1. Introduction
The population growth and increasing waste generation in low, medium and high income lands poses a huge challenge concerning the waste management and its final destination [
1,
2]. Lignin-cellulosic based biomass is class of waste to be considered, due to its volume generated, particularly those associated to agricultural activities [
3].
Açaí (Euterpe oleracea, Mart.), a palm of native occurrence in the floodplains of the Amazon [
4,
5,
6], has great economic importance not only for the agroindustry, but also for rural communities whose economic income is based on
extractive activities in the state of Pará-Brazil [
7]. The pulp of Açaí (Euterpe oleracea, Mart.) fruits
in nature is processed with warm H
2O to produce a thick, purple juice [
3,
6], generating a residue [
1,
2], the seeds.
The seeds of Açaí (Euterpe oleracea, Mart.), a rich lignin-cellulosic biomass, have great potential for energy and fuels [
8,
9,
10,
11,
12]. In the 2017 crop year, around 1200-1274 million tons of Açaí fruits were produced in Brazil, and the state of Pará the main producer in Brazil (94%), generating a large amount of solid waste [
7,
13].
Pyrolysis is a thermo-chemical process with great potential to convert biomass into energy & fuels [
8,
11]. The reaction products include a gas, a liquid (bio-oil) and a solid (bio-char) [
8,
11]. The yield and properties of reaction products is a function of biomass characteristics, type of pyrolysis process, type of reactors, operating mode, and process parameters, particularly, the temperature, type of catalyst, and catalyst-to-biomass ratio [
14,
15,
16].
In the last years, studies on the pyrolysis of residual Açaí seeds [
8,
11,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29], most of them aiming to produce a carbonaceous solid with activated carbon characteristics, were reported in the literature [
8,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29].
