Submitted:
02 March 2023
Posted:
03 March 2023
You are already at the latest version
Abstract
Keywords:
Introduction
Measurement of Human Intelligence and Neurocognition
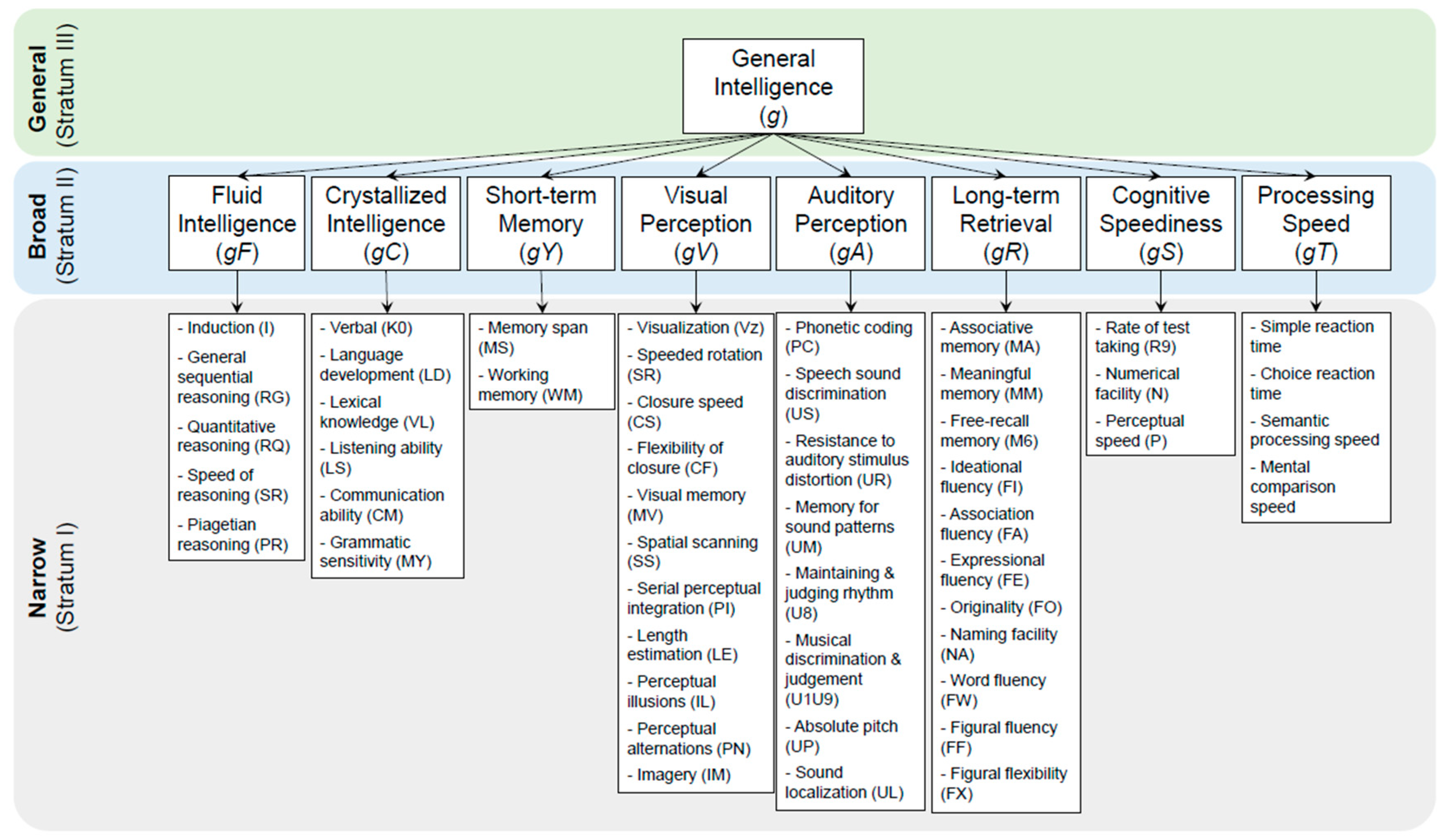
Theories linking brain structure and neurocognitive function
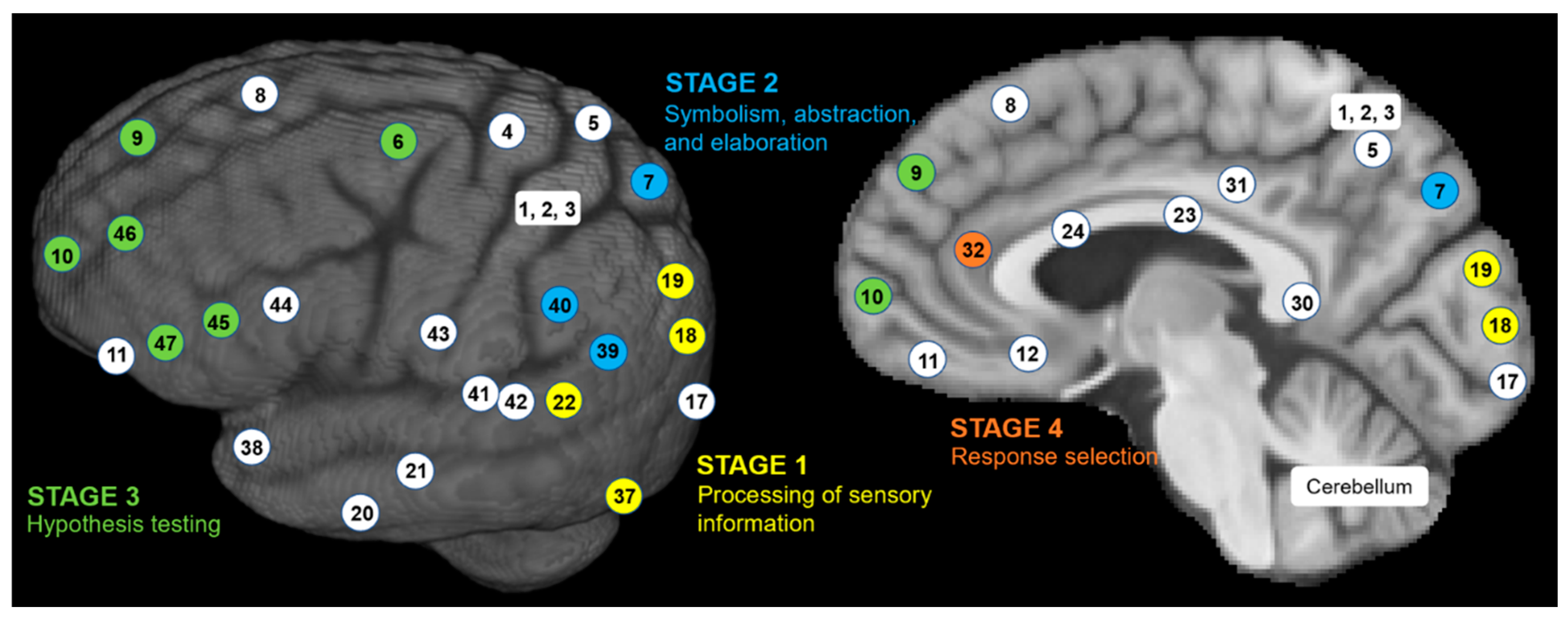
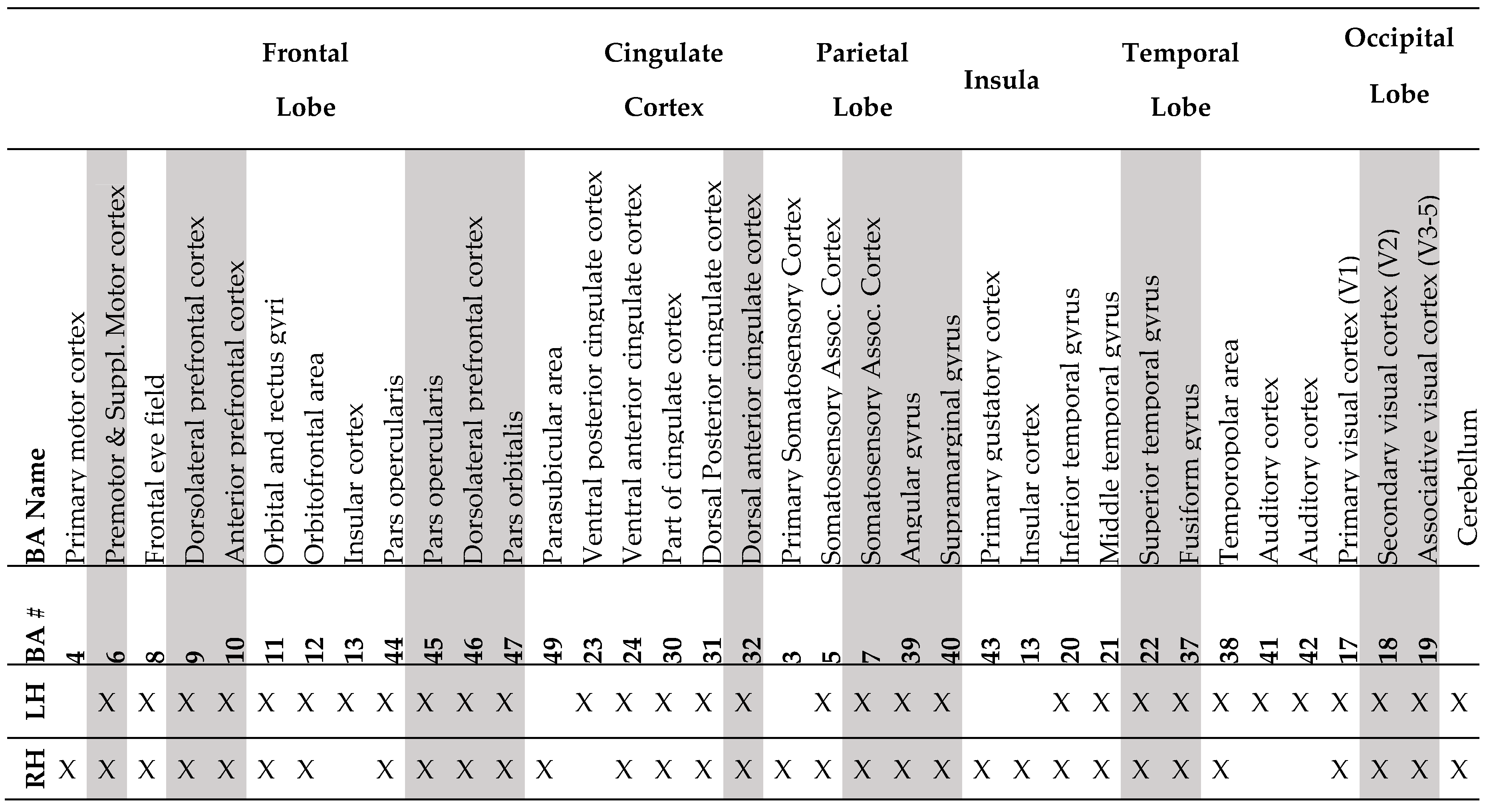
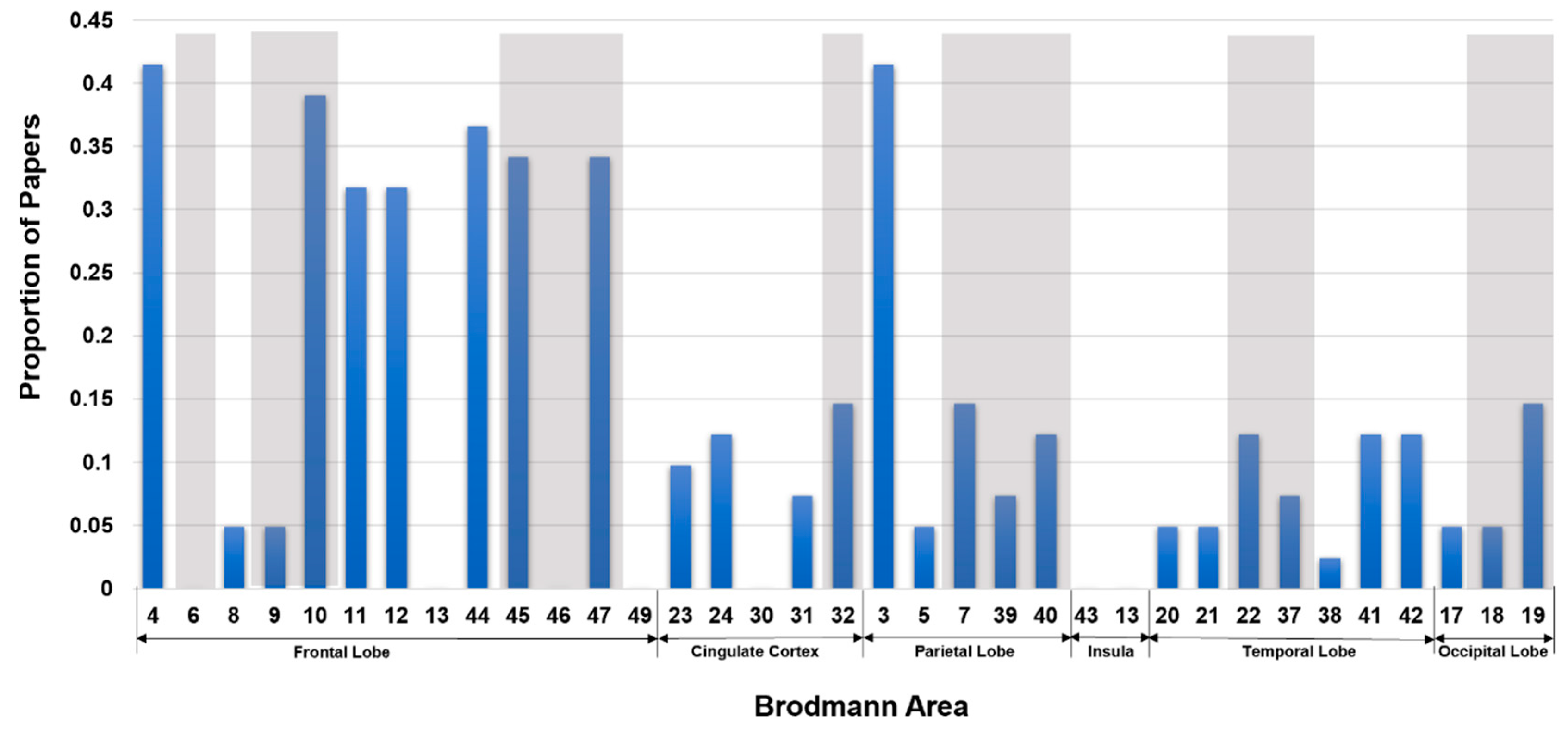
Structural MRI to Infer Intelligence and Neurocognition
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Saha et al.70 | 2021 | 7709 | 9-10 | ABCD | T1-w | CNN learned features and volumes of manually identified brain regions | GM regions of left/right hippocampus, parahippocampal gyrus, thalamus, precentral gyrus and caudate nucleus; WM region of the pons. | 34, 4 | NIH-TCB | Normal | CNN and MLP | Correlation between the actual and predicted gF = 0.1 (p < 0.05) |
| Hilger et al.76 | 2020 | 380 | 18-60 | NKI-Rockland-Enhanced | T1-w | GM volume per voxel | Frontoparietal network, default mode network, Dorsal attention network, and cerebellum | 38, 25, 23, 31, 4, 17, 18, 19, 8, 7, 6, 9 | WASI | Normal | PCA | MSE and correlation between the actual and estimated FSIQ is 320 (p = 0.279) and 0.11, respectively (for true residual FSIQ in the range of [39, 136]) |
| Chiang et al.60 | 2019 | 8669 | 9-10 | ABCD | T1-w | Total volume, mean signal intensity, and entropy | Visual, frontoparietal, somatosensory, motor, default mode network, and cingulo opercular network. | 6, 8, 9, 22, 41, 42, 17, 18, 19, 1, 2, 3, 5, 7, 4 | NIH-TCB | Normal | CNN, and LASSO | Mean Square Error (gF) = 95.38 (for true residual gF in the range of [-40, 30]) |
| Shrivastava et al.61 | 2019 | 8669 | 9-10 | ABCD | T1-w | Volume, mean intensity, and count of GM voxels | Gyrus rectus, hippocampus, inferior frontal gyrus, middle frontal gyrus, postcentral gyrus, precentral gyrus, precuneus, superior frontal gyrus and supramarginal gyrus. | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | CNN, SVR, RF, gradient boosting, and XGBoost | Mean Square Error (gF) = 93.68 (for true residual gF in the range of [-40, 30]) |
| Ren et al.62 | 2019 | 8669 | 9-10 | ABCD | T1-w | ROI volumes | GM | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | Bagging and boosting of LR, RR, RF, envelope-based reduced-rank regression, LASSO, Elastic-Net regressor, and KNN | Mean Square Error (gF) = 92.99 (for true residual gF in the range of [-40, 30]) |
| Tamez-Pena et al.63 | 2019 | 8669 | 9-10 | ABCD | T1-w | ROI volumes | GM, WM, CSF, and cerebellum | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | Ensemble of SVM, RF, and bootstrapped step wise model selection | Mean Square Error (gF) = 100.89 (for true residual gF in the range of [-40, 30]) |
| Brueggeman et al.64 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes | GM, WM, CSF | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | RF | Mean Square Error (gF) = 92.49 (for true residual gF in the range of [-40, 30]) |
| Mihalik et al.65 | 2019 | 8669 | 9-10 | ABCD | T1-w | Voxel intensities and probabilistic tissue-type labels | GM, WM | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | Kernel ridge regressor | Mean Square Error (gF) = 92.13 (for true residual gF in the range of [-40, 30]) |
| Ranjbar et al.66 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes | GM, WM, CSF | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | CNN and RF | Mean Square Error (gF) = 93.64 (for true residual gF in the range of [-40, 30]) |
| Wlaszczyk et al.67 | 2019 | 8669 | 9-10 | ABCD | T1-w | ROI volumes, signal intensity, anterior and posterior cross-sectional area from corpus callosum | GM and corpus callosum |
11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | RF | Mean Square Error (gF) = 92.93 (for true residual gF in the range of [-40, 30]) |
| Zhang-James et al.55 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes | GM, WM, CSF | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | Nu SVM | Mean Square Error (gF) = 95.63 (for true residual gF in the range of [-40, 30]) |
| Kao et al.68 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes | GM, WM, CSF | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | StackNet consisting of random forest, random tree, ridge regressor, and gradient boosting | Mean Square Error (gF) = 94.25 (for true residual gF in the range of [-40, 30]) |
| Li et al.69 | 2019 | 8669 | 9-10 | ABCD | T1-w | ROI volumes, # detected surface holes, the globus pallidus volume, the mean curvatures of precentral gyrus, postcentral gyrus, and banks of Superior Temporal Sulcus | Right posterior cingulate gyrus, left caudate nucleus, entorhinal white matter, globus pallidus, precentral gyrus, postcentral gyrus, and superior temporal sulcus | 23, 31, 28, 4, 1, 2, 3, 22 | NIH-TCB | Normal | BlockPC-XGBoost | Mean Square Error (gF) = 93.16 (for true residual gF in the range of [-40, 30]) |
