Submitted:
07 March 2023
Posted:
08 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
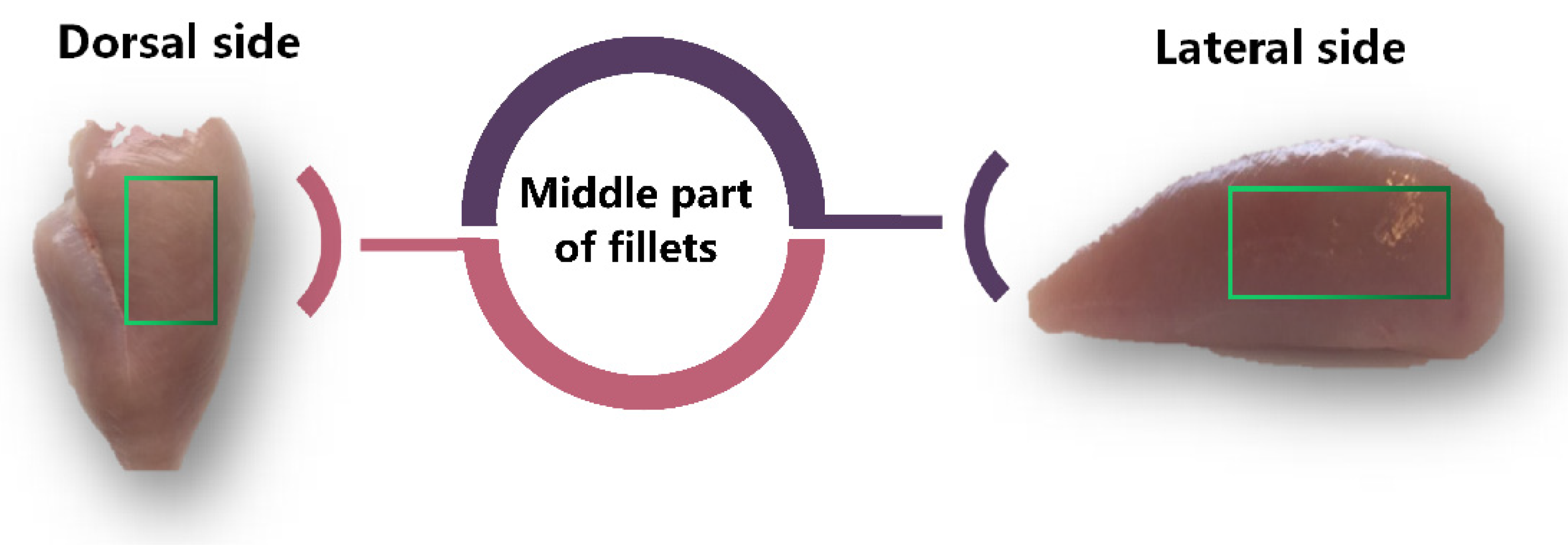
2.1. Sample Collection and Chemicals
2.2. Compression Test
2.3. Drip Loss Measurements
2.4. Collagen Profiles Measurements
2.5. Filter Residue and Thermal Properties of IMCT Analysis
2.6. Protein Extraction Analysis and Thiol Groups Content Measurements
2.7. Statistical Analyses
3. Results and Discussion
3.1. Compression Values and Purge Loss
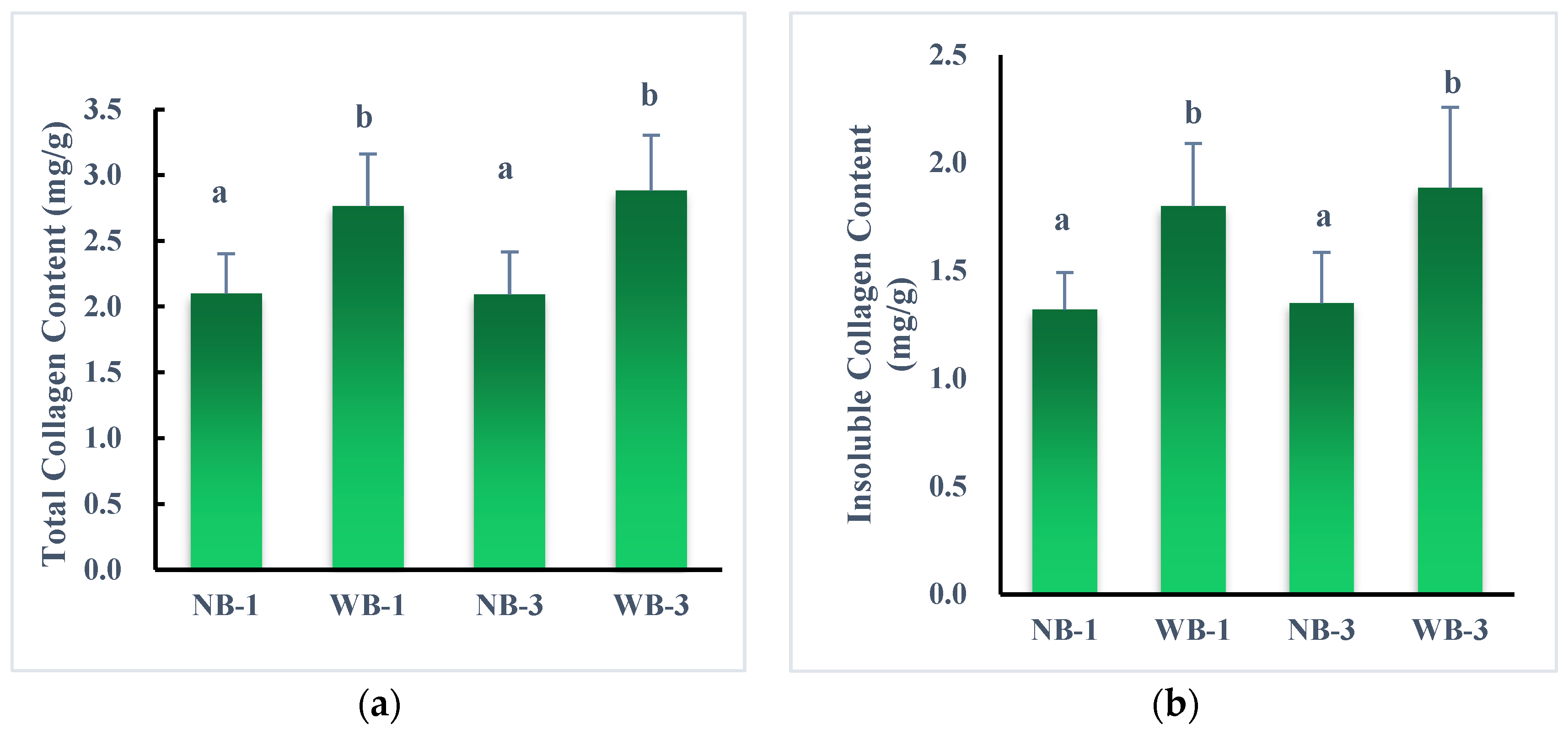
3.2. Collagen Profile
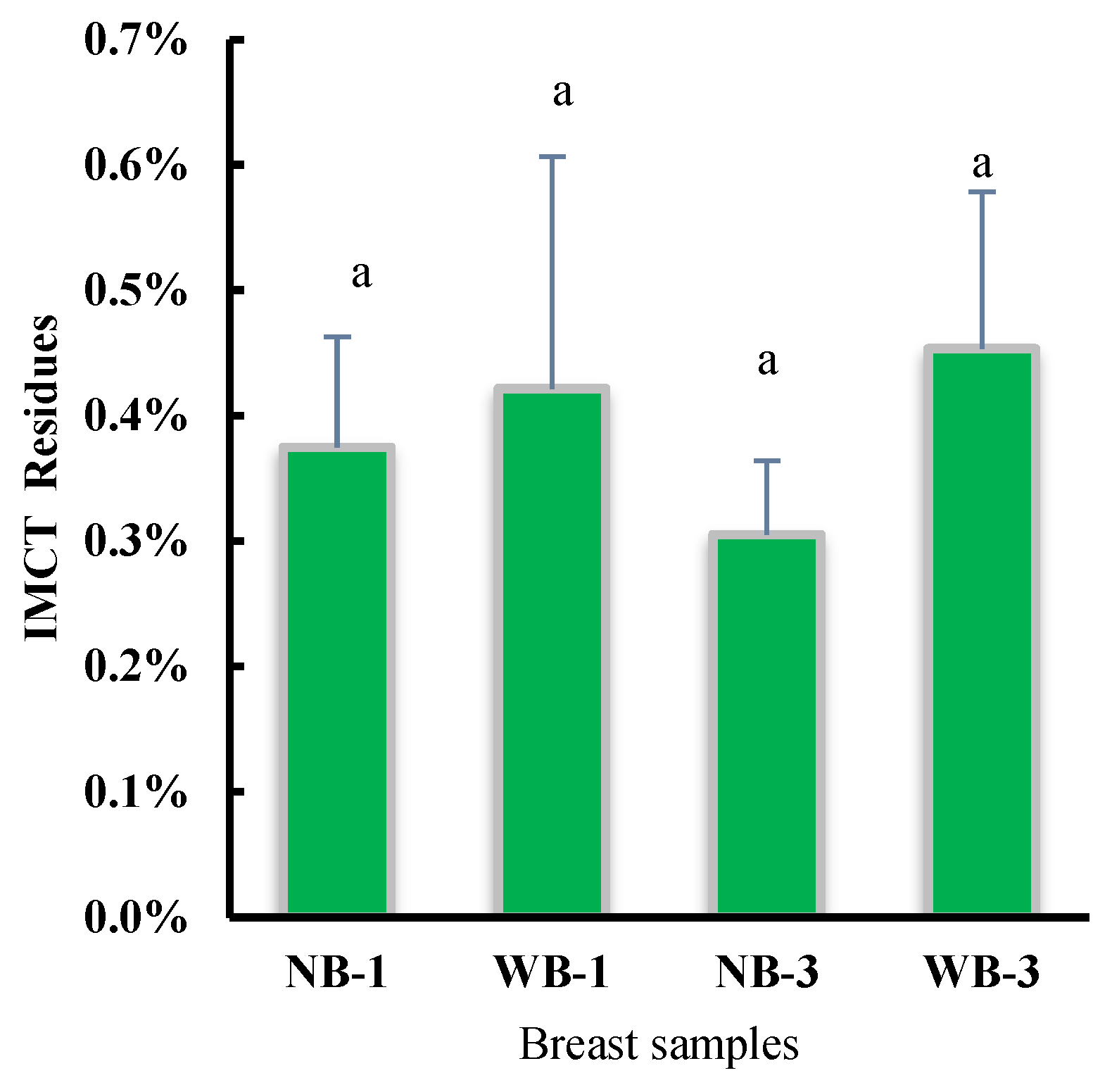
3.3. Filter Residue and Thermal Properties of Intramuscular Connective Tissue
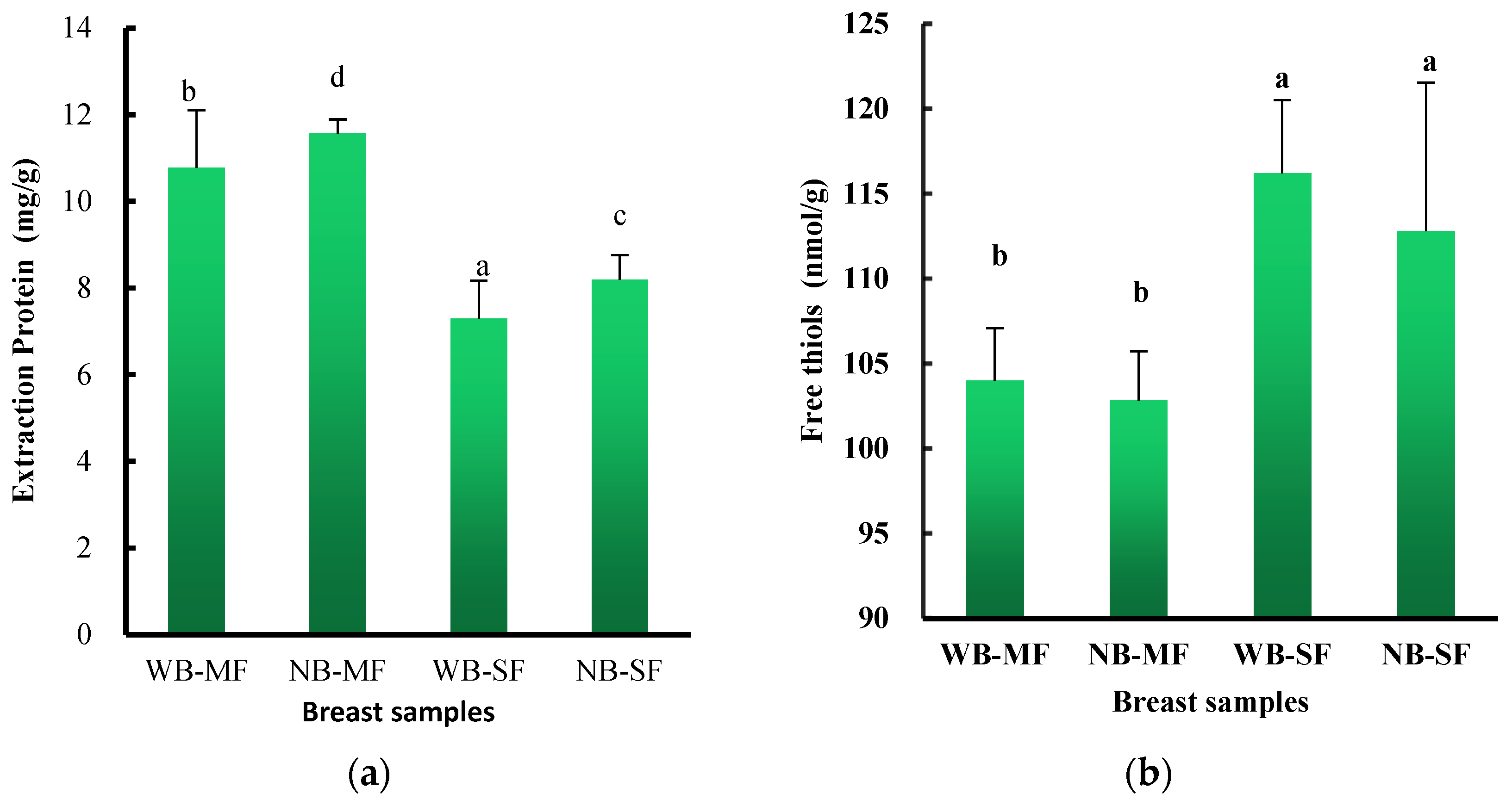
3.4. Protein Extraction Characteristics
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dransfield, E.; Sosnicki, A.A. Relationship between muscle growth and poultry meat quality. Poultry Science 1999, 78, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Kuttappan, V.A.; Shivaprasad, H.L.; Shaw, D.P.; Valentine, B.A.; Hargis, B.M.; Clark, F.D.; McKee, S.R.; Owens, C.M. Pathological changes associated with white striping in broiler breast muscles. Poultry Science 2013, 92, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Mudalal, S.; Bonfiglio, A.; Cavani, C. Occurrence of white striping under commercial conditions and its impact on breast meat quality in broiler chickens. Poultry Science 2013, 92, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Petracci, M.; Mudalal, S.; Soglia, F.; Cavani, C. Meat quality in fast-growing broiler chickens. Worlds Poultry Science Journal 2015, 71, 363–373. [Google Scholar] [CrossRef]