Despite studies on the pyrolysis of residual Açaí seeds
in nature [
17,
18,
19], and activated residual Açaí seeds [
8,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29], until the moment, no systematic study has investigated the effect of acid and alkalis activation of residual Açaí seeds on the yield, chemical composition and acidity of bio-oil and aqueous phase.
This work aims to investigate the effect of temperature and acid (HCl) and alkalis (KOH) chemical activation of residual Açaí seeds on the yield, hydrocarbon content and acidity of bio-oil, as well as on the chemical composition and acidity of aqueous phase by pyrolysis of residual Açaí seeds at 350, 400, and 450 °C and 1.0 atmosphere, activated with aqueous solutions of 2.0 M KOH and HCl, in laboratory scale.
5. Conclusions
XRD analysis of biochar produced by pyrolysis of Açaí seeds at 350, 400, and 450 °C, 1.0 atmosphere, with 2.0 M KOH, in laboratory scale, shows the presence of Kalicinite (KHCO3), the dominant crystalline phase in biochar.
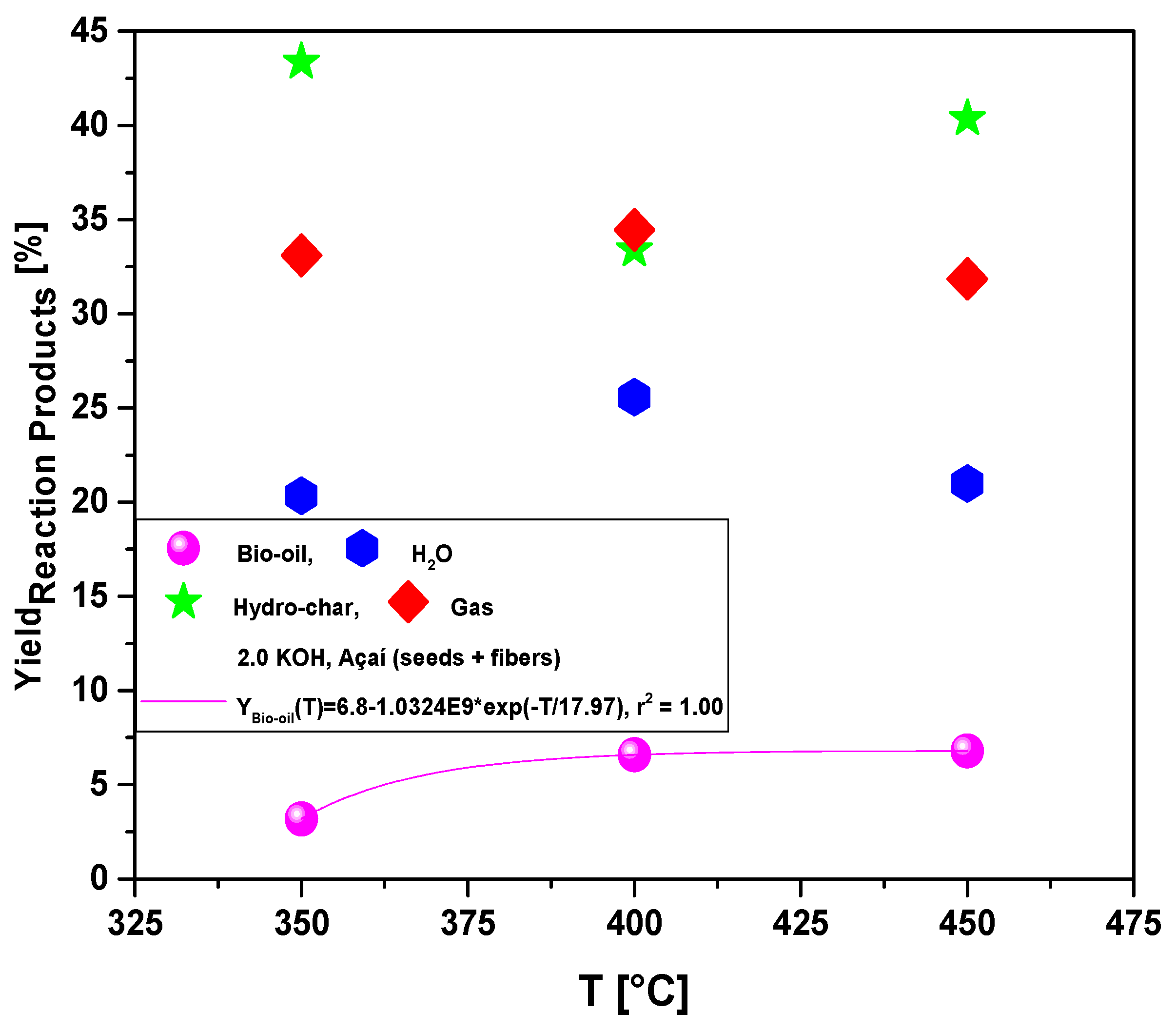
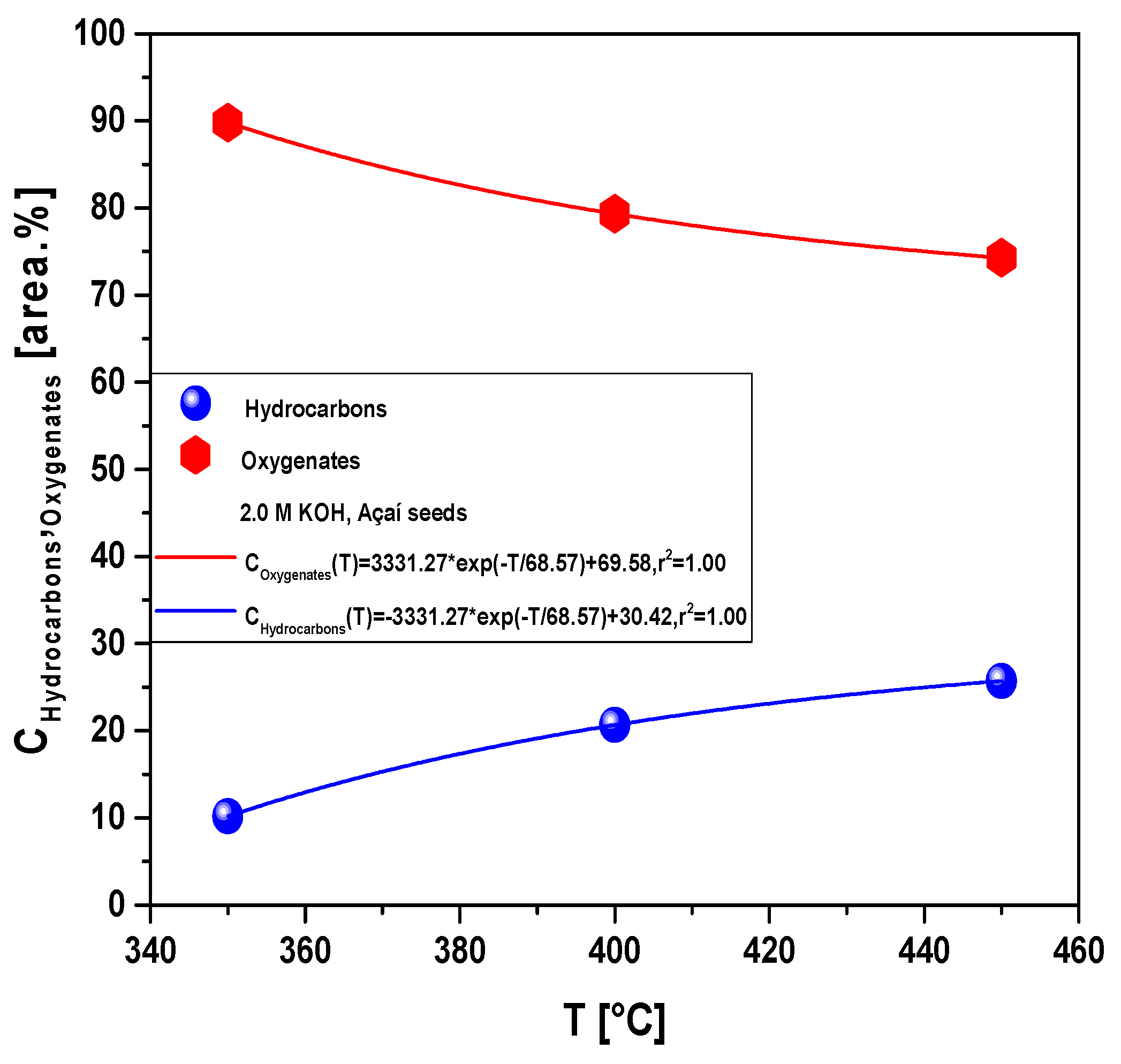
The yields of bio-oil by pyrolysis of activated Açaí seeds at 350, 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH, in laboratory scale, show a smooth increase with temperature, being correlated with a first order exponential decay model. In addition, by increasing the temperature, the concentrations of acyclic saturated/unsaturated hydrocarbons and heterocyclic hydrocarbons in bio-oil increase, while that of oxygenates (cresols, phenols, and ketones) decreases with increasing temperature, showing that higher pyrolysis temperatures favors the formation of hydrocarbons and disfavor the formation of oxygenates [
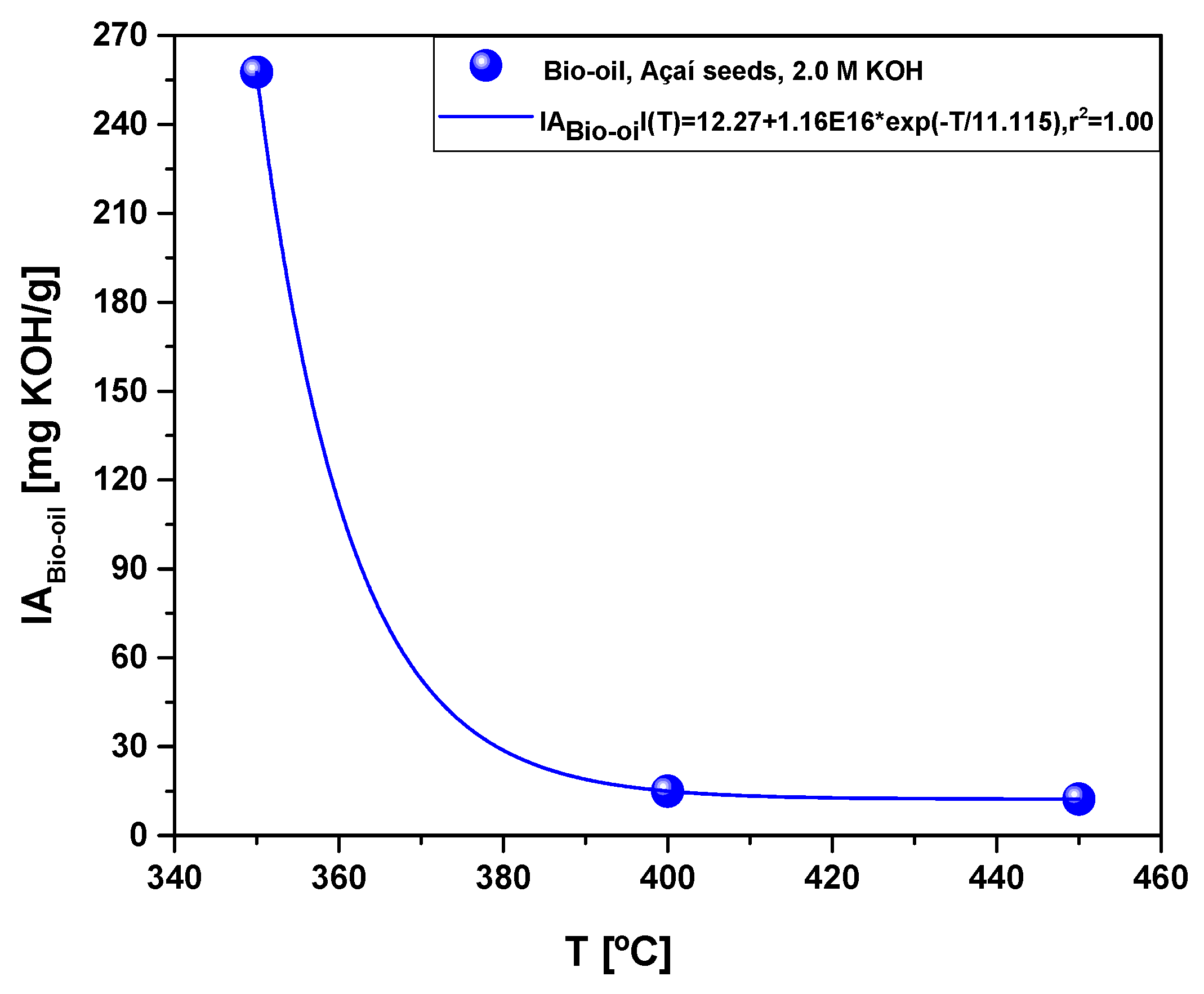
57], as proved by a sharp decrease on the acidity of bio-oil, from 257.6 to 12.3 (mgKOH/g), due to a drastic decrease on the concentration of oxygenates.
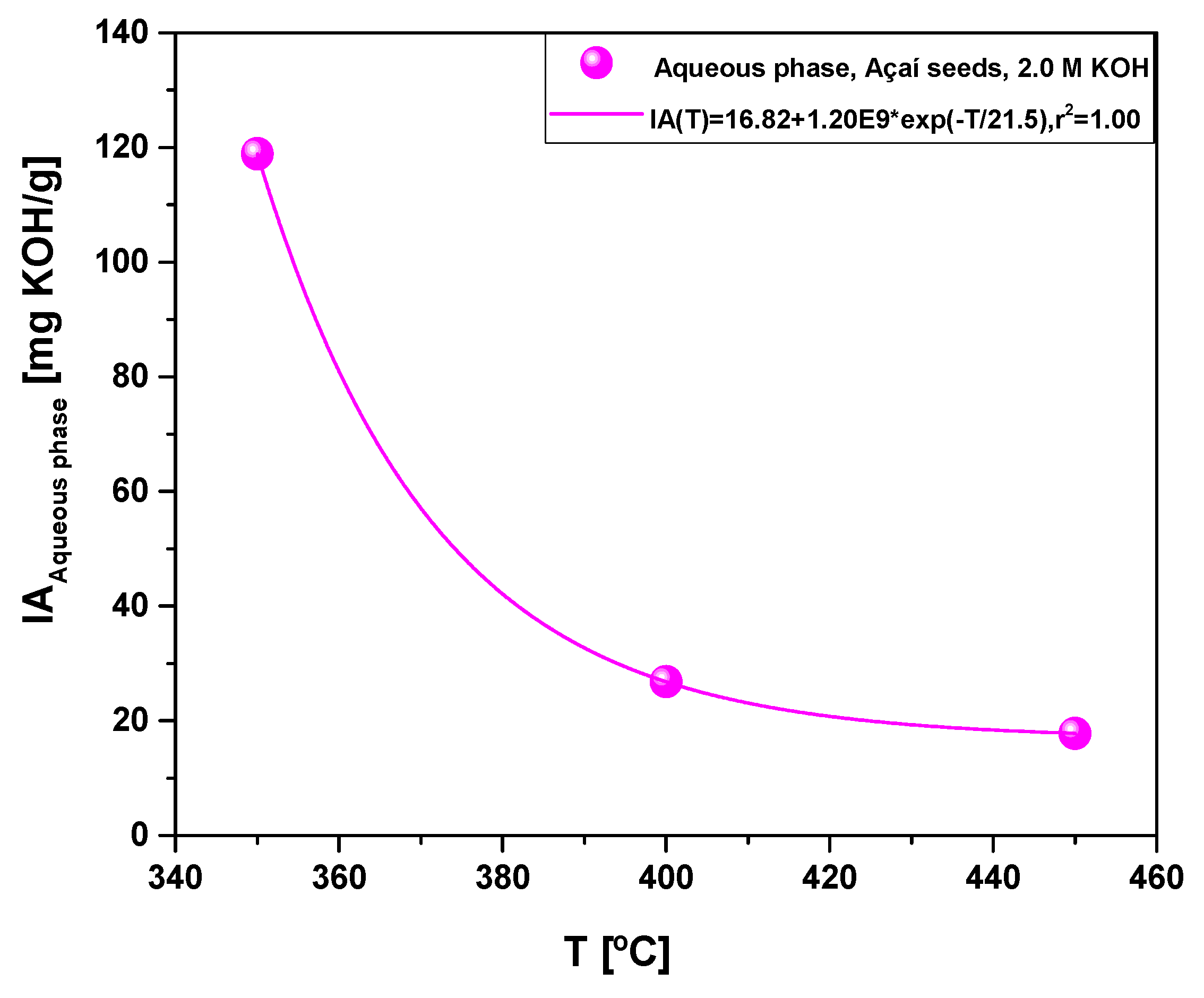
The composition of aqueous phase produced by pyrolysis of activated Açaí seeds at 350, 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH, in laboratory scale, is according to similar studies reported in the literature [
57,
58,
59], being identified the presence of carboxylic acids, ketones, alcohols, phenols, among other compounds. The acidity of aqueous phase decreases sharply with temperature, as the concentration of ketones in the aqueous phase decreases.
The yields of bio-oil by pyrolysis of activated Açaí seeds at 350, 400, and 450 °C, 1.0 atm, activated with 2.0 M HCl, in laboratory scale, decrease with increasing temperature, showing that activation of Açaí seeds with 2.0 M HCl do not favor the thermo-chemical transformation of biomass into bio-oil. In addition, it has not been identified the presence of hydrocarbons in bio-oil, only oxygenates, proving that acid (HCl) pre-treatment of Açaí seeds did not enhance the yield of bio-oil oil nor favors the formation of hydrocarbons in bio-oil.
Supplementary Materials
The following are available. Table S1: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350 °C, 1.0 atmosphere, in laboratory scale. Table S2: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 400 °C, 1.0 atmosphere, in laboratory scale. Table S3: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 450 °C, 1.0 atmosphere, in laboratory scale. Table S4: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350 °C, 1.0 atmosphere, in laboratory scale. Table S5: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 400 °C, 1.0 atmosphere, in laboratory scale. Table S6: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 450 °C, 1.0 atmosphere, in laboratory scale. Table S7: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350 °C, 1.0 atmosphere, in laboratory scale. Table S8: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 400 °C, 1.0 atmosphere, in laboratory scale. Table S9: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 450 °C, 1.0 atmosphere, in laboratory scale. Table S10: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350 °C, 1.0 atmosphere, in laboratory scale. Table S11: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 400 °C, 1.0 atmosphere, in laboratory scale. Table S12: Classes of compounds, summation of peak areas, CAS number, and retention times of chemical compounds identified by CG-MS in aqueous phase by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 450 °C, 1.0 atmosphere, in laboratory scale.