| Morsing et al.74 | 2018 | 74 | 7-8 | Skane University Hospital in Lund, Sweden | T1-w | ROI volumes | ICV, GM, WM, CSF, and thalamus. | N/A | WISC-III | P-FGR, PT-AGA, and T-AGA | Chi-square and ANOVA | The mean (SD) FSIQ was 80 (17) in the PT-FGR group and 103 (12) in the PT-AGA group |
| Ogawa et al. 75 | 2018 | 232 | 21-69 | Advanced Telecommunication Research Institute International, Kyoto | T1-w | GM volume | Right insula, right middle cingulate cortex/precuneus | 13, 14, 16, 4 | Insight test battery (ITB) | Normal | Pearson correlation | ITB score was positively correlated with the GM volumes in the mentioned region (p < 0.001) |
| Paul et al.73 | 2016 | 211 | 18-44 | University of Illinois Urbana-Champaign | T2-w | Volume fractions across tissue types | GM, WM, CSF | 23, 31 | BOMAT, Number Series, and Letter Set | Normal | Bivariate correlation | GM volume is found positively correlated with quantitative reasoning (r = 0.26; p < 0.01) and working memory (r = 0.21; p < 0.01), and gF (r = 0.16; p < 0.01) |
|
Grazioplene et al.
79 |
2015 | 517 | 18-40 | University of Minnesota, University of New Mexico in Albuquerque, Yale University | T1-w MPRAGE | Caudate volume | Caudate nucleus | N/A | WAIS-III, WAIS-IV, WASI |
Normal | LR | Regression of IQ onto bilateral caudate volume indicated a significant positive correlation between caudate volume and FSIQ (r = 0.24; p = 0.01) |
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Zhang et al.96 | 2020 | 23 | 0-4 | UNC Chapel Hill Early Brain Development Study | T1-w, T2-w |
Cortical thickness, mean curvature, local gyrification index, vertex area, vertex volume, sulcal depth in string distance, and sulcal depth in Euclidean distance | Parcellation of the cerebral cortex into 70 anatomically meaningful ROIs | Not specified | VR, FM, RL, EL, and ELC (MSEL) | Normal | CNN | RMSE between the predicted and actual VR, FM, RL, and EL scores is 0.067 |
| Li et al.90 | 2020 | 68 | 8 | Arkansas Children's Nutrition Center | T1-w | Gray matter volume, surface area, and cortical thickness | Orbitofrontal gyrus, transverse temporal gyri, left superior temporal gyrus, and right anterior cingulate gyrus | 11, 12, 41, 42, 22, 24, 32, 33 | RIAS | Normal | Spearman’s correlation test | RIAS scores showed significant correlations (r = [0.38-0.44], p = [0.005-0.046]) with cortical metrices |
| Tadayon et al.91 | 2020 | 740 | 21-35 | HCP | T1-w | Cortical thickness, cortical surface area, and cortical gyrification | Superior parietal, left supramarginal, left caudal middle frontal, left pars-opercularis, left inferior temporal, right inferior and middle temporal, right medial orbitofrontal, and right rostral middle frontal regions | 7, 40, 22, 44, 20, 21, 11, 12, 10 | PMAT and NIH-TCB | Normal | Linear regression | Correlation between the local gyrification, and surface area with gF and gC are 0.29 and 0.22 (p < 0.001), 0.28 and 0.28 (p < 0.001), respectively |
| Oxtoby et al.84 | 2019 | 8669 | 9-10 | ABCD | T1-w | Cortical morphology as graph | A structural co-variance network graph considers small cortical regions (3 voxels cubed) as nodes, and structural similarity (morphology) between nodes as edges. | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | Event-based model of progression, and SVR | Mean Square Error (gF) = 93.83 (for true residual gF in the range of [-40, 30]) |
| Rebsamen et al.85 | 2019 | 8669 | 9-10 | ABCD | T1-w | Subcortical volumes, cortical thicknesses, curvatures, and surface areas | Middle temporal gyrus, superior temporal gyrus | 21, 22 | NIH-TCB | Normal | SVR | Mean Square Error (gF) = 93.03 (for true residual gF in the range of [-40, 30]) |
| Valverde et al.86 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes in the gray matter, white matter, and cerebrospinal fluid, 78 contrast and 78 cortical thickness measures, gender, age, and scanner manufacturer | Gray matter, white matter, and cerebrospinal fluid | Not specified | NIH-TCB | Normal | Fully connected neural network | Mean Square Error (gF) = 94.02 (for true residual gF in the range of [-40, 30]) |
| Pölsterl et al.87 |
2019 | 8669 | 9-10 | ABCD | T1-w | Cortical thickness and volumes of 122 ROIs in the gray matter, white matter, and cerebrospinal fluid | Left/right parahippocampal gyrus, pons white matter, hippocampus, posterior cingulate gyrus, cuneus, left lingual gyrus, left middle frontal gyrus, supramarginal gyrus, right fusiform gyrus, superior temporal gyrus, right anterior cingulate gyrus, etc. |