- Petracci, M.; Soglia, F.; Madruga, M.; Carvalho, L.; Ida, E.; Estevez, M. Wooden-Breast, White Striping, and Spaghetti Meat: Causes, Consequences and Consumer Perception of Emerging Broiler Meat Abnormalities. Compr. Rev. Food. Sci. Food Saf. 2019, 18, 565–583. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Immonen, K.; Puolanne, E. Myodegeneration With Fibrosis and Regeneration in the Pectoralis Major Muscle of Broilers. Veterinary Pathology 2014, 51, 619–623. [Google Scholar] [CrossRef]
- Velleman, S.G.; Clark, D.L. Histopathologic and Myogenic Gene Expression Changes Associated with Wooden Breast in Broiler Breast Muscles. Avian Diseases 2015, 59, 410–418. [Google Scholar] [CrossRef]
- Norring, M.; Valros, A.; Valaja, J.; Sihvo, H.K.; Immonen, K.; Puolanne, E. Wooden breast myopathy links with poorer gait in broiler chickens. Animal 2019, 13, 1690–1695. [Google Scholar] [CrossRef] [PubMed]
- Mutryn, M.F.; Brannick, E.M.; Fu, W.; Lee, W.R.; Abasht, B. Characterization of a novel chicken muscle disorder through differential gene expression and pathway analysis using RNA-sequencing. Bmc Genomics 2015, 16. [Google Scholar] [CrossRef]
- Sihvo, H.K.; Airas, N.; Linden, J.; Puolanne, E. Pectoral Vessel Density and Early Ultrastructural Changes in Broiler Chicken Wooden Breast Myopathy. Journal of Comparative Pathology 2018, 161, 1–10. [Google Scholar] [CrossRef]
- Soglia, F.; Petracci, M.; Puolanne, E. Sarcomere lengths in wooden breast broiler chickens. Italian Journal of Animal Science 2020, 19, 569–573. [Google Scholar] [CrossRef]
- Soglia, F.; Gao, J.; Mazzoni, M.; Puolanne, E.; Cavani, C.; Petracci, M.; Ertbjerg, P. Superficial and deep changes of histology, texture and particle size distribution in broiler wooden breast muscle during refrigerated storage. Poultry Science 2017, 96, 3465–3472. [Google Scholar] [CrossRef]
- Latorre, M.E.; Lifschitz, A.L.; Purslow, P.P. New recommendations for measuring collagen solubility. Meat Science 2016, 118, 78–81. [Google Scholar] [CrossRef]
- Chang, H.-J.; Xu, X.-L.; Zhou, G.-H.; Li, C.-B.; Huang, M. Effects of Characteristics Changes of Collagen on Meat Physicochemical Properties of Beef Semitendinosus Muscle during Ultrasonic Processing. Food and Bioprocess Technology 2012, 5, 285–297. [Google Scholar] [CrossRef]
- Voutila, L.; Ruusunen, M.; Puolanne, E. Comparison of the thermal characteristics of connective tissue in loose structured and normal structured porcine M. semimembranosus. Meat Science 2008, 80, 1024–1030. [Google Scholar] [CrossRef]
- Zhu, X.; Ruusunen, M.; Gusella, M.; Zhou, G.; Puolanne, E. High post-mortem temperature combined with rapid glycolysis induces phosphorylase denaturation and produces pale and exudative characteristics in broiler Pectoralis major muscles. Meat Science 2011, 89, 181–188. [Google Scholar] [CrossRef]
- Bao, Y.; Boeren, S.; Ertbjerg, P. Myofibrillar protein oxidation affects filament charges, aggregation and water-holding. Meat Science 2018, 135, 102–108. [Google Scholar] [CrossRef]
- Mudalal, S.; Lorenzi, M.; Soglia, F.; Cavani, C.; Petracci, M. Implications of white striping and wooden breast abnormalities on quality traits of raw and marinated chicken meat. Animal 2015, 9, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Tonniges, J.R.; Clark, D.L.; Velleman, S.G. The Effect of the Wooden Breast Fibrotic Myopathy in Broilers on Fibrillar Collagen Organization and Decorin-Collagen Binding. Avian Diseases 2019, 63, 48–60. [Google Scholar] [CrossRef]
- Tasoniero, G.; Bertram, H.C.; Young, J.F.; Zotte, A.D.; Puolanne, E. Relationship between hardness and myowater properties in Wooden Breast affected chicken meat: A nuclear magnetic resonance study. LWT-Food Sci. Technol. 2017, 86, 20–24. [Google Scholar] [CrossRef]
- Pang, B.; Yu, X.; Bowker, B.; Zhang, J.; Yang, Y.; Zhuang, H. Effect of meat temperature on moisture loss, water properties, and protein profiles of broiler pectoralis major with the woody breast condition. Poultry Science 2021, 100, 1283–1290. [Google Scholar] [CrossRef] [PubMed]
- Purslow, P.P. Contribution of collagen and connective tissue to cooked meat toughness; some paradigms reviewed. Meat Science 2018, 144, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Ertbjerg, P.; Failla, S.; Sanudo, C.; Richardson, R.I.; Nute, G.R.; Olleta, J.L.; Panea, B.; Alberti, P.; Juarez, M.; et al. Relationship between collagen characteristics, lipid content and raw and cooked texture of meat from young bulls of fifteen European breeds. Meat Science 2011, 87, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, M. Proteoglycan and its possible role in “ Wooden Breast “ condition in Broilers. Nepalese Journal of Agricultural Sciences 2015, 0, 253–260. [Google Scholar]
- Kopp, J.; Bonnet, M.; Renou, J.P. EFFECT OF COLLAGEN CROSSLINKING ON COLLAGEN-WATER INTERACTIONS (A DSC INVESTIGATION). Matrix 1990, 9, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.L.; Velleman, S.G. Spatial influence on breast muscle morphological structure, myofiber size, and gene expression associated with the wooden breast myopathy in broilers. Poultry Science 2016, 95, 2930–2945. [Google Scholar] [CrossRef] [PubMed]
- Lepetit, J. A theoretical approach of the relationships between collagen content, collagen cross-links and meat tenderness. Meat Science 2007, 76, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G.; Clark, D.L.; Tonniges, J.R. Fibrillar Collagen Organization Associated with Broiler Wooden Breast Fibrotic Myopathy. Avian Diseases 2017, 61, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Sanden, K.W.; Bocker, U.; Ofstad, R.; Pedersen, M.E.; Host, V.; Afseth, N.K.; Ronning, S.B.; Pleshko, N. Characterization of Collagen Structure in Normal, Wooden Breast and Spaghetti Meat Chicken Fillets by FTIR Microspectroscopy and Histology. Foods 2021, 10. [Google Scholar] [CrossRef]
- Ali, S.; Zhang, W.; Rajput, N.; Khan, M.A.; Li, C.-b.; Zhou, G.-h. Effect of multiple freeze-thaw cycles on the quality of chicken breast meat. Food Chemistry 2015, 173, 808–814. [Google Scholar] [CrossRef]
- Baldi, G.; Soglia, F.; Laghi, L.; Tappi, S.; Rocculi, P.; Tavaniello, S.; Prioriello, D.; Mucci, R.; Maiorano, G.; Petracci, M. Comparison of quality traits among breast meat affected by current muscle abnormalities. Food Research International 2019, 115, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Baldi, G.; Yen, C.-N.; Daughtry, M.R.; Bodmer, J.; Bowker, B.C.; Zhuang, H.; Petracci, M.; Gerrard, D.E. Exploring the Factors Contributing to the High Ultimate pH of Broiler Pectoralis Major Muscles Affected by Wooden Breast Condition. Frontiers in Physiology 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Zhao, Z.R.; Zhao, X.; Xu, X.L.; Zhang, L.; Gao, F. Enhanced transforming growth factor-beta signaling and fibrosis in the pectoralis major muscle of broiler chickens affected by wooden breast myopathy. Poultry Science 2021, 100. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, D.E.; Latorre, M.E. Physicochemical, thermal and mechanical characterization study of perimysial collagen of two bovine muscles. International Journal of Biological Macromolecules 2019, 136, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dong, X.; Puolanne, E.; Ertbjerg, P. Effect of wooden breast degree on lipid and protein oxidation and citrate synthase activity of chicken pectoralis major muscle. LWT-Food Sci. Technol. 2022, 154. [Google Scholar] [CrossRef]
- Carvalho, L.M.; Delgado, J.; Madruga, M.S.; Estevez, M. Pinpointing oxidative stress behind the white striping myopathy: depletion of antioxidant defenses, accretion of oxidized proteins and impaired proteostasis. Journal of the Science of Food and Agriculture 2021, 101, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Abasht, B.; Mutryn, M.F.; Michalek, R.D.; Lee, W.R. Oxidative Stress and Metabolic Perturbations in Wooden Breast Disorder in Chickens. Plos One 2016, 11. [Google Scholar] [CrossRef]
- Bao, Y.; Ertbjerg, P. Relationship between oxygen concentration, shear force and protein oxidation in modified atmosphere packaged pork. Meat Science 2015, 110, 174–179. [Google Scholar] [CrossRef]
- Thanatsang, K.V.; Malila, Y.; Arayamethakorn, S.; Srimarut, Y.; Tatiyaborworntham, N.; Uengwetwanit, T.; Panya, A.; Rungrassamee, W.; Visessanguan, W. Nutritional Properties and Oxidative Indices of Broiler Breast Meat Affected by Wooden Breast Abnormality. Animals 2020, 10, 2272. [Google Scholar] [CrossRef]
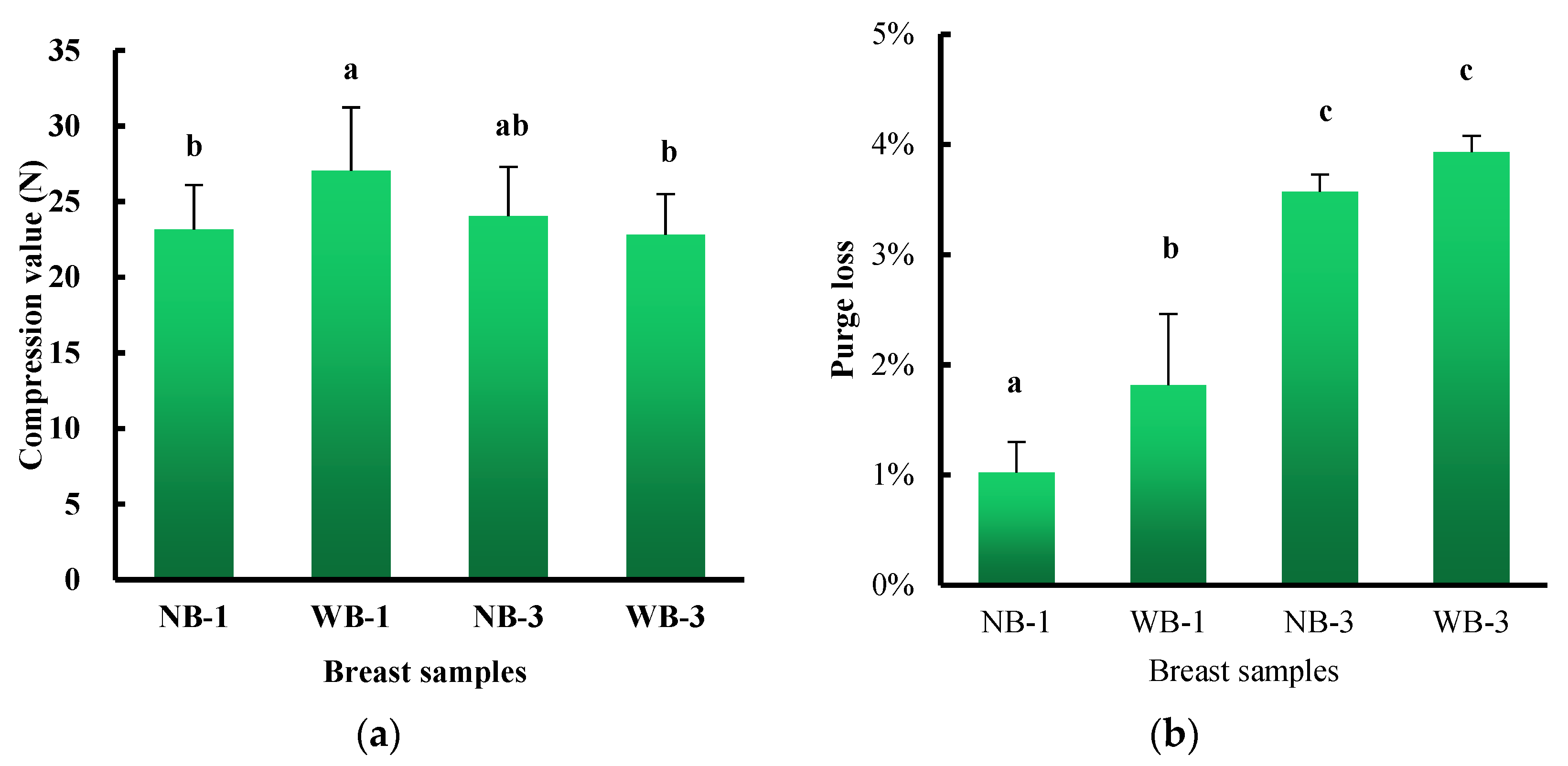
| WB-1d | NB-1d | WB-3d | NB-3d | |
|---|---|---|---|---|
| onset T (°C) | 58.22±0.82a | 57.24±2.77a | 56.70±0.76a | 57.66±2.33a |
| peak T (°C) | 63.73±0.36a | 63.58±2.17a | 62.81±0.25a | 64.04±1.87a |
| endset T (°C) | 71.55±0.66a | 72.62±1.59a | 69.91±0.90b | 72.84±1.13a |
| ΔH (J/g) | 9.76±4.34a | 14.71±5.04b | 7.86±3.69a | 14.16±3.62b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





