognosis [
8]. Breast MRI is the gold standard for the assessment of the primitive tumor before NAC, and
18F-FDG PET/CT represents an accurate tool to support MRI in the assessment of response to therapy [
9,
10]. Achievement of pathologic complete response (pCR) after NAC is associated with better prognosis in BC patients, especially when more aggressive subtypes are present [
11]. Nevertheless, few articles evaluated the prognostic value of
18F-FDG PET/CT before NAC and assessed the impact of PET-derived semiquantitative parameters on survival outcomes related to differentiation grade and pathologic characteristics [
12,
13].
In this study, we aimed to investigate whether 18F-FDG PET-derived semiquantitative parameters could predict disease-free survival (DFS) in patients with grade III BC of different molecular types scheduled for NAC.
Methods
Patients
Patients were retrospectively retrieved from the databases of two Italian hospitals. Inclusion criteria were: 1) histologically proven diagnosis of grade III BC; 2) history of NAC; 3) execution of a baseline 18F-FDG PET/CT scan before the start of NAC; 4) a minimum follow-up time of 3 years from the date of the baseline PET/CT scan.
Patients were classified into 5 molecular subgroups (Luminal A, Luminal B, Luminal B + HER-2, HER-2 enriched, triple-negative) according to the St. Gallen consensus [
14], as already described in a previous paper of our group [
15].
A pathological complete response (pCR) was defined as complete absence of invasive residual tumour cells on microscopy both in the breast and in the axillary or distant lymph nodes. For assessing the response to NAC, the Sataloff criteria were considered [PMID: 7874340].
Informed consent was signed by every patient for inclusion in potential retrospective research studies, in accordance with the local requirements. This study was approved by the local institutional review boards with a waiver of authorization due to its retrospective design.
18. F-FDG-PET/CT scanning and analysis
All patients fasted 8 hours before PET/CT scan.
18F-FDG injection (3,7MBq/1Kg) was performed 60 ± 10 minutes before image acquisitions following a standard acquisition protocol according to the EANM guidelines [
7].
18F-FDG PET/CT scan was performed on both institutions on a dedicated PET/CT system (both scanners were by Siemens) that combines a “full ring” PET scanner and a spiral CT scanner. Data acquired with CT component of the scan were used for attenuation correction. PET/CT data was reconstructed using a dedicated commercial workstation along axial, coronal and sagittal.
A volume of interest (VOI) was manually defined by two nuclear medicine physicians on the primary tumor. Further VOIs were placed on each patient in all the suspicious lymph node and distant metastases. For each 18F-FDG-PET/CT scan, the following parameters were calculated in the primary tumor (SUVmax, SUVmean, MTV, TLG) and whole-body (WB_SUVmax, WB_MTV, and WB_TLG), obtained through the sum of the semiquantitative value of every lesion detected in the scan.
Statistical Analysis
The DFS was defined as the time from the 18F-FDG PET/CT scan to the first documented date of progression or death. Receiver operating characteristic (ROC) analysis was used for each parameter to assess the prediction capability for DFS. DFS differences between groups were assessed using Kaplan-Meier curves and compared by log-rank test. A p value of 0.05 was used as the minimum threshold for significance. Moreover, To investigate the association between PET parameters able to predict DFS and pCR, student’s t test was used, whereas non-parametric statistic was employed to assess any difference among each molecular subtype.
Statistical analysis was performed using MedCalc Statistical Software version 19.1.3 (MedCalc Software, Ostend, Belgium;
https://www.medcalc.org; 2020).
Results
Whole group analysis
Ninety-five grade III BC patients with different molecular types were retrieved (luminal A: 5; luminal B: 34; luminal B- HER2: 22; HER2-enriched: 7; triple-negative: 27). Patient characteristics are summarised in
Table 1.
After NA, 22 patients achieved pCR whereas 61 did not; no information on pCR was available in 12 patients. pCR occurred in 4/27 (15%) patients with luminal B, 6/13 (46%) with luminal B-HER2, 3/6 (50%) with HER2-enriched, and 9/15 (60%) with triple negative BC.