34, 23, 31, 19, 10, 40, 37, 22, 24, 32, 33 | NIH-TCB | Normal | An ensemble of gradient boosted trees, and a linear ridge regressor. | Mean Square Error (gF) = 94.25 (for true residual gF in the range of [-40, 30]) |
| Pölsterl et al.88 | 2019 | 8669 | 9-10 | ABCD | T1-w | Cortical thickness and volumes of 122 ROIs in the gray matter, white matter, and cerebrospinal fluid | Left/right parahippocampal gyrus, pons white matter, hippocampus, posterior cingulate gyrus, cuneus, left lingual gyrus, left middle frontal gyrus, supramarginal gyrus, right fusiform gyrus, superior temporal gyrus, right anterior cingulate gyrus, etc. |
34, 23, 31, 19, 10, 40, 37, 22, 24, 32, 33 | NIH-TCB | Normal | AutoML ensembles of 14 classifiers | Mean Square Error (gF) = 94.25 (for true residual gF in the range of [-40, 30]) |
| Guerdan et al.89 | 2019 | 8669 | 9-10 | ABCD | T1-w | Volume, elongation, surface area, roundness, and flatness of grey matter ROIs. | Gray matter, white matter, and cerebrospinal fluid |
Not specified | NIH-TCB | Normal | LASSO, ridge regressor, SVR, gradient boosting, and AdaBoost regressors. | Mean Square Error (gF) = 94.48 (for true residual gF in the range of [-40, 30]) |
| Girault et al.93 | 2019 | 487 | 1-2 | University of North Carolina (UNC) Chapel Hill Early Brain Development Study | T1-w, T2-w |
Cortical thickness, and surface area | Bilateral superior frontal and middle frontal gyri, right medial superior frontal gyrus, right occipital superior gyrus, bilateral superior parietal cortices, left primary motor cortex, bilateral anterior cingulate and precuneus, and right superior and middle temporal cortices areas | 10, 19, 7, 4, 24, 32, 33. 22 |
GM, VR, FM, RL, EL, and ELC (MSEL) | Normal | Pearson correlation, Linear mixed effect model | Correlations between average cortical thickness at age 1 and GM, FM, EL, and RL scores at age 1 (r = 0.137, p = 0.025; r = 0.186, p = 0.002; r = 0.147, p = 0.016; r = 0.120, p = 0.049, respectively), |
| Adeli et al.94 | 2019 | 24 | 0-4 | UNC Chapel Hill Early Brain Development Study | T1-w, T2-w, DWI |
Cortical thickness, mean curvature, local gyrification index, vertex area, vertex volume, sulcal depth in string distance, and sulcal depth in Euclidean distance | Parcellation of the cerebral cortex into 70 anatomically meaningful ROIs | Not specified | VR, FM, RL, EL, and ELC (MSEL) | Normal | Multi-task multi-linear regression | RMSE between the predicted and actual VR, FM, RL, and EL scores is 0.18. |
| Zhang et al.95 | 2018 | 23 | 0-4 | UNC Chapel Hill Early Brain Development Study | T1-w, T2-w |
Cortical thickness, mean curvature, local gyrification index, vertex area, vertex volume, sulcal depth in string distance, and sulcal depth in Euclidean distance | Parcellation of the cerebral cortex into 70 anatomically meaningful ROIs | Not specified | VR, FM, RL, EL, and ELC (MSEL) | Normal | Multi-task multi-linear regression | RMSE between the predicted and actual VR, FM, RL, and EL score is 0.158. |
| Bajaj et al.92 | 2018 | 56 | 18-45 | McLean Hospital and Partners Healthcare, and the U.S. Army Human Research Protections Office | T1-w | Cortical thickness, cortical surface area, cortical volume, and cortical gyrification | Posterior frontal, superior and inferior parietal lobes, left insula, and inferior frontal gyrus | 7, 39, 40, 13, 14, 16, 44, 45, 47 | WASI-II | Normal | Generalized linear model | Significant positive relationships between thicker cortex and higher IQ at a liberal CFT of p < 0.05 as well as at a strict CFT of p < 0.01 is observed. |
| Wang et al. 99 | 2015 | 164 | 6-15 | ABIDE | T1-w | Cortical thickness, surface area, sulcal depth, curvature |
Bilateral transverse temporal gyri, bilateral thalamus, left parahippocampal gyrus, left hippocampus, right opercular part of inferior frontal gyrus, left anterior cingulate gyrus, right amygdala, left lingual gyrus, left superior parietal lobule, right inferior parietal lobule, left angular gyrus, left paracentral lobule, and left caudate nucleus |
41, 42, 34, 44, 45, 47, 32, 7, 40, 39, 1, 2, 3, 4 | - | Normal | Multi/single kernel support vector regressor | Correlation between the actual and estimated IQ is 68.4% |
| Squeglia et al.97 | 2013 | 185 | 12-14 | San Diego area public middle schools | T1-w | Cortical thickness | Left and right inferior parietal cortices, and left and right superior parietal cortices | 39, 40, 7 | WISC-III, WAIS-IV |
Normal | Hierarchical linear regressions | For both males and females, thinner parietal association cortices corresponded with better neurocognitive functioning above and beyond age alone. |
| Yang et al. 98 | 2013 | 78 | 17-27 | Seoul National University, Catholic University of Korea | T1-w | Cortical thickness, surface area, sulcal depth and absolute mean curvature in 78 parcellated ROIs | Cerebral cortex |
34, 35, 37 | WAIS | Normal | Partial least square regression |
Correlation between the Actual and predicted FSIQ is 30% (p < 0.01) |
| Choi et al. 100 | 2008 | 225 | 20.9±2.9 | Seoul National University, Catholic University of Korea | T1-w | The thickness of the gray matter of the cerebral cortex | Gray matter of cerebral cortex | 38, 20, 21, 40 | WASI, RPM-II | Normal | Multivariate regression model | gC is correlated to cortical thickness and gF is related to BOLD signals. |
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Hidese et al.104 | 2020 | 266 | 45.6±12.9 | Volunteer data from Kodaira city, Tokyo | T1-w, DTI |
Regional gray matter volumes in the VBM and the white matter FA values in the DTI | Left gyrus rectus and anterior cingulate gyrus, left posterior insula, left superior and middle frontal gyri | 11, 24, 32, 33, 13, 14, 16, 10 | WAIS-III | Normal | Pearson correlation | VIQ correlated positively with the specified brain regional volumes with p < 0.005. |
| McDermott et al.105 | 2019 | 623 | 5-25 | National Institute of Mental Health Intramural Research Program | T1-w | Surface-based shape | Left inferior and middle temporal, left inferior parietal, and left medial frontal regions | 20, 21, 39, 40, 25 | WASI, WISC, WAIS | Normal | Linear mixed-effect model | Positive associations (β > 100; p < 0.001) between FSIQ and cortical anatomy is observed. |
| Ramsden et al.106 | 2011 | 33 | 14.1±1.0 | Department of Psychological Sciences, Birkbeck College, University of London | T1-w | Changes in gray matter density | Motor speech area, and anterior cerebellum | 4, 6 | WISC, WAIS | Normal | Linear regression | Correlation between change in VIQ and change in grey matter density were 0.876 (p < 0.01) for high, 0.797 (p < 0.05) for average and 0.660 (p < 0.05) for low ability groups, respectively. For PIQ, correlation was 0.492 (p > 0.05) for high, 0.788 (p < 0.05) for average and 0.715 (p < 0.01) for low ability groups, respectively. |
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Chiang et al.60 | 2019 | 8669 | 9-10 | ABCD | T1-w | Total volume, mean signal intensity, and entropy | Visual, fronto-parietal, somatosensory, motor, default mode network, and cingulo opercular network. | 6, 8, 9, 22, 41, 42, 17, 18, 19, 1, 2, 3, 5, 7, 4, 6 | NIH-TCB | Normal | CNN, and LASSO | Mean Square Error (gF) = 95.38 (for true residual gF in the range of [-40, 30]) |
| Ranjbar et al.66 | 2019 | 8669 | 9-10 | ABCD | T1-w | 122 ROI volumes in the gray matter, white matter, and cerebrospinal fluid | Gray matter, white matter, and cerebrospinal fluid | 11, 44, 45, 47, 4, 1, 2, 3, 10, 12, 40 | NIH-TCB | Normal | CNN and random forest | Mean Square Error (gF) = 93.64 (for true residual gF in the range of [-40, 30]) |
| Vang et al.107 | 2019 | 8669 | 9-10 | ABCD | T1-w | CNN-learned features | Gray matter, white matter, and cerebrospinal fluid |
Not specified | NIH-TCB | Normal | CNN with gradient boosting machine | Mean Square Error (gF) = 96.18 (for true residual gF in the range of [-40, 30]) |
| Pominova et al.108 | 2019 | 8669 | 9-10 | ABCD | T1-w | CNN-learned features | Gray matter | Not specified | NIH-TCB | Normal | VoxCNN | Mean Square Error (gF) = 93.838 (for true residual gF in the range of [-40, 30]) |
| Zou et al.109 | 2019 | 8669 | 9-10 | ABCD | T1-w | CNN-learned features | Bilateral transverse temporal gyri, bilateral thalamus, left parahippocampal gyrus, left hippocampus, right opercular part of inferior frontal gyrus, left anterior cingulate gyrus, right amygdala, left lingual gyrus, left superior parietal lobule, right inferior parietal lobule, left angular gyrus, left paracentral lobule, and left caudate nucleus. | 41, 42, 34, 44, 45, 47, 24, 32, 33, 19, 7, 39, 40, 1, 2, 3, 4 |
NIH-TCB | Normal | 3D CNN | Mean Square Error (gF) = 92.74 (for true residual gF in the range of [-40, 30]) |
| Liu et al.110 | 2019 | 8669 | 9-10 | ABCD | T1-w | CNN-learned features | Skull-stripped whole brain | Not specified | NIH-TCB | Normal | UNet-like encoder/decoder | Mean Square Error (gF) = 102.25 (for true residual gF in the range of [-40, 30]) |
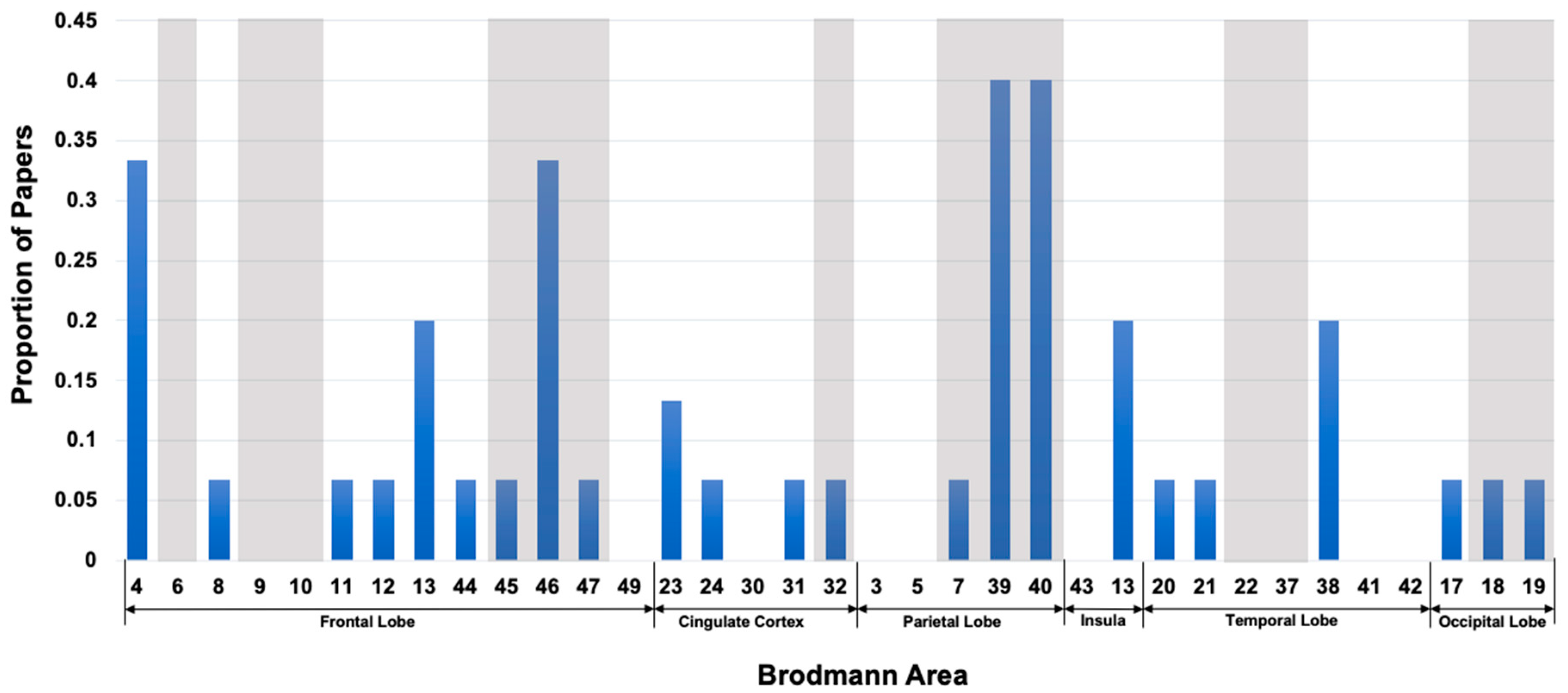
Diffusion MRI to Infer Intelligence and Neurocognition