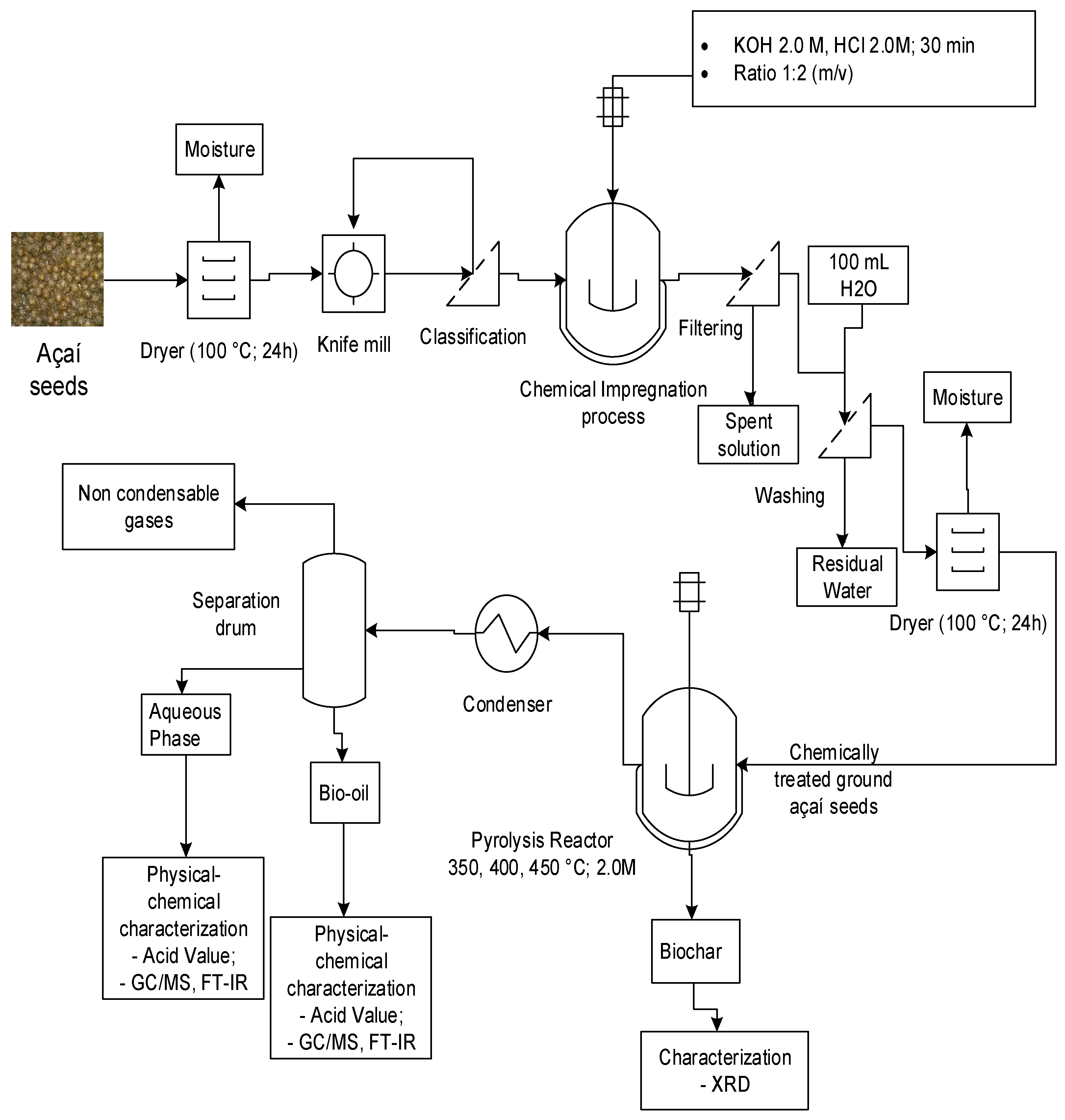
Figure 1.
Process flow schema of bio-oil production by pyrolysis of Açaí seeds at 350, 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH and HCl, using a fixed bed reactor, in laboratory scale.
Figure 1.
Process flow schema of bio-oil production by pyrolysis of Açaí seeds at 350, 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH and HCl, using a fixed bed reactor, in laboratory scale.
Figure 2.
Seeds of Açaí (Euterpe oleracea, Mart.) discharged on the sidewalks and streets by a small store of Açaí commercialization, located in the District of Jurunas, Belém-Pará-Brazil.
Figure 2.
Seeds of Açaí (Euterpe oleracea, Mart.) discharged on the sidewalks and streets by a small store of Açaí commercialization, located in the District of Jurunas, Belém-Pará-Brazil.
Figure 3.
Açaí seeds after drying, milling, and sieving process [Dried Açaí seeds (a); Knife cutting mill (b); Mechanical sieve shaker (c); Dried, grinded and sieved Açaí seeds (d)].
Figure 3.
Açaí seeds after drying, milling, and sieving process [Dried Açaí seeds (a); Knife cutting mill (b); Mechanical sieve shaker (c); Dried, grinded and sieved Açaí seeds (d)].
Figure 4.
Chemical activation of dried, grinded and sieved Açaí seeds with 2.0 M KOH solution [Açaí seeds fine powders mixed with 2.0 M KOH solution (a); washing/filtration of Açaí pasty cake (b); KOH activated Açaí fine powders seeds (c)].
Figure 4.
Chemical activation of dried, grinded and sieved Açaí seeds with 2.0 M KOH solution [Açaí seeds fine powders mixed with 2.0 M KOH solution (a); washing/filtration of Açaí pasty cake (b); KOH activated Açaí fine powders seeds (c)].
Figure 5.
Laboratory scale borosilicate glass pyrolysis reactor.
Figure 5.
Laboratory scale borosilicate glass pyrolysis reactor.
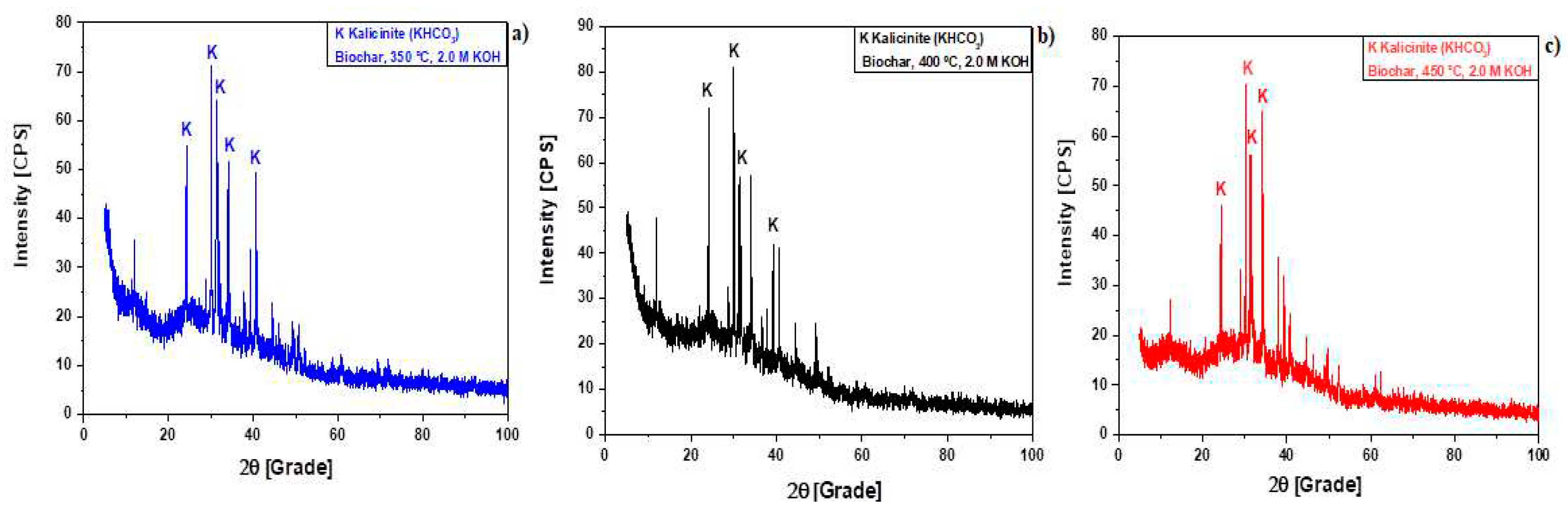
Figure 6.
XRD of biochar produced by pyrolysis of Açaí seeds at 350 , 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH, in laboratory scale [350 °C (a); 400 °C (b); 450 °C (c)].
Figure 6.
XRD of biochar produced by pyrolysis of Açaí seeds at 350 , 400, and 450 °C, 1.0 atm, activated with 2.0 M KOH, in laboratory scale [350 °C (a); 400 °C (b); 450 °C (c)].
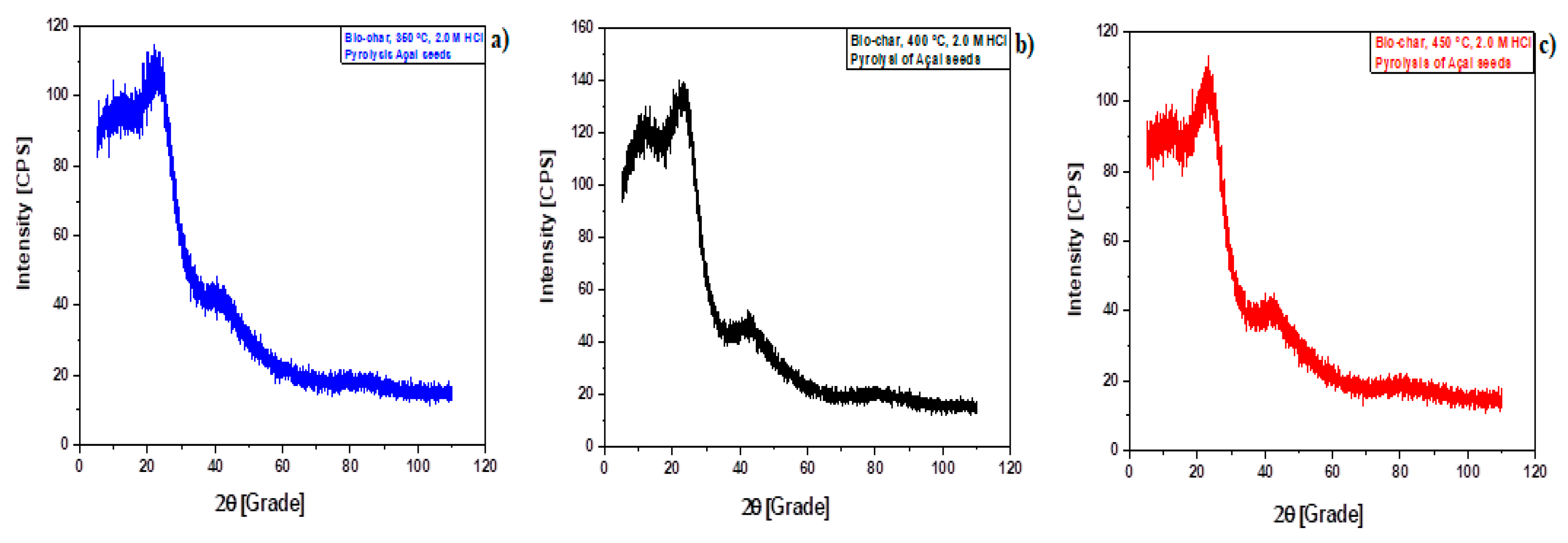
Figure 7.
XRD of biochar produced by pyrolysis of Açaí seeds at 350 , 400, and 450 °C, 1.0 atm, activated with 2.0 M HCl, in laboratory scale [350 °C (a); 400 °C (b); 450 °C (c)].
Figure 7.
XRD of biochar produced by pyrolysis of Açaí seeds at 350 , 400, and 450 °C, 1.0 atm, activated with 2.0 M HCl, in laboratory scale [350 °C (a); 400 °C (b); 450 °C (c)].
Figure 8.
Yield of reaction products (bio-oil, H2O, hydro-char, gas) by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 8.
Yield of reaction products (bio-oil, H2O, hydro-char, gas) by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 9.
Concentration of hydrocarbons and oxygenates in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 9.
Concentration of hydrocarbons and oxygenates in bio-oil by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 10.
Acidity of bio-oil obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 10.
Acidity of bio-oil obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
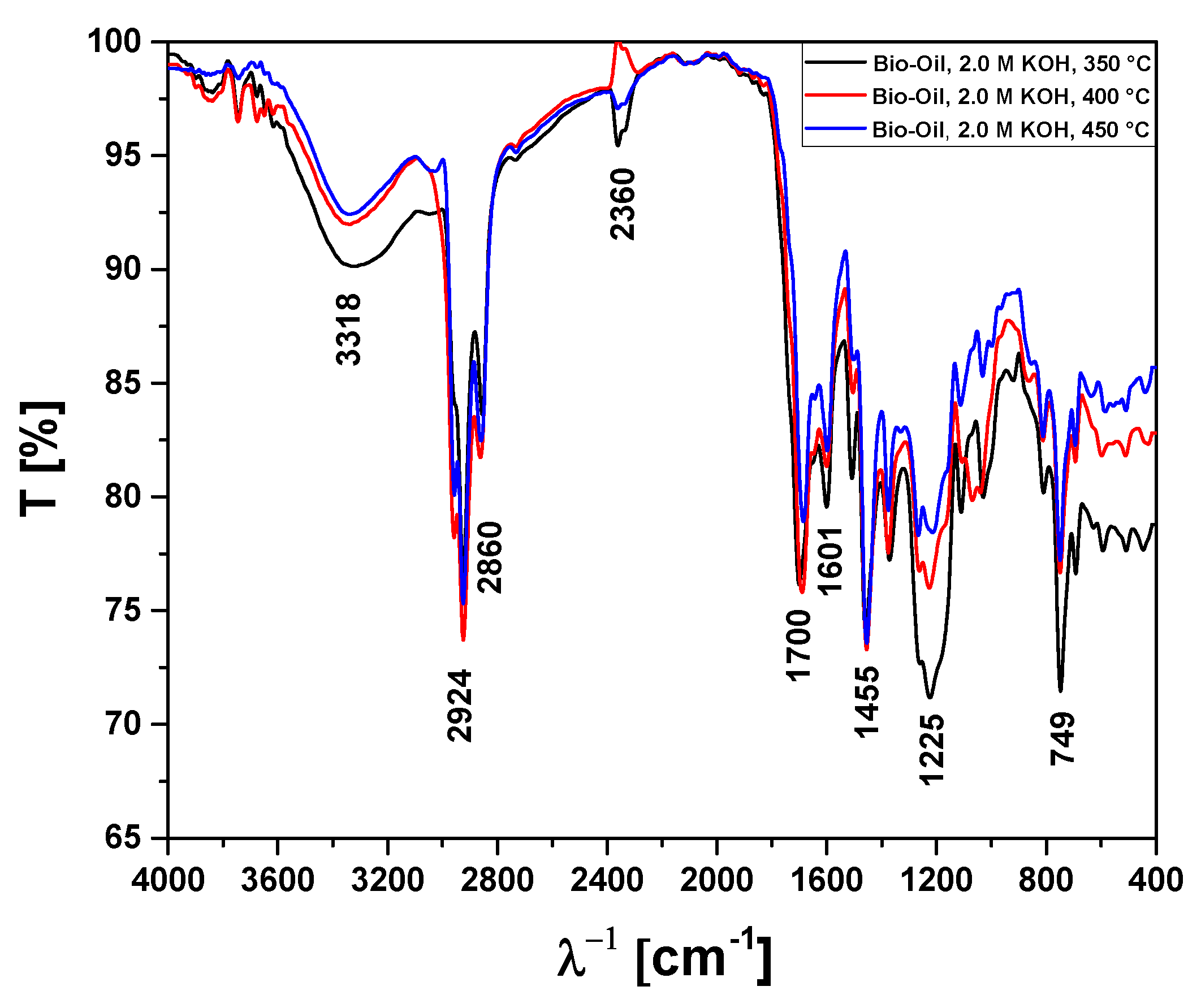
Figure 11.
FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 11.
FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 12.
Acidity of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 12.
Acidity of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
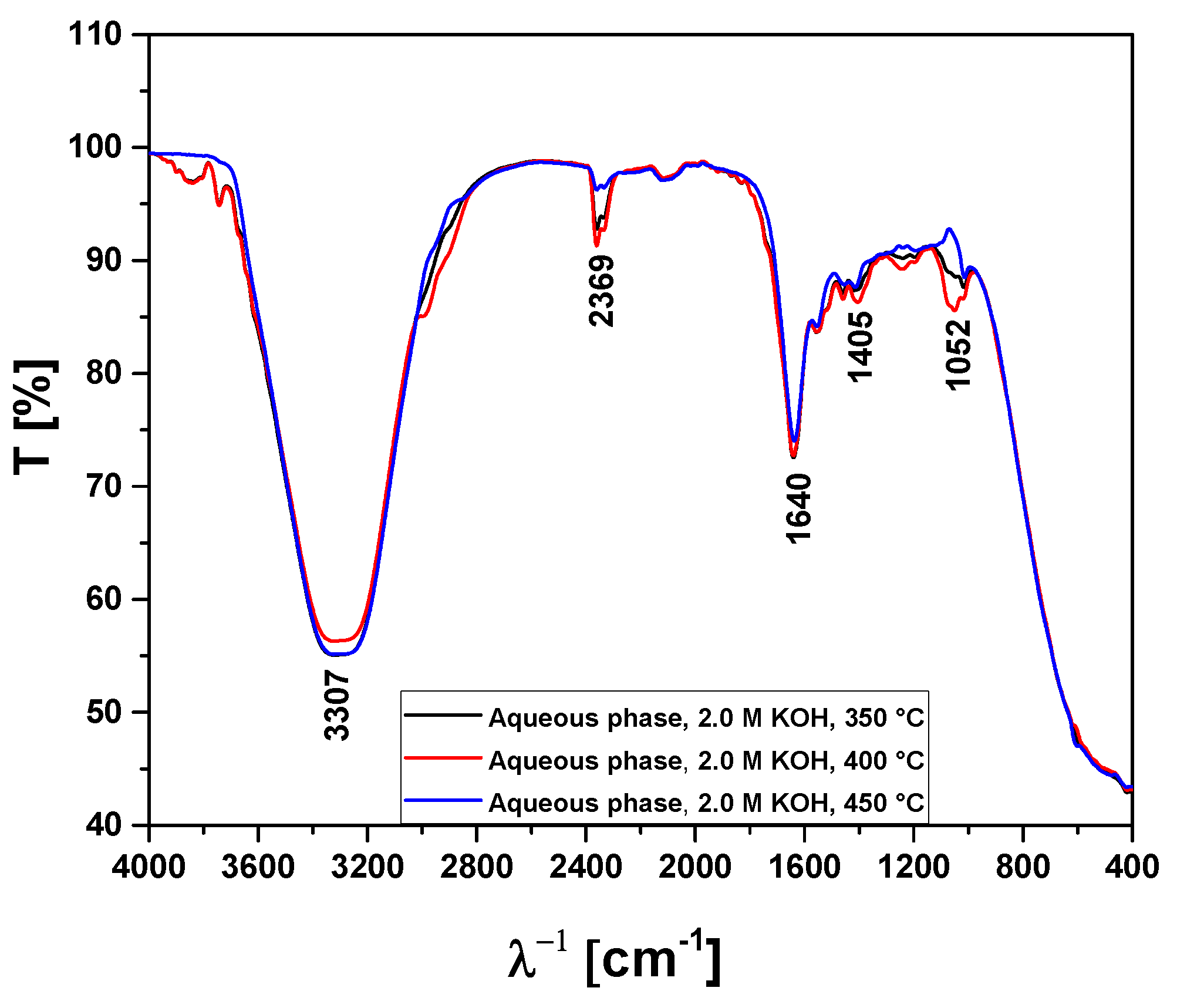
Figure 13.
FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 13.
FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
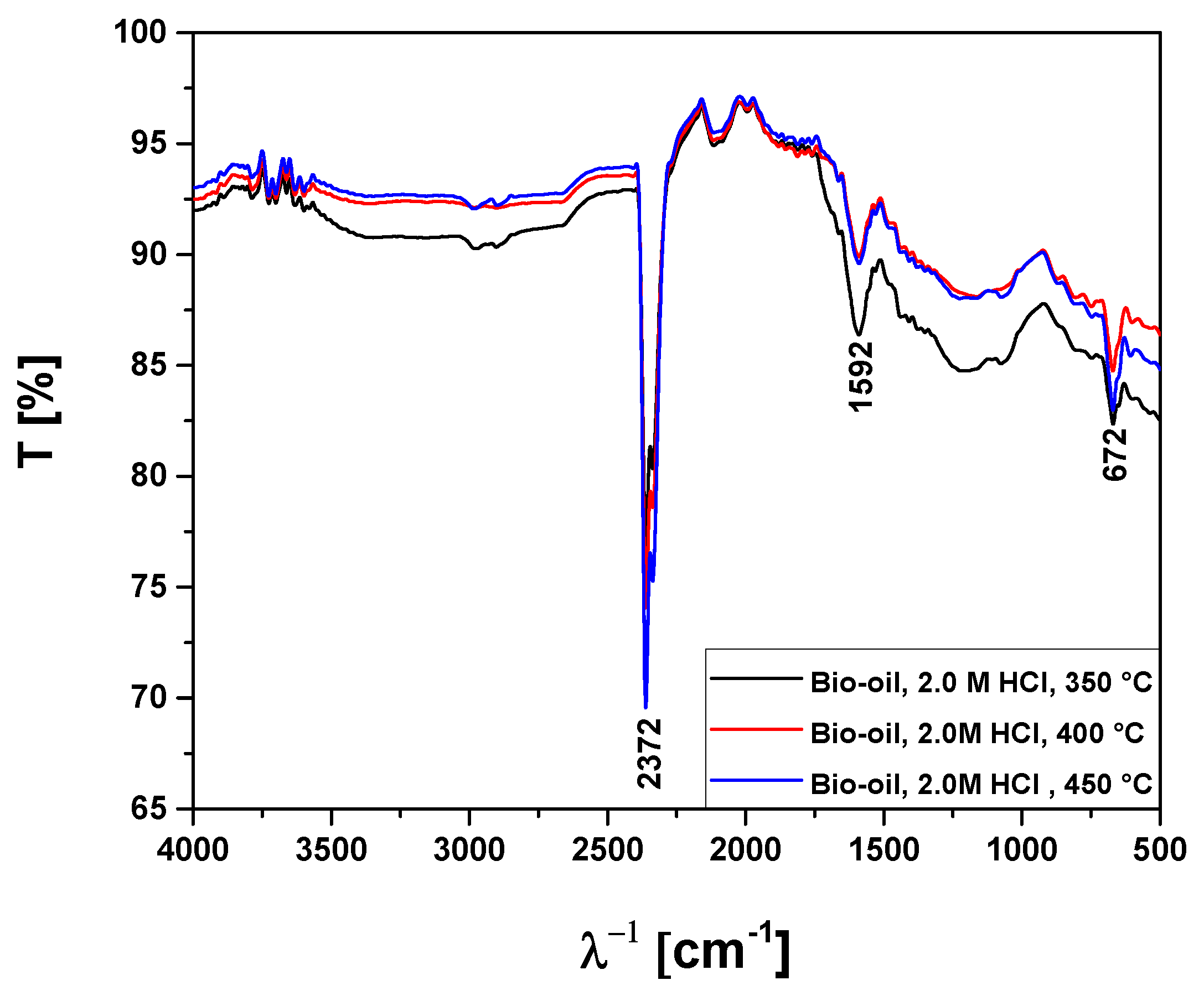
Figure 14.
FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 14.
FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
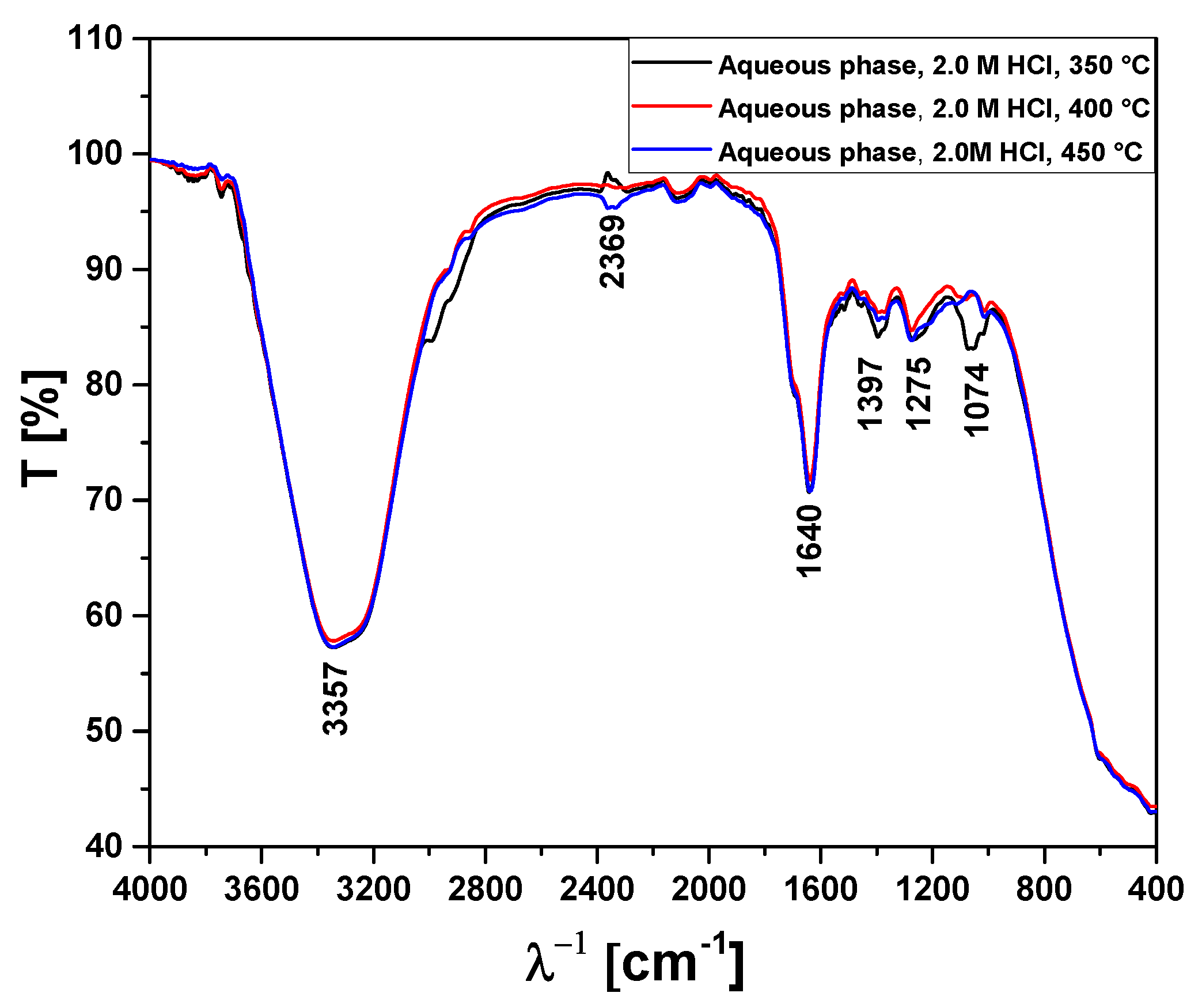
Figure 15.
FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Figure 15.
FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Table 1.
Analysis of peaks characteristics (Intensity, Position: 2θ, Percentage: %) of crystalline phases identified by XRD in bio-chars by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
Table 1.
Analysis of peaks characteristics (Intensity, Position: 2θ, Percentage: %) of crystalline phases identified by XRD in bio-chars by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
| Temperature |
Peaks Intensity, Position (2), and Percentage (%)
|
| Medium |
Medium |
High |
| 2 |
(%) |
2 |
(%) |
2 |
(%) |
| 350 °C |
24.2 |
66.8 |
40.6 |
68.6 |
30.0 |
100 |
| 400 °C |
Medium |
High |
High |
| 2 |
(%) |
2 |
(%) |
2 |
(%) |
| 31.3 |
62.2 |
24.1 |
81.73 |
30.0 |
100 |
| 450 °C |
High |
High |
High |
| 2 |
(%) |
2 |
(%) |
2 |
(%) |
| 30.2 |
100.0 |
31.3 |
79.9 |
34.2 |
92.1 |
Table 2.
Process parameters, mass balances, yields of reaction products (liquids, solids, H2O, and gas), and acidity of bio-oils by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
Table 2.
Process parameters, mass balances, yields of reaction products (liquids, solids, H2O, and gas), and acidity of bio-oils by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
| Process Parameters |
2.0 M KOH |
| 350 °C |
400 °C |
450 °C |
| Mass of Açaí seeds (g) |
40.12 |
40.12 |
40.06 |
| Cracking time (min) |
72 |
72 |
72 |
| Yield of Bio-oil (wt.%) |
3.19 |
6.58 |
6.79 |
| Yield of H2O (wt.%) |
20.34 |
25.57 |
20.99 |
| Yield of Hydro-char (wt.%) |
43.37 |
33.40 |
40.36 |
| Yield of Gas (wt.%) |
33.10 |
34.45 |
31.85 |
| Acidity (mg KOH/g) |
257.6 |
15.0 |
12.3 |
Table 3.
Absorption bands and chemical functions/groups identified by FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Table 3.
Absorption bands and chemical functions/groups identified by FT-IR analysis of bio-oils obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
| Absorption Bands |
Chemical Functions/Chemical Bonds |
| 3400-3200 cm-1
|
ν-OH, hydrogen bonds of alcohol and H2O. |
| 2870-2840 cm-1
|
νs-CH2, methylene group CH2. |
| 2930-2920 cm-1
|
νas-CH2, methylene group CH2. |
| 1709 cm-1
|
ν-C=O, carbonyl group of carboxylic acids and ketones. |
| 1601 cm-1
|
νC=C-C, C=C-C ring-related stretching associated to phenols. |
| 1465-1440 cm-1
|
δasCH3, methyl group (C-H). |
| 1200-1125 cm-1
|
ν-C-O, saturated alcohols (C-O). |
| 1000-650 cm-1
|
γ =C-H, Alkenes (=C-H). |
Table 4.
Chemical composition and acidity (alcohols, carboxylic acids, ketones, phenols, and other oxygenates) of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale, identified by GC-MS.
Table 4.
Chemical composition and acidity (alcohols, carboxylic acids, ketones, phenols, and other oxygenates) of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale, identified by GC-MS.
| Chemical Composition Ci (area.%) |
2.0 M KOH |
| 350 °C |
400 °C |
450 °C |
| Alcohols |
2.34 |
20.74 |
26.62 |
| Carboxylic Acids |
4.05 |
15.02 |
9.23 |
| Ketones |
52.81 |
44.38 |
19.69 |
| Oxygenates |
40.80 |
19.86 |
44.46 |
|
100.00 |
100.00 |
100.00 |
| Acidity (mg KOH/g) |
118.9 |
26.8 |
17.9 |
|
Table 5.
Absorption bonds and chemical functions identified by FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
Table 5.
Absorption bonds and chemical functions identified by FT-IR analysis of aqueous phase obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M KOH solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale.
| Absorption Bands |
Chemical Functions/Chemical Bonds |
| 3400-3200 cm-1
|
-OH, hydrogen bond of alcohols and H2O. |
| 2369 cm-1
|
ass-CO2, axial asymmetric deformation of CO2. |
| 1648-1636 cm-1
|
C=C-C, C=C-C ring-related stretching associated to phenols. |
| 1052 cm-1
|
C-O-C, C-O, C-H, C-O-C bond of esters, C-O bonds, and C-H bonds of benzene rings. |
Table 6.
Process parameters, mass balances, yields of reaction products (liquids, solids, H2O, and gas), and acidity of bio-oils by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
Table 6.
Process parameters, mass balances, yields of reaction products (liquids, solids, H2O, and gas), and acidity of bio-oils by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, using a borosilicate glass reactor, in laboratory scale.
| Process Parameters. |
2.0 M HCl |
| 350 °C |
400 °C |
450 °C |
| Mass of Açaí seeds (g) |
42.100 |
40.480 |
40.433 |
| Cracking time (min) |
72 |
72 |
72.0 |
| Yield of Bio-oil (wt.%) |
3.37 |
2.84 |
2.13 |
| Yield of H2O (wt.%) |
31.19 |
32.85 |
22.91 |
| Yield of Hydro-char (wt.%) |
47.53 |
35.08 |
37.32 |
| Yield of Gas (wt.%) |
17.91 |
29.22 |
37.64 |
| Acidity (mg KOH/g) |
127.1 |
128.9 |
218.5 |
Table 7.
Chemical composition of major compounds (carboxylic acids, phenols) and oxygenates (alcohols, ketones, aldehydes, etc.) of bio-oil obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale, identified by GC-MS.
Table 7.
Chemical composition of major compounds (carboxylic acids, phenols) and oxygenates (alcohols, ketones, aldehydes, etc.) of bio-oil obtained by pyrolysis of Açaí seeds (Euterpe Oleracea, Mart), activated with 2.0 M HCl solution, at 350, 400, and 450 °C, 1.0 atmosphere, in laboratory scale, identified by GC-MS.
| Chemical Composition Ci (area.%) |
2.0 M HCl |
| 350 °C |
400 °C |
450 °C |
| Carboxylic Acids |
53.056 |
43.540 |
61.175 |
| Phenols |
35.945 |
32.700 |
26.682 |
| Oxygenates (alcohols, aldehydes, ketones, cresols) |
10.999 |
23.860 |
12.143 |
|
100.00 |
100.00 |
100.00 |