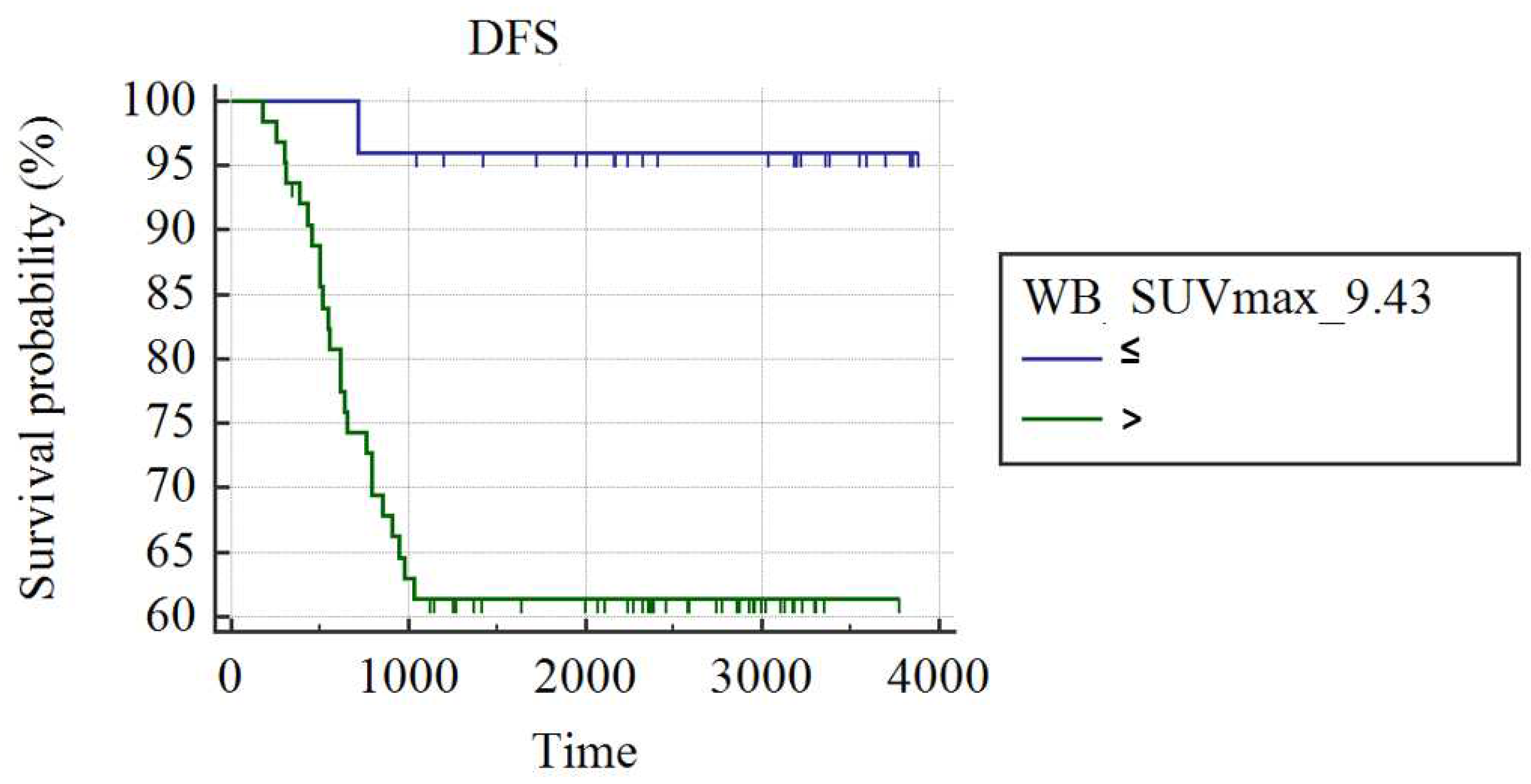
In the whole group of 95 patients, WB_SUVmax was the best predictor of recurrence (AUC: 0.66; best cut-off: WB_SUVmax>9.43; SS: 96%; SP: 38.1%; p<0.008). Patients with a WB_SUVmax>9.43 presented a higher rate of recurrence at the end of follow-up and shorter DFS compared to patients with WB_SUVmax≤9.43 (38.10% vs. 4%; mean DFS: 2552±196 days
vs. 3756±124, respectively; p=0.001;
Figure 1). Among 61 patients not achieving pCR, women with WB_SUVmax≤9.43 (n=20) demonstrated a significantly longer DFS compared to patients with WB_SUVmax>9.43 (2546±227 days vs. 1769±180 days: p=0.011).
Subgroup analysis
Patients with luminal A and HER-2-enriched BC subtypes were excluded from the molecular subgroup analysis due to the small samples (5 and 7, respectively).
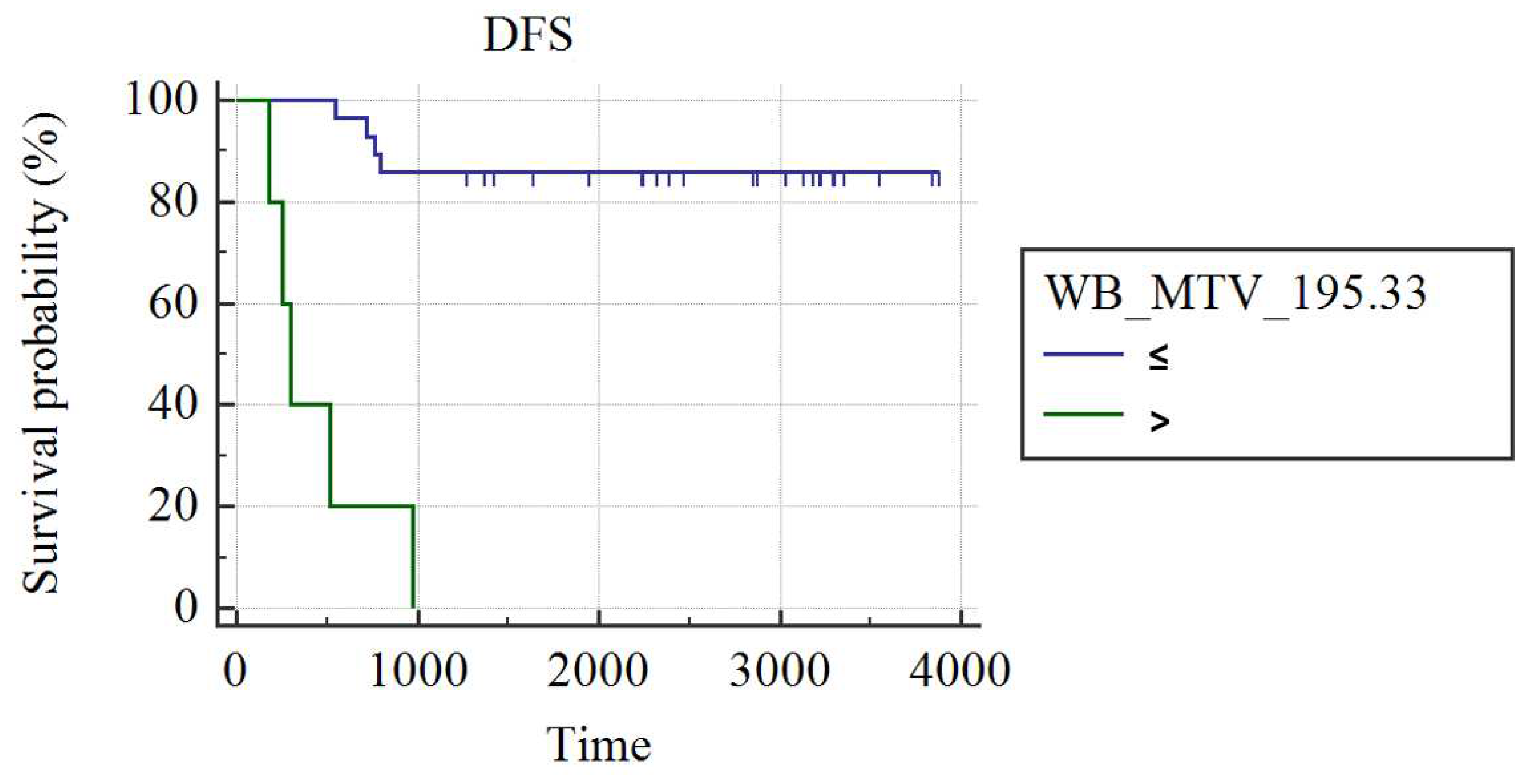
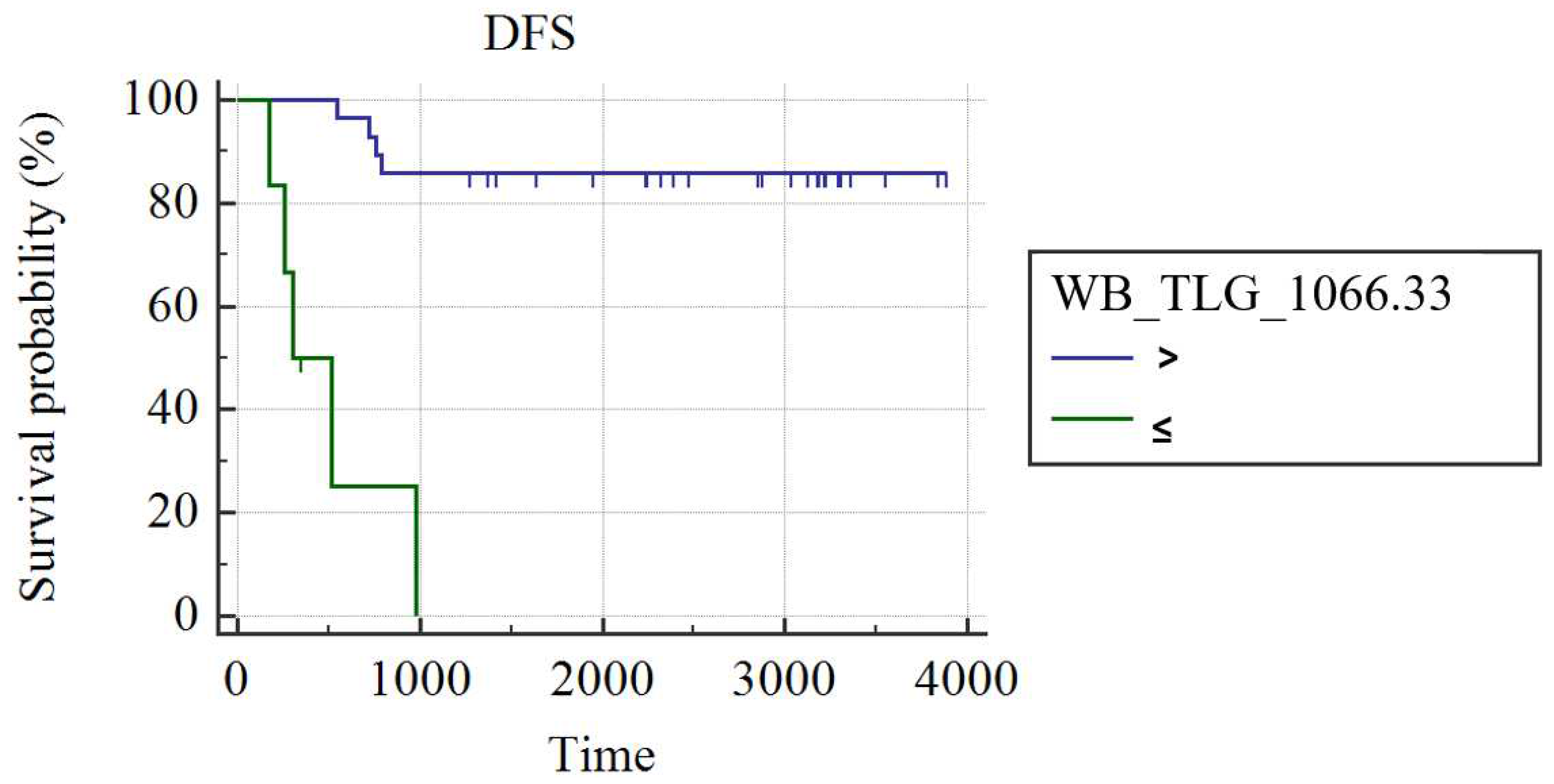
In grade III luminal B patients (n=34), WB_MTV (AUC: 0.75; best cut-off: WB_MTV>195.33; SS: 55.56%, SP: 100%; p=0.002) and WB_TLG (AUC: 0.73; best cut-off: WB_TLG>1066.21; SS: 55.56%, SP: 100%; p=0.05) were the best predictors of DFS. Patients had a higher probability of DFS in case of a WB MTV≤195.33 (3429±210 vs. 446±144 day; p<0.001;
Figure 2) or WB_TLG≤ 1066.33 (937.35±19 vs. 496±131 days; p<0.001;
Figure 3). In luminal B patients, there was a trend (p=0.1) for lower median WB_MTV (5.2 vs. 23.18) and WB_TLG (16.93 vs. 137.38) in patients achieving pCR compared to patients not achieving pCR.
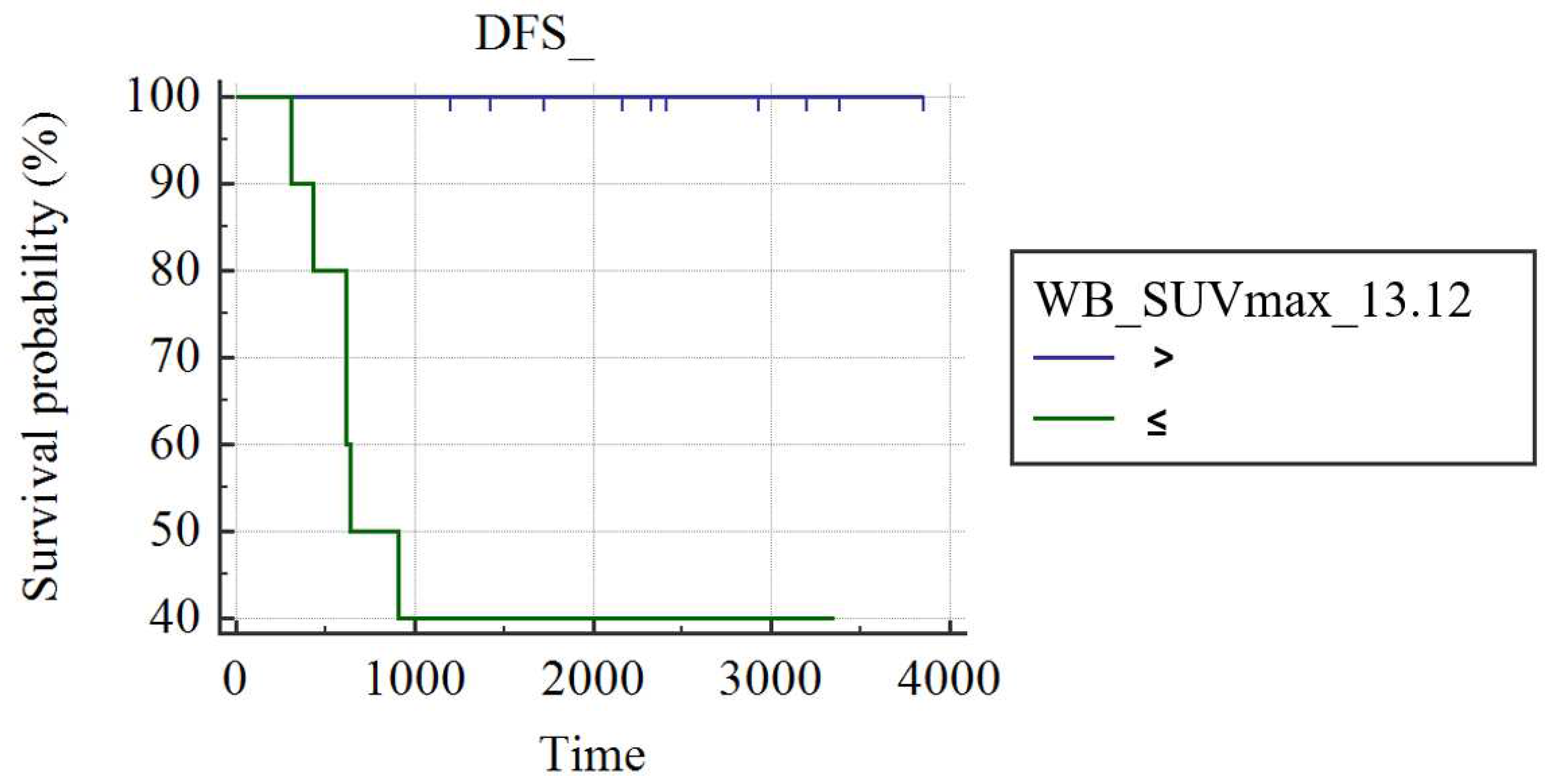
In grade III luminal B-HER2 patients (n=22), WB_SUVmax was the only predictor of DFS (AUC: 0.857; best cut-off: WB_SUVmax>13.12; SS: 100%; SP: 71.43%; p<0.001); patients with WB_SUVmax≤13.12 had a significantly longer DFS (3857 days) than patients with a WB_SUVmax >13.12 (1693±430 days; p<0.004;
Figure 4).
No parameter resulted significant in the prediction of DFS in 27 patients with grade III triple-negative cancer.
Discussion
Baseline
18F-FDG PET/CT appears a useful tool for predicting prognosis [
8,
12,
13,
16,
17] and response to NAC [
15,
18] in patients with BC with luminal-B and Luminal B-HER2 subtypes.
Luminal-B BC has a higher risk of recurrence and metastasis. Patients with endocrine therapy resistance and chemotherapy insensitivity have a poor prognosis. Indeed, low androgen receptor (AR) expression is associated with poor prognosis in luminal B BC patients. High AR/ER and residual tumor Ki67 were associated with poor DFS in NAC group. In our study we focused on patients with grade III BC, independently from the AR/ER ratio, since these patients have higher risk of lymph node involvement and recurrence than patients with lower grade [
19,
20,
21].
One of the main issue in BC therapy management regards the possibility to stratify patients with major risk of a progressive disease. Our study, in the whole cohort, without differentiating patients based on molecular type, the only predictor of recurrence was WB_SUVmax (>9.43). Indeed, it was able to stratify the outcomes of both patients with the diverse molecular BC and those with/without pCR at NAC. WB_SUVmax may mirror a more reliable estimate of tumor burden compared to SUVmax measured in the primary lesion. Indeed WB_SUVmax can provide a more accurate estimate of the tumor burden and glucose metabolism of tumor cells in the whole body and predict prognosis, as demonstrated in previous clinical studies with lymphoma [
22] and non-small cell lung cancer [
23]. In patients with luminal B-HER2, the cut-off value of WB_SUVmax able to predict a higher risk of recurrence at the 3-years follow-up was superior to 13.12, possibly due to the high rate of osseous, pulmonary, and hepatic metastases occurring in this luminal subtype during follow-up [
24]. Furthermore, Kwon and colleagues demonstrated in a large population of BC patients (n=284), a gradual increase of FDG uptake (measured as SUVmax) from hormonal types to triple negative, suggesting also a trend for higher SUV in HER2-positive tumors compared to tumors without HER2 (p = 0.093) [
25].
In luminal B patients, also the volume-based parameters, such as WB_MTV and WB_TLG, resulted the best predictors of 3-years DFS, possibly due to the high prognostic power of tumor volume in this luminal type and the low FDG uptake compared to other subtypes [
26]. Partially in keeping with our results, another study with 143 stage II–III ER+/HER2- BC patient without distant metastases at baseline
18F-FDG PET demonstrated the inverse correlation of SUVmax, MTV and TLG with the event-free survival [
27]. Volumetric parameters (MTV and TLG) seem more promising as prognostic data than metabolic parameters (SUVmax, SUVpeak, and SUVmean) in patients with grade III Luminal B. Furthermore, WB_TLG and WB_MTV showed a different trend in patients who achieved a pCR after NAC, thus underlining their additional role in predicting the response to therapy before surgery. Currently, the different predictive and prognostic role of PET parameters can be difficulty to explain, but future studies could confirm this preliminary data.
As expected, in view of the intrinsic poor prognosis of patients with grade III triple-negative BC,
the results of the study showed the absence of significant parameters able to improve the prediction of 3Y-DFS in this population.
We postulate that the lack of PET-based parameter able to predict DFS in TN patients may firstly derive from the main limit of the study, namely the limited sample of the patient cohort. However, TNBC is the most aggressive subtype of BC and any imaging-related outcome predictor would be of great impact for patients’ management. Hopefully, radiomics and artificial intelligence could provide an added value to answer this unmet clinical need [
18]
, although the lack of standardization is still an obstacle to overcome. Secondly, the lack of correlation with other parameters as Ki67 values and other biomarkers (eg. Ca15.3) represents a further limit for a more accurate patients stratification. Probably, the combination of more factors could be the best solution.
Nevertheless, the statistical significance of the results in this bicentric investigation suggest the possibility of validating our findings in multicentre studies with larger study samples.
Conclusions
18F-FDG PET volume-based parameters demonstrate the potentiality of predicting survival outcomes in patients with grade III luminal B and luminal B-He breast cancer undergoing NAC. Larger study samples are needed to confirm these preliminary findings and the potential application in clinical scenario.
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Tryfonidis, K.; Senkus, E.; Cardoso, M.J.; Cardoso, F. Management of locally advanced breast cancer-perspectives and future directions. Nat Rev Clin Oncol 2015, 12, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, A.; Roarty, K.; Rosen, J.M. The mammary stem cell hierarchy: A looking glass into heterogeneous breast cancer landscapes. Endocrine-related cancer 2015, 22, T161–176. [Google Scholar] [CrossRef] [PubMed]
- Heil, J.; Kuerer, H.M.; Pfob, A.; Rauch, G.; Sinn, H.P.; Golatta, M.; Liefers, G.J.; Vrancken Peeters, M.J. Eliminating the breast cancer surgery paradigm after neoadjuvant systemic therapy: Current evidence and future challenges. Annals of oncology : official journal of the European Society for Medical Oncology 2020, 31, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Schwarzenböck, S.M.; Krause, B.J. Fdg pet hybrid imaging. Recent results in cancer research. Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 2020, 216, 625–667. [Google Scholar] [PubMed]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A.; et al. Fdg pet/ct: Eanm procedure guidelines for tumour imaging: Version 2.0. European journal of nuclear medicine and molecular imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- Evangelista, L.; Cervino, A.R.; Michieletto, S.; Saibene, T.; Ghiotto, C.; Guarneri, V.; Conte, P.; Reccia, P.; Saladini, G. Diagnostic and prognostic impact of fluorine-18-fluorodeoxyglucose pet/ct in preoperative and postoperative setting of breast cancer patients. Nucl Med Commun 2017, 38, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, D.; Chen, B.; Tang, H.; Xie, X.; Wei, W. Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: A meta-analysis including 12,993 patients. Disease markers 2018, 2018, 9863092. [Google Scholar] [CrossRef]
- Negrão, E.M.S.; Bitencourt, A.G.V.; de Souza, J.A.; Marques, E.F. Accuracy of breast magnetic resonance imaging in evaluating the response to neoadjuvant chemotherapy: A study of 310 cases at a cancer center. Radiologia brasileira 2019, 52, 299–304. [Google Scholar] [CrossRef]
- Asaoka, M.; Narui, K.; Suganuma, N.; Chishima, T.; Yamada, A.; Sugae, S.; Kawai, S.; Uenaka, N.; Teraoka, S.; Miyahara, K.; et al. Clinical and pathological predictors of recurrence in breast cancer patients achieving pathological complete response to neoadjuvant chemotherapy. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2019, 45, 2289–2294. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Urso, L.; Caracciolo, M.; Stracuzzi, F.; Panareo, S.; Cistaro, A.; Catalano, O. Fdg pet/ct volume-based quantitative data and survival analysis in breast cancer patients: A systematic review of the literature. Current medical imaging 2022. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Quartuccio, N.; Caracciolo, M.; Evangelista, L.; Schirone, A.; Frassoldati, A.; Arnone, G.; Panareo, S.; Bartolomei, M. Impact on the long-term prognosis of fdg pet/ct in luminal-a and luminal-b breast cancer. Nucl Med Commun 2022, 43, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the st gallen international expert consensus on the primary therapy of early breast cancer 2013. Annals of oncology : official journal of the European Society for Medical Oncology 2013, 24, 2206–2223. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Evangelista, L.; Alongi, P.; Quartuccio, N.; Cittanti, C.; Rambaldi, I.; Ortolan, N.; Borgia, F.; Nieri, A.; Uccelli, L.; et al. The value of semiquantitative parameters derived from (18)f-fdg pet/ct for predicting response to neoadjuvant chemotherapy in a cohort of patients with different molecular subtypes of breast cancer. Cancers 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Al-Nahhas, A.; Rubello, D.; Muzzio, P.C. Tumor marker-guided pet in breast cancer patients-a recipe for a perfect wedding: A systematic literature review and meta-analysis. Clin Nucl Med 2012, 37, 467–474. [Google Scholar] [CrossRef]
- Panareo, S.; Urso, L.; Nieri, A.; Caracciolo, M.; Valpiani, G.; Torricelli, P.; Frassoldati, A.; Cittanti, C.; Rollo, M.; Bartolomei, M. Clinical-diagnostic relevance of breast "incidentaloma" detected during 18f-fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography: Correlation with radiological imaging and histopathology. Indian J Nucl Med 2021, 36, 385–390. [Google Scholar]
- Urso, L.; Manco, L.; Castello, A.; Evangelista, L.; Guidi, G.; Castellani, M.; Florimonte, L.; Cittanti, C.; Turra, A.; Panareo, S. Pet-derived radiomics and artificial intelligence in breast cancer: A systematic review. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Inic, Z.; Zegarac, M.; Inic, M.; Markovic, I.; Kozomara, Z.; Djurisic, I.; Inic, I.; Pupic, G.; Jancic, S. Difference between luminal a and luminal b subtypes according to ki-67, tumor size, and progesterone receptor negativity providing prognostic information. Clinical Medicine Insights. Oncology 2014, 8, 107–111. [Google Scholar] [CrossRef]
- Courtney, D.; Davey, M.G.; Moloney, B.M.; Barry, M.K.; Sweeney, K.; McLaughlin, R.P.; Malone, C.M.; Lowery, A.J.; Kerin, M.J. Breast cancer recurrence: Factors impacting occurrence and survival. Irish Journal of Medical Science (1971 -) 2022, 191, 2501–2510. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. JNCI: Journal of the National Cancer Institute 2021, 114, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.H.; Ding, C.Y.; Gale, R.P.; Wang, L.; Xu, J.; Qu, X.Y.; Fan, L.; Li, T.L.; Li, J.Y.; Xu, W. Prognostic value of whole-body suvmax of nodal and extra-nodal lesions detected by 18f-fdg pet/ct in extra-nodal nk/t-cell lymphoma. Oncotarget 2017, 8, 1737–1743. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, F.R.A.; Santos, A.O.; de Lima, M.; Toro, I.F.C.; de Souza, T.F.; Amorim, B.J.; Barbeiro, A.S.; Etchebehere, E. The ratio between the whole-body and primary tumor burden, measured on (18)f-fdg pet/ct studies, as a prognostic indicator in advanced non-small cell lung cancer. Radiologia brasileira 2021, 54, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, B.N.; Ferianto, D.; Pieter, J., Jr. Evaluation of breast cancer metastasis and mortality rates based on molecular subtype: A description study. Breast disease 2022, 41, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.W.; Lee, J.H.; Pahk, K.; Park, K.H.; Kim, S. Clustering subtypes of breast cancer by combining immunohistochemistry profiles and metabolism characteristics measured using fdg pet/ct. Cancer Imaging 2021, 21, 55. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Giacchetti, S.; Moretti, J.-L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.-S.; Cuvier, C.; Vercellino, L.; et al. Correlation of high 18f-fdg uptake to clinical, pathological and biological prognostic factors in breast cancer. European journal of nuclear medicine and molecular imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Groheux, D.; Martineau, A.; Teixeira, L.; Espié, M.; de Cremoux, P.; Bertheau, P.; Merlet, P.; Lemarignier, C. 18fdg-pet/ct for predicting the outcome in er+/her2- breast cancer patients: Comparison of clinicopathological parameters and pet image-derived indices including tumor texture analysis. Breast Cancer Research 2017, 19, 3. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).