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Malpas et al.114 | 2016 | 91 | 18-55 | Nathan Kline Institute/Rockland Sample | DTI | FA | 42 Brodmann regions were specified in each hemisphere | 1, 3, 4, 5, 6, 7, 8, 9, 11, 24, 25, 29, 32, 44, 45, 46, 47, 13, 22, 34, 35, 36, 38, 41, 42, 39, 40, 43, 17, 18 | WASI | Normal |
t statistic regression analysis |
FA was positively correlated with FSIQ with r = 0.53 (95% CI 0.35–0.66). |
| Konrad et al. 116 | 2012 | 30 | 22.8±1.5 | Institute of Neuroradiology of the Johannes Gutenberg University Mainz, Germany | T1-w, DTI |
FA, MD | Left-hemispheric Brocaʼs area |
44, 45, 22 | Hamburg–Wechsler Intelligenztest (HAWIE-R) - equivalent to WAIS-R | Normal | Voxel-wise t statistic regression analysis, Pearson correlation |
VIQ performance is negatively correlated to the FA in the mentioned regions (r = - 0.73; p < 0.001). |
| Feng et al.115 | 2019 | 38 | 0-2 | Arkansas Children’s Nutrition Center | DTI | FA | White matter tracts | Not specified | BSID-III | Normal | Voxel-wise tract-based spatial statistics (TBSS) |
Correlations between FA at 2 weeks of age and BSID subfields scores at 2 years of age are 0.35~0.48. |
| Casson et al.117 | 2014 | 45 | 30-60 | Wayne State University | T1-w, SWI, DTI | FA-based dysarthria, pyramidal system dysfunction, extrapyramidal system dysfunction, and cerebellar dysfunction | Gray matter, white matter, and cerebrospinal fluid | Not specified | MMSE | Normal/ abnormal | Chi-square test | The number of football-related concussions was associated with isolated neurocognitive abnormalities in 24% of population. |
| Adeli et al.94 | 2019 | 24 | 0-4 | UNC Chapel Hill Early Brain Development Study | T1-w, T2-w, DWI |
Cortical thickness, mean curvature, local gyrification index, vertex area, vertex volume, sulcal depth in string distance, and sulcal depth in Euclidean distance | Parcellation of the cerebral cortex into 70 anatomically meaningful ROIs | Not specified | VR, FM, RL, EL, and ELC (MSEL) | Normal | Multi-task multi-linear regression | Correlation between predicted and true ELC is 0.70 (p < 0.001) |
| Lee et al.118 | 2017 | 535 | 0-2 | UNC Chapel Hill Early Brain Development Study | DTI | Axial diffusivity (AD), radial diffusivity (RD), and FA |
White matter | Not specified | MSEL: ELC | Normal | Distance correlation coefficient | Correlation between AD, RD and FA with ELC are 0.13~0.20 (p < 0.05) |
| Zhang et al. 119 | 2019 | 1076 | - | HCP | DWI | Count of streamlines, connected surface area (CSA) and weighted CSA, mean and maximum values of FA and MD, cluster number, average length, and mean deviations from a template streamline | ROIs in the whole cortex |
Not specified |
Raven's Progressive Matrices |
Normal | Tensor network principal components analysis |
Correlation between actual and estimated gF is 24.11% (p < 0.001). |
Functional MRI to Infer Intelligence and Neurocognition
| Study | Year | N |
Age (years) |
Dataset | MRI type | MRI features | Regions | Probable BAs |
IQ/ Neuro. Test |
Normal/ Abnormal |
Method |
Correlation/ Finding |
| Song et al. 122 | 2008 | 59 | 18.5–33.3 | Xuanwu Hospital of Capital Medical University | fMRI | Functional connectivity | bilateral dorsolateral prefrontal cortices | 9 | WAIS | Normal | Stepwise linear regression | FSIQ is correlated to the functional connectivity in bilateral dorsolateral prefrontal cortices (r = 0.47; p = 0.0002). |
| Kwak et al.123 | 2021 | 795 | 46-96 | OASIS-3, KSHAP | T1-w, fMRI |
Functional connectivity from BOLD signals | Region of frontoparietal network and central brain | 9, 4, 39, 40, 46, 10, 13, 1, 2, 3 | MMSE | Normal | Ridge regression | Correlation between behavioral test scores and FC-predicted score is 0.12~0.44 (p < 0.001). |
| Finn et al. 124 | 2015 | 126 | 22-35 | Human Connectome Project (HCP) | fMRI | Positive and negative edges, frontoparietal networks | Frontoparietal region | 9, 4, 39, 40, 46, 10, 13 | Raven's Progressive Matrices | Normal | CPM | Correlation between actual and estimated gF is 0.5 (p < 0.01) |
| Powell et al. 125 | 2017 | 841 | 22-37 | HCP | fMRI | Voxel-wise local structural connectome | Region of frontoparietal network | 9, 4, 39, 40, 46, 10, 13 | NIH-TCB | Normal | LASSO Principal Component Regressor | Correlation between the actual and predicted NIH picture sequence memory test is 0.097 (p < 0.001) |
| Sripada et al. 126 | 2020 | 2013 | 9-10 | ABCD | fMRI | Resting-state functional connectivity pattern | Default mode network, frontoparietal network, salience network, dorsal attention network | 8, 9, 10, 21, 28, 36, 23, 24, 32, 29, 30, 31, 39, 40 | NIH-TCB | Normal | Brain basis set (BBS) modeling (combination of PCA and linear regression) | General neurocognitive ability score is highly correlated to the mentioned networks (r = 0.31; p < 0.0001) |
| Jiang et al. 127 | 2017 | 360 | 17-24 | University of Electronic Science and Technology, China | fMRI | Functional connectivity | Superior frontal gyrus, inferior and superior parietal lobules |
10, 11, 12, 39, 40, 7 | WAIS-RC | Normal | RelieF+LASSO | Correlation between actual and estimated FSIQ is 51% (p < 0.001) |
| Hart et al.128 | 2006 | 25 | 14-29 | UNC Pediatric Endocrinology Turner Syndrome Clinic | fMRI | Activated voxels in fMRI | Left and right middle frontal gyri, inferior frontal gyri, intraparietal sulci and inferior temporal gyri | 10, 44, 45, 47, 20 | WASI | Normal/ abnormal |
ANOVA | Individuals with Turner syndrome and controls had significantly different verbal IQs (p < 0.0001) |
| Greene et al. 129 | 2018 | 1086 | 8-36 | HCP, Philadelphia Neurodevelopmental Cohort (PNC) | fMRI | Whole brain functional connectivity | Cortical and subcortical grey matter, cerebellum | Not specified | Raven's Progressive Matrices | Normal | CPM |
Correlation between actual and estimated gF is 19% in resting state (p = 0.039) |
| He et al. 131 | 2018 | 9821 | 22-69 | HCP, UK-Biobank | fMRI | Functional Connectivity Matrix | Whole-brain spatially independent components | Not specified | Raven's Progressive Matrices | Normal | Kernel Regression, Feedforward NN, CNN | Correlation between actual and estimated gF is 23.9% (p < 0.001) using the Kernel regression |
| Li et al. 132 | 2018 | 100 | - | HCP | fMRI | Amplitude of low-frequency fluctuation of left anterior cingulate cortex | Right prefrontal cortex, left anterior cingulate cortex | 8, 24, 32, 33 | Raven's Progressive Matrices | Normal | Support vector regressor | Correlation between actual and estimated gF is 32.5% (p = 0.031) |
| Dubois et al. 133 | 2018 | 884 | 22-36 | HCP | fMRI | Functional Connectivity Matrix | Cortical and subcortical grey matter | Not specified | Raven's Progressive Matrices | Normal | Univariate correlation filtering + Elastic net regression | Correlation between actual and estimated gF is 22% using the univariate model (p < 0.001) |
| Yoo et al. 134 | 2019 | 575 | 22-56 | HCP | fMRI | Functional Connectivity Matrix | Regions of frontoparietal and default mode networks | 9, 4, 39, 40, 46, 10, 13, 38, 25, 23, 31 | Raven's Progressive Matrices | Normal | CPM-based Multivariate distance correlation | Correlation between actual and estimated cognitive ability is 9.5% (p < 0.01) |
| Noble et al. 135 | 2017 | 618 | 22-56 | HCP | fMRI | 10 functionally coherent networks | Whole gray matter | Not specified | Raven's Progressive Matrices | Normal | CPM | Correlation between actual and estimated gF is 22% (p < 0.0001) |
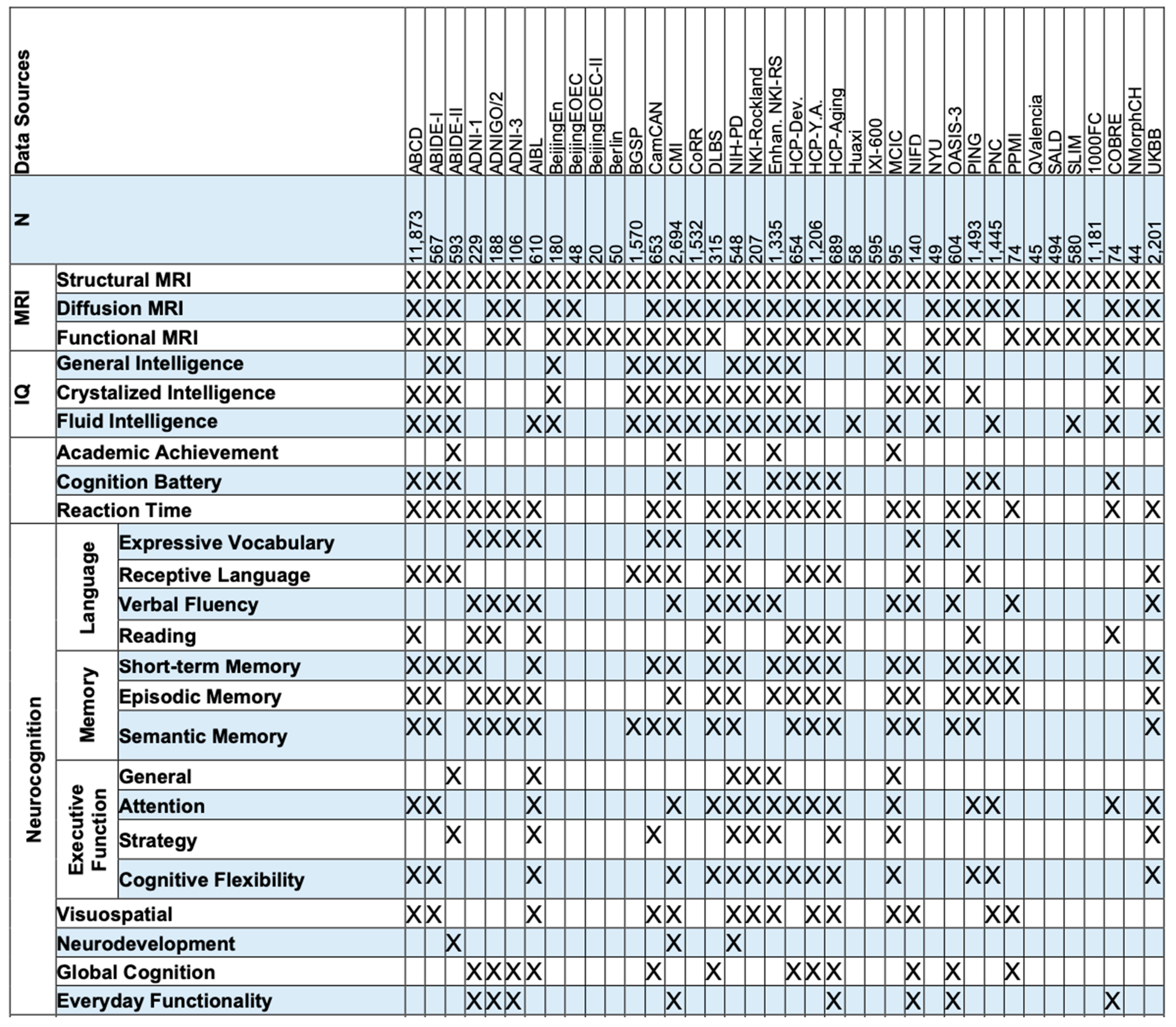
Opportunities and Challenges
Conclusion
References
- Morley JE, Morris JC, Berg-Weger M, et al. Brain health: the importance of recognizing cognitive impairment: an IAGG consensus conference. J Am Med Dir Assoc. 2015, 16, 731–739. [Google Scholar] [CrossRef]
- Latal B, Patel P, Liamlahi R, Knirsch W, Tuura RO, von Rhein M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr Res. 2016, 80, 531–537. [Google Scholar] [CrossRef]
- Kessler N, Feldmann M, Schlosser L, et al. Structural brain abnormalities in adults with congenital heart disease: prevalence and association with estimated intelligence quotient. Int J Cardiol. 2020, 306, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Watson CG, Stopp C, Wypij D, Bellinger DC, Newburger JW, Rivkin MJ. Altered white matter microstructure correlates with IQ and processing speed in children and adolescents post-fontan. J Pediatr. 2018, 200, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Dubois J, Galdi P, Paul LK, Adolphs R. A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Philos Trans R Soc B Biol Sci. 2018, 373, 20170284. [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Liamlahi R, Latal B. Neurodevelopmental outcome of children with congenital heart disease. Handb Clin Neurol. 2019, 162, 329–345. [Google Scholar]
- Urschel S, Bond GY, Dinu IA, et al. Neurocognitive outcomes after heart transplantation in early childhood. J Heart Lung Transplant. 2018, 37, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Pallas, SL. Intrinsic and extrinsic factors that shape neocortical specification. Trends Neurosci. 2001, 24, 417–423. [Google Scholar] [CrossRef]
- Spitzka, EA. Brain-weight, cranial capacity and the form of the head, and their relations to the mental powers of man. Science. 1903, 17, 753–754. [Google Scholar] [CrossRef]
- Pol HEH, Schnack HG, Posthuma D, et al. Genetic contributions to human brain morphology and intelligence. J Neurosci. 2006, 26, 10235–10242. [Google Scholar] [CrossRef] [PubMed]
- Rushton JP, Ankney CD. Whole brain size and general mental ability: a review. Int J Neurosci. 2009, 119, 692–732. [Google Scholar] [CrossRef] [PubMed]
- Deary IJ, Bastin ME, Pattie A, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006, 66, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK. Cognitive functions correlate with white matter architecture in a normal pediatric population: a diffusion tensor MRI study. Hum Brain Mapp. 2005, 26, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Jensen, AR. Clocking the Mind: Mental Chronometry and Individual Differences. Elsevier; 2006.
- Poldrack RA, Gorgolewski KJ. Making big data open: data sharing in neuroimaging. Nat Neurosci. 2014, 17, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Graham SA, Lee EE, Jeste DV, et al. Artificial intelligence approaches to predicting and detecting cognitive decline in older adults: A conceptual review. Psychiatry Res. 2020, 284, 112732. [Google Scholar] [CrossRef]
- Dizaji AS, Vieira BH, Khodaei MR, et al. Linking Brain Biology to Intellectual Endowment: A Review on the Associations of Human Intelligence With Neuroimaging Data. Basic Clin Neurosci. 2021, 12, 1. [Google Scholar] [CrossRef]
- Naef N, Schlosser L, Brugger P, et al. Brain volumes in adults with congenital heart disease correlate with executive function abilities. Brain Imaging Behav. Published online 2021, 1–9. [Google Scholar]
- Fontes K, Rohlicek CV, Saint-Martin C, et al. Hippocampal alterations and functional correlates in adolescents and young adults with congenital heart disease. Hum Brain Mapp. 2019, 40, 3548–3560. [Google Scholar] [CrossRef]
- Pike NA, Roy B, Moye S, et al. Reduced hippocampal volumes and memory deficits in adolescents with single ventricle heart disease. Brain Behav. 2021, 11, e01977. [Google Scholar] [CrossRef]
- Ehrler M, Latal B, Kretschmar O, von Rhein M, Tuura RO. Altered frontal white matter microstructure is associated with working memory impairments in adolescents with congenital heart disease: a diffusion tensor imaging study. NeuroImage Clin. 2020, 25, 102123. [Google Scholar] [CrossRef] [PubMed]
- McGrew, KS. CHC Theory and the Human Cognitive Abilities Project: Standing on the Shoulders of the Giants of Psychometric Intelligence Research. Elsevier; 2009.
- Carroll, JB. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. Cambridge University Press; 1993.
- Horn JL, Cattell RB. Refinement and test of the theory of fluid and crystallized general intelligences. J Educ Psychol. 1966, 57, 253. [Google Scholar] [CrossRef] [PubMed]
- Spearmen, C. General intelligence objectively determined and measured. Am J Psychol. 1904, 15, 107–197. [Google Scholar] [CrossRef]
- Cattell, RB. Theory of fluid and crystallized intelligence: A critical experiment. J Educ Psychol. 1963, 54, 1. [Google Scholar] [CrossRef]
- Schneider WJ, McGrew KS. The Cattell–Horn–Carroll theory of cognitive abilities. Published online 2018.
- McGrew, K. Cattell-Horn-Carroll CHC (Gf-Gc) Theory: Past, Present & Future.
- Kaufman, AS. Contemporary Intellectual Assessment: Theories, Tests, and Issues. Guilford Publications; 2018.
- Hartman, DE. Wechsler Adult Intelligence Scale IV (WAIS IV): return of the gold standard. Appl Neuropsychol. 2009, 16, 85–87. [Google Scholar] [CrossRef] [PubMed]
- Benson N, Hulac DM, Kranzler JH. Independent examination of the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV): what does the WAIS-IV measure? Psychol Assess. 2010, 22, 121. [Google Scholar] [CrossRef]
- Assessing the psychometric utility of IQ scores: A tutorial using the Wechsler intelligence scale for children–fifth edition. Sch Psychol Rev. Published online 2021, 1-15.
- Wechsler, D. WASI-II: Wechsler Abbreviated Scale of Intelligence. PsychCorp; 2011.
- Woodcock RW, McGrew KS, Mather N, Schrank FA. Woodcock-Johnson III diagnostic supplement to the tests of cognitive abilities. Itasca IL Riverside. 2003, 10, 003435520104400407. [Google Scholar]
- Bornman J, Romski M, Tonsing K, et al. Adapting and translating the Mullen Scales of Early Learning for the South African context. S Afr J Commun Disord. 2018, 65, 1–9. [Google Scholar]
- dos Santos ESL, de Kieviet JF, Königs M, van Elburg RM, Oosterlaan J. Predictive value of the Bayley scales of infant development on development of very preterm/very low birth weight children: a meta-analysis. Early Hum Dev. 2013, 89, 487–496. [Google Scholar] [CrossRef]
- Kubinger, KD. Psychologische Diagnostik: Theorie Und Praxis Psychologischen Diagnostizierens. Hogrefe Verlag; 2006.
- Wegenschimmel B, Leiss U, Veigl M, et al. Do we still need IQ-scores? Misleading interpretations of neurocognitive outcome in pediatric patients with medulloblastoma: a retrospective study. J Neurooncol. 2017, 135, 361–369. [Google Scholar] [CrossRef]
- Zgaljardic DJ, Temple RO. Neuropsychological Assessment Battery (NAB): Performance in a sample of patients with moderate-to-severe traumatic brain injury. Appl Neuropsychol. 2010, 17, 283–288. [Google Scholar] [CrossRef]
- Akshoomoff N, Beaumont JL, Bauer PJ, et al. VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 2013, 78, 119–132. [Google Scholar] [CrossRef] [PubMed]
- Denboer JW, Nicholls C, Corte C, Chestnut K. National Institutes of Health Toolbox Cognition Battery. Oxford University Press; 2014.
- Barbey, AK. Network neuroscience theory of human intelligence. Trends Cogn Sci. 2018, 22, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends Neurosci. 2000, 23, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J. The multiple-demand (MD) system of the primate brain: mental programs for intelligent behaviour. Trends Cogn Sci. 2010, 14, 172–179. [Google Scholar] [CrossRef]
- Kovacs K, Conway AR. Process overlap theory: A unified account of the general factor of intelligence. Psychol Inq. 2016, 27, 151–177. [Google Scholar] [CrossRef]
- Jung RE, Haier RJ. The Parieto-Frontal Integration Theory (P-FIT) of intelligence: converging neuroimaging evidence. Behav Brain Sci. 2007, 30, 135–154. [Google Scholar] [CrossRef]
- Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010, 11, 201–211. [Google Scholar] [CrossRef]
- Colom R, Karama S, Jung RE, Haier RJ. Human intelligence and brain networks. Dialogues Clin Neurosci. Published online 2022.
- Lerch JP, Van Der Kouwe AJ, Raznahan A, et al. Studying neuroanatomy using MRI. Nat Neurosci. 2017, 20, 314–326. [Google Scholar] [CrossRef]
- Fischl, B. FreeSurfer. Neuroimage. 2012, 62, 774–781. [Google Scholar] [CrossRef]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. Fsl. Neuroimage. 2012, 62, 782–790. [Google Scholar] [CrossRef]
- Cox, RW. AFNI: what a long strange trip it’s been. Neuroimage. 2012, 62, 743–747. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff SB, Yeo BT, Genon S. Imaging-based parcellations of the human brain. Nat Rev Neurosci. 2018, 19, 672–686. [Google Scholar] [CrossRef]
- Zhang-James Y, Glatt SJ, Faraone SV. Nu Support Vector Machine in Prediction of Fluid Intelligence Using MRI Data. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 92-98.
- Chen H, Dou Q, Yu L, Qin J, Heng PA. VoxResNet: Deep voxelwise residual networks for brain segmentation from 3D MR images. NeuroImage. 2018, 170, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Tsukahara JS, Harrison TL, Draheim C, Martin JD, Engle RW. Attention control: The missing link between sensory discrimination and intelligence. Atten Percept Psychophys. 2020, 82, 3445–3478. [Google Scholar] [CrossRef] [PubMed]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. J Cogn Neurosci. 2000, 12, 1–47. [Google Scholar] [CrossRef]
- MacDonald AW, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Chiang JN, Reggente N, Dell’Italia J, Zheng ZS, Lutkenhoff ES. Predicting Fluid Intelligence Using Anatomical Measures Within Functionally Defined Brain Networks. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 143-149.
- Srivastava S, Eitel F, Ritter K. Predicting fluid intelligence in adolescent brain mri data: An ensemble approach. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 74-82.
- Ren H, Wang X, Wang S, Zhang Z. Predict Fluid Intelligence of Adolescent Using Ensemble Learning. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 66-73.
- Tamez-Pena J, Orozco J, Sosa P, Valdes A, Nezhadmoghadam F. Ensemble of svm, random-forest and the bswims method to predict and describe structural associations with fluid intelligence scores from t1-weighed mri. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 47-56.
- Brueggeman L, Koomar T, Huang Y, et al. Ensemble Modeling of Neurocognitive Performance Using MRI-Derived Brain Structure Volumes. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 124-132.
- Mihalik A, Brudfors M, Robu M, et al. ABCD Neurocognitive Prediction Challenge 2019: predicting individual fluid intelligence scores from structural MRI using probabilistic segmentation and kernel ridge regression. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 133-142.
- Ranjbar S, Singleton KW, Curtin L, et al. Sex differences in predicting fluid intelligence of adolescent brain from T1-weighted MRIs. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 150-157.
- Wlaszczyk A, Kaminska A, Pietraszek A, Dabrowski J, Pawlak MA, Nowicka H. Predicting Fluid Intelligence from Structural MRI Using Random Forest regression. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 83-91.
- Kao PY, Zhang A, Goebel M, Chen JW, Manjunath BS. Predicting Fluid Intelligence of Children using T1-weighted MR Images and a StackNet. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 9-16.
- Li T, Wang X, Luo T, et al. Adolescent Fluid Intelligence Prediction from Regional Brain Volumes and Cortical Curvatures Using BlockPC-XGBoost. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019, 167-175.
- Saha S, Pagnozzi A, Bradford D, Fripp J. Predicting fluid intelligence in adolescence from structural MRI with deep learning methods. Intelligence. 2021, 88, 101568. [Google Scholar] [CrossRef]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychon Bull Rev. 2002, 9, 637–671. [Google Scholar] [CrossRef]
- Conway AR, Cowan N, Bunting MF, Therriault DJ, Minkoff SR. A latent variable analysis of working memory capacity, short-term memory capacity, processing speed, and general fluid intelligence. Intelligence. 2002, 30, 163–183. [Google Scholar] [CrossRef]
- Paul EJ, Larsen RJ, Nikolaidis A, et al. Dissociable brain biomarkers of fluid intelligence. Neuroimage. 2016, 137, 201–211. [Google Scholar] [CrossRef]
- Morsing E, Malova M, Kahn A, et al. Brain Volumes and Developmental Outcome in Childhood Following Fetal Growth Restriction Leading to Very Preterm Birth. Front Physiol. 2018, 9, 1583. [Google Scholar] [CrossRef]
- Ogawa T, Aihara T, Shimokawa T, Yamashita O. Large-scale brain network associated with creative insight: combined voxel-based morphometry and resting-state functional connectivity analyses. Sci Rep. 2018, 8, 1–11. [Google Scholar]
- Hilger K, Winter NR, Leenings R, et al. Predicting intelligence from brain gray matter volume. Brain Struct Funct. 2020, 225, 2111–2129. [Google Scholar] [CrossRef] [PubMed]
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002, 25, 563–593. [Google Scholar] [CrossRef] [PubMed]
- Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. J Cogn Neurosci. 2006, 18, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Grazioplene RG, G. Ryman S, Gray JR, Rustichini A, Jung RE, DeYoung CG. Subcortical intelligence: Caudate volume predicts IQ in healthy adults. Hum Brain Mapp. 2015, 36, 1407–1416. [Google Scholar] [CrossRef]
- Westlye LT, Walhovd KB, Bjørnerud A, Due-Tønnessen P, Fjell AM. Error-related negativity is mediated by fractional anisotropy in the posterior cingulate gyrus—a study combining diffusion tensor imaging and electrophysiology in healthy adults. Cereb Cortex. 2009, 19, 293–304. [Google Scholar] [CrossRef]
- Bar M, Tootell RB, Schacter DL, et al. Cortical mechanisms specific to explicit visual object recognition. Neuron. 2001, 29, 529–535. [Google Scholar] [CrossRef] [PubMed]
- McCandliss BD, Cohen L, Dehaene S. The visual word form area: expertise for reading in the fusiform gyrus. Trends Cogn Sci. 2003, 7, 293–299. [Google Scholar] [CrossRef]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nat Rev Neurosci. 2003, 4, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Oxtoby NP, Ferreira FS, Mihalik A, et al. ABCD Neurocognitive Prediction Challenge 2019: Predicting individual residual fluid intelligence scores from cortical grey matter morphology. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:114-123.
- Rebsamen M, Rummel C, Mürner-Lavanchy I, