Submitted:
03 March 2023
Posted:
07 March 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
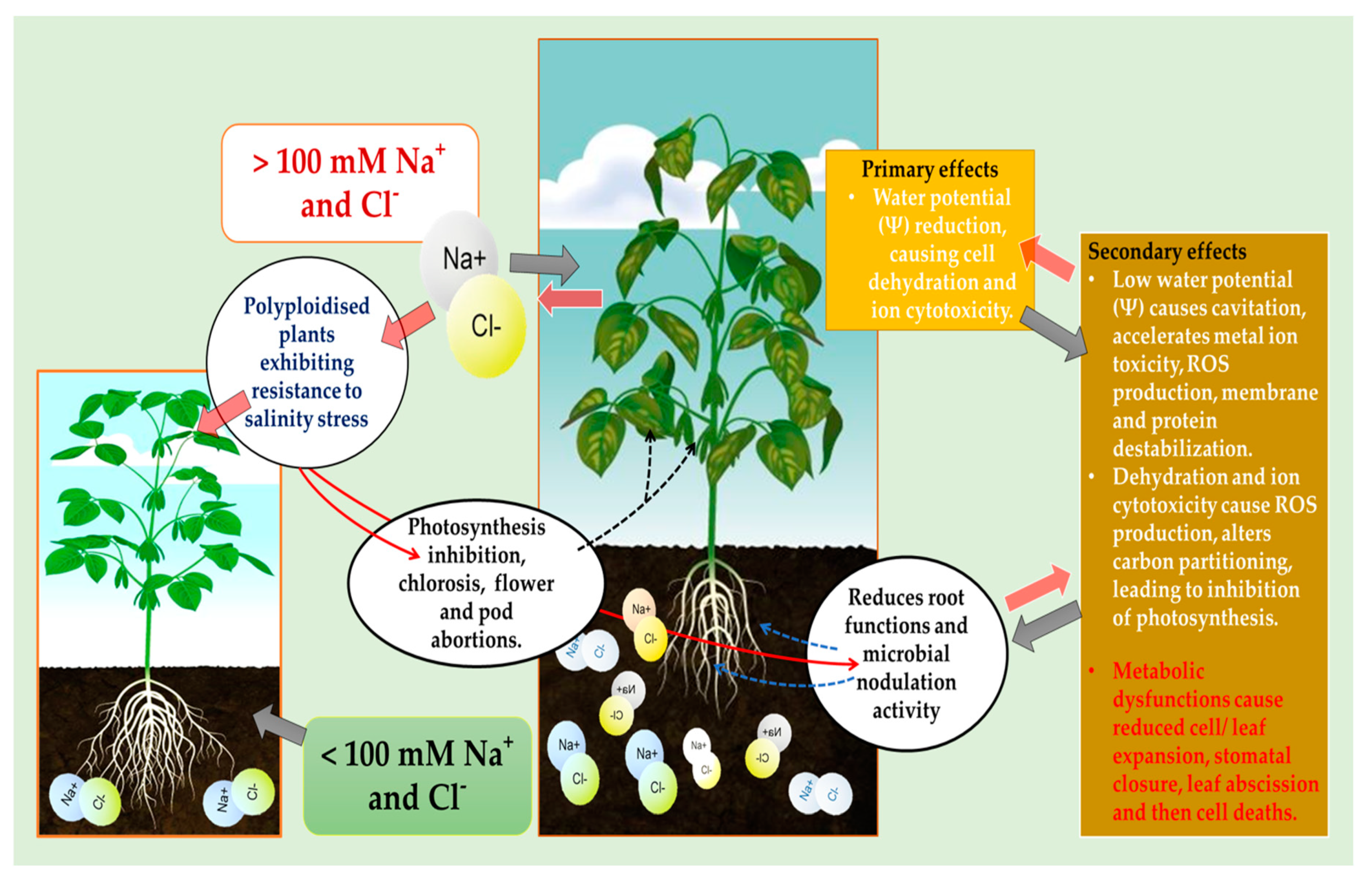
2. Genetic Architecture and Response to Salinity Stress in Soybean
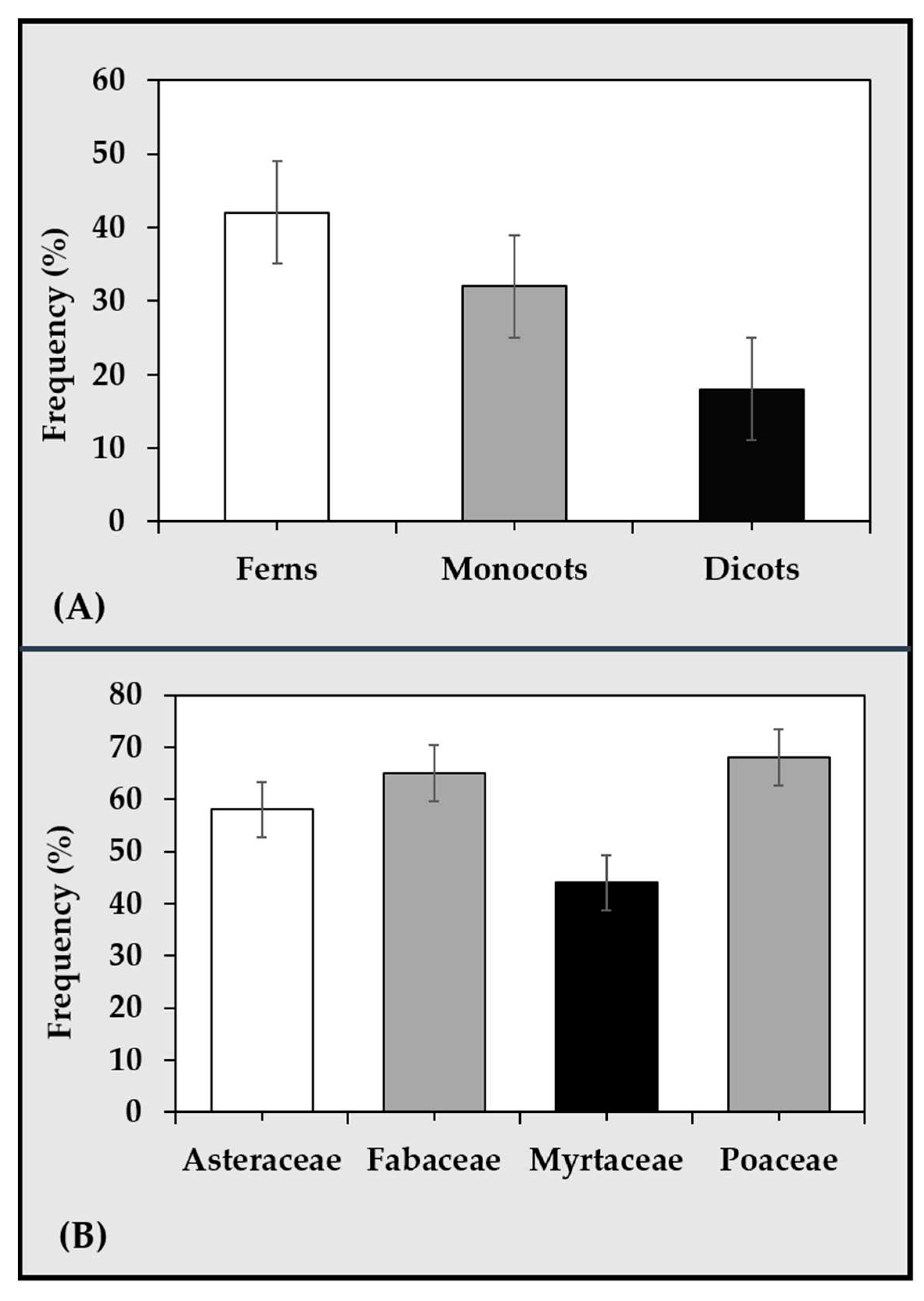
3. Improvement of Salt Stress Tolerance via Polyploidy Induction in Plants
3.1. Vegetative Growth Characteristics for Salinity Tolerance
3.2. Enhanced Biochemical and Physiological Responses
4. Ploidy Stability and Molecular Profile for Salinity Stress Resistance in Soybean
5. Potential Undesirable Ploidy Effects
6. Future Prospects and Final Considerations
5. Conclusions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kim, K.D.; El-Baidouri, M.; Abernathy, B.; Iwato-Otsubo, A.; Chavarro, C.; Gonzales, M.; Libaut, M.; Grimwood, J.; Jackson, S.A. A comparative epigeonomic analysis of polyploidy-derived genes in soybean and common bean. Plant Physiol 2015, 168(4), 1433–1447. [CrossRef]
- Manzoor, A.; Ahmad, T.; Bashir, A.; Hafiz, I.A.; Silvestri, C. Studies on colchicine induced chromosome doubling for enhancement of quality traits in ornamental plants. Plants (Basel) 2019, 8(7), 194. [CrossRef]
- Juliao, S.A.; Ribeiro, C.V.; Lopes, J.M.L.; de Matos, E.M.; Reis, A.C.; Peixoto, P.H.P.; Machado, M.A.; Azevedo, A.L.S.; Grazul, R.M.; Campos, J.M.S.; Viccini, L.F. Induction of synthetic polyploids and assessment of genomic stability in Lippia alba. Front Plant Sci 2020, 11, 292. [CrossRef]
- Sedivy, E.J.; Wu, F.; Hanzawa, Y. Soybean domestication: The origin, genetic architecture and molecular bases. New Physiol 2017, 214(2), 539–553. [CrossRef]
- Oyegbami, A.; Fadairo, A.O.; Oyedokun, M.O. Women’s knowledge of the nutritional benefits and perceived constraints in soybean utilization in Oyo State, Nigeria. S. Afr J Agric Ext 2020, 48(2), 166–175.
- Kang, Z.; Xiaowu, W.; Feng, C. Plant polyplidy: Origin, evolution and its influence on crop domestication. Horti Plant J 2019, 5(6), 231–239. [CrossRef]
- Al Nebaihi, H.; Le, T.S.; Davies, N.M.; Brocks, D.R. Liquid chromatography tandem mass spectrometric analytical method for study of colchicine in rats given low doses. Processes 2021, 9(11), 2007. [CrossRef]
- Katukojvala, S.; Barlett, K.N.; Lotesta, S.D.; Williams, L.J.; Spirodiepoxides in total synthesis: Epoxomicin. J Am Chem Soc 2004, 126(47), 15348–15349. [CrossRef]
- Kantin, G.P.; Krasavin, M.Y. Microwave promoted reaction of N-(alk-1-enyl)chloroacetamides with sodium azide unexpectedly yields 1H-imidazol-5(4H)-ones. Mendocomm 2017, 27(1), 95–96. [CrossRef]
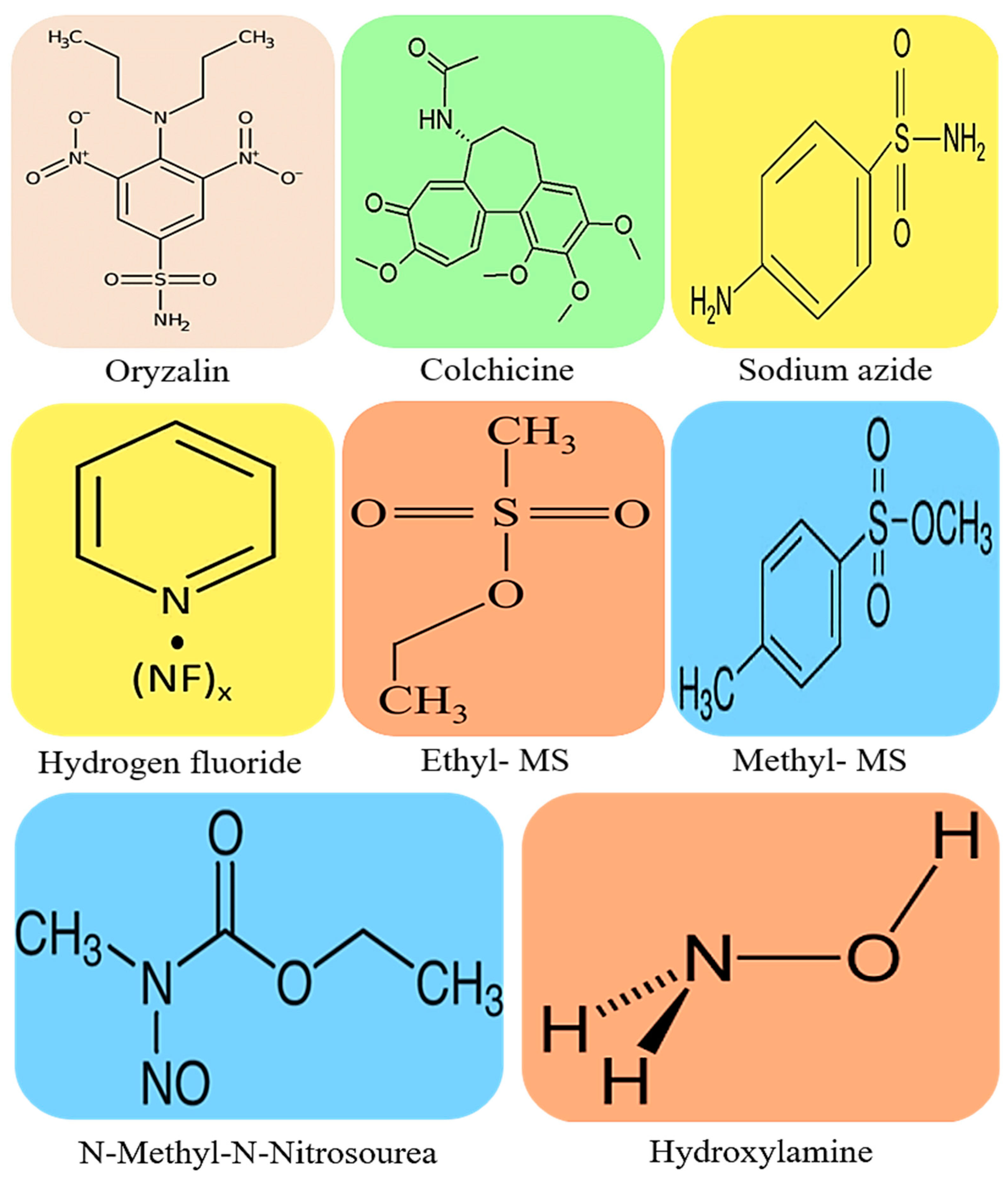
- Honma, M. An assessment of mutagenicity of chemical substances by (quantitative) structure-activity relationship. Gene Environ 2020, 42, 23. [CrossRef]
- Simontacchi, M.; Galatro, A.; Ramos-Artuso, F.; Santa-Maria, GE. Plant survival in changing environment: The role of nitric oxide in plant response to abiotic stress. Front Plant Sci 2015, 6, 977. [CrossRef]
- Espina, M.J.; Ahmed, C.M.S.; Bernardini, A.; Adeleke, E.; Yadegari, Z.; Arielli, P.; Pantalone, V.; Taheri, A. Development and phenotypic screening of ethyl methane sulfonate mutant population in soybean. Front Plant Sci 2018, 9, 394. DOI: 3389/fpls.2018.00394.
- Mastuti, R.; Munawarti, A.; Afiyanti, M. The effect of colchicine on in vitro growth of ciplukan (Physalis angulate). IOC Conf Ser: Earth Environ Sci 2022, 1097, 1–7. [CrossRef]
- Van der Hoorn, R.A.L.; Colby, T.; Nickel, S.; Richau, K.H.; Schmidt, J.; Kaiser, M. Mining the active proteome of Arabidopsis thaliana. Front Plant Sci 2021, 2, 89. [CrossRef]
- Schwarz, K.; Giul, R.; Schmidtke, G.; Kostka, S.; van der Broek, M.; Kim, K.B.; Crews, C.M.; Kraft, R.; Groettrup, M. The selective proteasome inhibitors lactacystatin and epoxomicin can be used to either up or down regulate antigen presentation at nontoxic does. J Immunol 2000, 164(12), 6147–6157. [CrossRef]
- Kisselev, A.F.; Goldberg, A.L. Proteosome inhibitor: From research tools to drug candidates. Chem Biol 2001, 8(8), 739–758.
- Levie, J.; Belvalet, H.D.; Sonon, S.; Ion, A.M.; Dumon, E.; Melser, S.; Lacombe, D.; Dupuy, J-W.; Lalou, C.; Benard, G. Ubiquitin-dependent degradation of mitochondrial protein regulates energy metabolism. Cell Rep 2018, 23, 2852–2862. [CrossRef]
- Meng, L.; Mohan, R.; Kwok, B.H.B.; Crews, C.M. Epoxomicin, a potent and selective proteasome inhibitor, exhibit in vivo anti-inflammatory activity. PNAS 1999, 96(18), 10403–10408. [CrossRef]
- Bouyer, D.; Geier, F.; Kragler, F.; Schnittger, A.; Pesch, M.; Wester, K.; Balkunde, R.; Timmer, J.; Fleck, C.; Hulskamp, M. Two-dimensional patterning by a trapping/ depletion mechanism: The role of TTGI and GL3 in Arabidopsis trichome formation. PLoS Biol 2008, 6(6), e141. [CrossRef]
- Silalahi, C.B.; Sinuraya, M.; Hanafiah, D.S.; Sipayung, R. The influence of oryzalin concentration on the plant growth of two tomato (Solanum lycopersicum L.) varieties. IOP Con Ser: Earth Environ Sci 2019, 454, 1–6. [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of plant response to salt stress. Int J Mol Sci 2021, 22, 4609. [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant salinity stress: Many unanswered questions remain. Front Plant Sci 2019, 10, 80. [CrossRef]
- Zahra, N.; Raza, Z.A.; Mahmood, S. Effect of salinity stress on various growth and physiological attributes of two contrasting maize genotypes. Braz Arch Biol Technol 2020, 63, e20200072.
- Morales, M.; Oakley, L.; Sartori, A.L.B.; Mogni, U=V.Y.; Atahuachi, M.; Vanni, R.O.; FRortunato, R.H.; Prado, D.E. Diversity and conservation of legumes in the Gran Chaco and biogeographical influences. PLoS One 2019, 14(8), e0220151. [CrossRef]
- Koenen, E.J.M.; Ojeda, D.I.; Bakker, F.T.; Weringa, J.J.; Kidner, C.; Hardy, O.J.; Pennington, R.T.; Herendeen, P.S.; Bruneau, A.; Hughes, C.E. The origin of the legumes is a complex paleopolyploid phylogenomic tangle closely associated with the Cretaceous-paleogene (K-Pg) mass extinction event. Systematic Biol 2020, 70(3), 508–526. [CrossRef]
- Gill, N.; Findley, S.; Walling, J.G.; Hans, C.; Ma, J.; Doyle, J.; Stacey, G.; Jackson, S.A. Molecular and chromosomal evidence for allopolyploidy in soybean. Plant Physiol 151(3), 1167–1174. [CrossRef]
- de la Estrella, M.; Forest, F.; Klitgard, B.; Lewis, G.P.; Mackinder, B.A.; de Queiroz, L.P.; Wieringa, J.J.; Bruneau, A. A new phylogeny-based tribal classification of subfamily Detarioideae, an early branching clade of florally diverse tropical arborescent legumes. Sci Rep 2018, 8, 6884. [CrossRef]
- Trytsman, M.; van Wyk, A.E.; Masemola, L. Systematics, diversity and forage value of indigenous legumes of South Africa, Lesotho and Swaziland. Afr J Biotechnol 2011, 10(63), 13773–13779. [CrossRef]
- Idowu, G.A.; Fletcher, A.J. The manufacture and characterization of Rosid angiosperms-derived biochars applied to water treatment. BioEnergy Res 2020, 13, 387–396. [CrossRef]
- Viviani, T.; Conte, L.; Cristofolini, G.; Speranza, M. Sero-systematic studies on the Phaseoleae (Leguminosae) and related tribes. Bot J Linnean Soc 2008, 105(2), 113–136. [CrossRef]
- Zhu, J-K. Abiotic stress signaling and responses in plants. Cell 2016, 167(2), 313–324. [CrossRef]
- Pi, E.; Qu, L.; Hu, J.; Huang, Y.; Qiu, L.; Lu, H.; Jiang, B.; Liu, C.; Peng, T.; Zhao, Y.; Wang, H.; Tsai, S-H.; Ngai, S.; Du, L. Mechanism of soybean roots’ tolerance to salinity revealed by proteomic and phosphoproteomic comparison between two cultivars. Mol Cell Proteomics 2016, 15(1), 266–288. [CrossRef]
- Cai, X.; Jia, B.; Sun, X. Insights into the regulation of wild soybean tolerance to salt-alkaline stress. Front Plant Sci 2022, 13, 1002302. [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Anee, T.I.; Parvin, K.; Nahar, K.; Mahmud, J.A.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidant 2019, 8(9), 384. [CrossRef]
- Lopez-Huertas, E.; Palma, J.M. Changes in glutathione, ascorbate, and antioxidant enzymes during olive fruit ripening. J Agric Food Chem 2020, 68(44), 12221–12228. [CrossRef]
- Peng, Z.; He, S.; Sun, J.; Pan, Z.; Gong, W.; Lu, Y.; Du, X. Na+ compartmentalization related to salinity stress tolerance in upland cotton (Gossypium hirsutum) seedlings. Sci Rep 2016, 6, 34548. [CrossRef]
- Mishra, A.; Tanna, B. Halophytes: Potential resources for salt stress tolerance genes and promoters. Front Plant Sci 2017, 8, 829. [CrossRef]
- Alam, H.; Razaq, M.; Salahuddin. Induced polyploidy as a tool for increasing tea (Camellia sinensis L.) production. J Northest Agric Univ 2015, 22(3), 43–47. [CrossRef]
- Forrester, N.J.; Ashman, T-L. The direct effects of plant polyploidy on the legume-rhizobia mutualism. Ann Bot 2028, 121(2), 209–220. [CrossRef]
- Boukar, O.; Abberton, M.; Oyatomi, O.; Togola, A.; Tripathi, L.; Fatokum, C. Introgression breeding in cowpea [Vigna unguiculata (L.) Walp.]. Front Plant Sci 2020, 11, 567425. [CrossRef]
- Munzbergova Z. Colchicine application significantly affect plant performance in the second generation of synthetic polyploids and its effects vary between populations. Ann Bot 2017, 120, 329–339. [CrossRef]
- Pavlikova, Z.; Pastova, L.; Munzbergova, Z. Synthetic polyploids in Vicia cracca: Methodology, effects on plant performance and aneuploidy. Plant Syst Evol 2017, 303, 827–839. [CrossRef]
- Mo, L.; Chen, J.; Lou, X.; Xu, Q.; Dong, R.; Tong, Z.; Huang, H.; Lin, E. Colchicine-induced polyploidization in Rhododendron fortunei Lindl. Plant 2020, 9, 424. [CrossRef]
- Noori, S.A.S.; Norouzi, M.; Karimzadeh, G.; Shirkool, K.; Niazian, M. Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tiss Org Cult 2017, 130, 534–551. [CrossRef]
- Luo, Z.; Iaffaldano, B.J.; Cornish, K. Colchicine-induced polyploidy has the potential to improve rubber yield in Taraxacum kok-saghyz. Ind Crops Prod 2018, 112, 75–81. [CrossRef]
- Jeloudar, N.I.; Chamani, E.; Shokouhian, A-a.; Zakaria, R.A. Induction and identification of polyploidy by colchicine treatment in Lilium regale. Cytologia 2019, 84(3), 271–276. [CrossRef]
- Kushwah, K.S.; Verma, R.C.; Patel, S.; Jain, N.K. Colchicine induced polyploidy in Chrysanthemum carinatum L. Phylogenetics Evol Biol 2018, 6, 1. [CrossRef]
- Palmar, R.G. Aneuploids in the soybean, Glycine max. Genome 2011, 16(2), 441–447. [CrossRef]
- Rauf, S.; Ortiz, R.; Malinowski, D.P.; Clarindo, W.R.; Kainat, W.; Schehzad, M.; Waheed, U.; Hassan, S.W. Induced polyploidy: A tool for forage species improvement. Agric 2021, 11(3), 210. [CrossRef]
- Debkeviciene, G.; Kemesyte, V.; Statkeviciute, G.; Lemeziene, N.; Brazauskas, G. Authopolyploids in fodder grass breeding: Induction and field performance. Span J Agric Res 2017, 15(4), e0706. [CrossRef]
- Innes, L.A.; Denton, M.D.; Dundas, I.S.; Pech, D.M.; Humphries, A.W. The effect of ploidy number on vigor, productivity, and potential adaptation to climate change in annual Medicago species. Crop Sci 2021, 61(1), 89–103. [CrossRef]
- Venial, L.R.; Mendonca, M.A.C.; Amaral-Silva, P.M.; Canal, G.B.; Passos, A.B.R.J.; Ferreira, A.; Soures, T.C.B.; Clarindo, W.R. Autotetraploid Coffea canephora and auto-alloctapleid Coffea arabica from in vitro chromosome set doubling: New germplasm for Coffea. Front Plant Sci 2020, 11, 154. [CrossRef]
- Lestari, P.; Roostika, I.; Nugroho, K.; Edison, H.S.; Rijzaani, H.; Mastar, M. Genetic stability of banana plant regenerated from floral axis organogenesis assessed by newly developed SSR markers. AGRIVTA J Agric Sci 2019, 41(2): 302–315. [CrossRef]
- Wendel, J.F.; Lisch, D.; Hu, G.; Manson, A.S. The long and short of doubling down: Polyploidy, epigenetics and the temporal dynamics of genome fractionation. Curr Opin Gnetic Dev 2018, 48: 1–7. [CrossRef]
- Kolar, F.; Certner, M.; Suda, J.; Schonswetter, P.; Husband, B.C. Mixed ploidy species: Progress and opportunities in polyploid research. Trends Plant Sci 2017; 22(12): 1041–1055. [CrossRef]
- Xue, H.; Zhang, F.; Zhang, Z-H.; Fu, J-F.; Wang, F.; Zhang, B.; Ma, Y. Differences in salt tolerance between diploid and autotetraploid apple seedlings exposed to salt stress. Sci Hort 2015, 190: 24–30. [CrossRef]
- Ruiz, M.; Quimones, A.; Martinez-Cuenca, M.R.; Aleza, P.; Morillon, R.; Navarro, L.; Primo-Millo, E.; Martinez-Alcantara, B. Tetraploidy enhances the ability to exclude chloride from leaves in Carrizo citrange seedlings. J. Plant Physiol 2016, 205: 1–10. [CrossRef]
- Oustric, J.; Quilichini, Y.; Morillon, R.; Herbette, S.; Luro, F.; Giannettini, J.; Berti, L.; Santini, J. Tetraploid Citrus seedlings subjected to long-term nutrient deficiency are less affected at the ultrastructural, physiological and biochemical levels than diploid ones. Plant Physiol Biochem 2019, 135: 375–384. [CrossRef]
- Rao, S.; Tian, Y.; Xia, X.; Li, Y.; Chen, J. Chromosome doubling mediates superior drought tolerance in Lycium ruthenicum via abscisic acid signaling. Hort Res 2020, 7: 40. [CrossRef]
- Chen, H.; Lu, Z.; Wang, J.; Chen, T.; Gao, J.; Zheng, J.; Zhang, J.; Zhang, S.; Xi, J.; Huang, X.; Guo, A.; Yi, K. Induction of new tetraploid genotypes and heat tolerance assessment in Asparagus officinalis L. Sci Hort 2020, 264: 109168. [CrossRef]
- Li, M.; Zhang, C.; Hou, L.; Yang, W.; Liu, S.; Pang, X.; Li, Y. Multiple responses contribute to the enhanced drought tolerance of the autotetraploid Ziziphus jujube Mill. Var. spinosa. Cell Biosci 2021, 11: 119. [CrossRef]
- Jin, Y.; Zhao, Y.; Ai, S.; Chen, X.; Liu, X.; Wang, H.; Han, Y.; Ma, F.; Li, C. Induction of polyploid Malus prunifolia and analysis of its salt tolerance. Tree Physiol 2022, 42(10): 2100–2115. [CrossRef]
- Otto, S.P. The evolutionary consequences of polyploidy. Cell 2007, 131: 452–462. [CrossRef]
- Weiss-Schneeweiss, H.; Emadzade, K.; Jang, T.S.; Schneeweiss, G.M. Evolutionary consequences, constraints and potential of polyplody in plants. Cytogenet Genome Res 2013, 140: 137–150. [CrossRef]
- Palmer, R.G.; Sandhu, D.; Curran, K.; Bhattacharyya, M.K. Molecular mapping of 36 soybean male-sterile, female-sterile mutants. Theor Appl Genet 2008, 117: 711–719. [CrossRef]
- Fox, D.T.; Soltis, D.E.; Solitis, P.S.; Ashman, T-L.; Van de Peer, Y. Polyploidy: A biological force from cells to ecosystems. Trend Cell Biol 2020, 30(9): 688–694. [CrossRef]
- Spoelhof, J.P.; Soltis, D.E.; Soltis, P.S. Habitat shape affects polyploid establishment in a spatial, stochastic model. Front Plant Sci 2020, 11: 592356. [CrossRef]
- Van de Peer, Y. Ashman, T-L.; Soltis, P.S.; Soltis, D.E. Polyploidy: An evolutionary and ecological force in stressful times. Plant Cell 2021, 33: 11–26. [CrossRef]
- Scholes, D.R.; Paige, K.N. Plasticity in ploidy: A generalized response to stress. Trend Plant Sci 2015; 20(3): 165–175. [CrossRef]
- Tossi, V.; Tosar, L.J.M.; Laino, L.E.; Lannicelli, J.; Regalado, J.J.; Escandon, A.S.; Baroli, I.; Causin, H.F.; Pitta-Alvarez, S.I. Impact of polyploidy on plant tolerance to abiotic and biotic stress. Front Plant Sci 2022, 13: 869423. [CrossRef]
- Khatoon, S.; Ali, S.I. Chromosome numbers and polyploidy in the legumes of Pakistan. Pak J Bot 2006, 38(4): 935–945.
- Singh, R.J.; Chung, G.H. Cytogenetics of soybean: Progress and perspectives. Nucleus 2007, 50(3): 403–425.
- Cai, D.T.; Chen, J.G.; Chen, D.L.; Dai, B.C.; Zhang, W.; Song, Z.J.; Yan, Z.F.; Du, C.D.; Tang, Z.Q.; He, Y.C.; Zhang, D.S.; He, G.C.; Zhu, Y.G. The breeding of two polyploid rice lines with the characteristics of polyploid meiosis stability. Sci China C Life Sci 2007, 50(3): 356–366. [CrossRef]
- Ijaz, U.; Adhikari, K.N.; Stoddard, F.L.; Trethwan, R.M. Rust resistance in faba bean (Vicia faba L.): Status and strategies form improvement. Aust Plant Pathol 2018, 47(1): 71–81. [CrossRef]
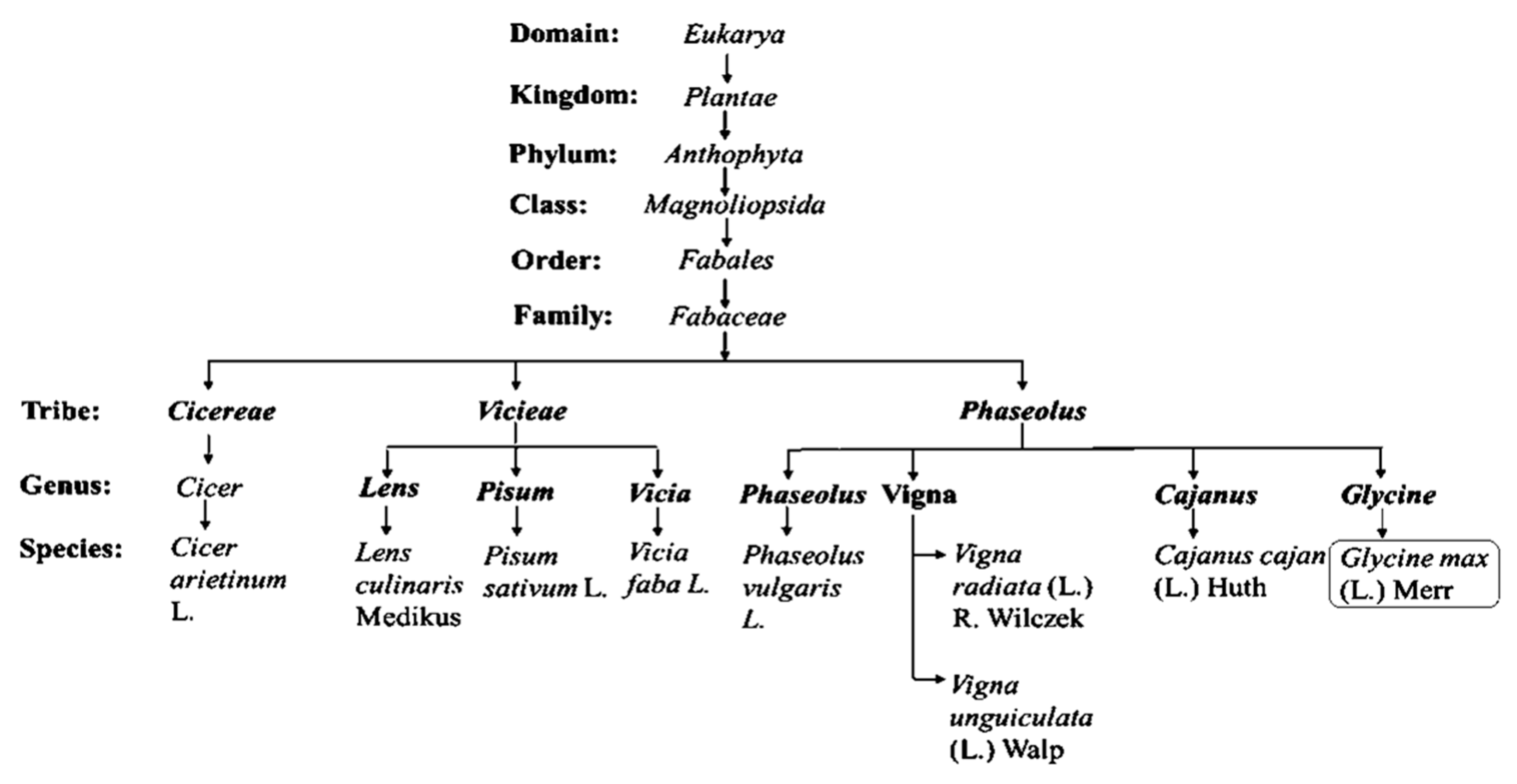
| Tribe | Species name | Common name |
|---|---|---|
| Phaseoleae | Cajanus cajan | Pigeon pea |
| Canavalia ensiformis | Horse bean | |
| Glycine max | Soybean | |
| Mucuna pruriens | Velvet bean | |
| Phaseolus lanatus | Butter bean | |
| Phaseolus acutifolius | Tepary bean | |
| Phaseolus vulgaris | Dry bean | |
| Vigna radiata | Mung bean | |
| Vigna mungo | Black gram | |
| Vigna umbellate | Rice bean | |
| Vigna unguiculata | Cowpea | |
| Vicieae | Lens culinaris | Lentil |
| Pisum sativum | Pea | |
| Vicia faba | Broad bean | |
| Cicereae | Cicer arietinum | Chickpea |
| Dalbergieae | Arachis hypogeae | Peanut |
| Genisteae | Lupinus luteus | Yellow lupin |
| Indigofereae | Cyamopsis tetragonoloba | Guar |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




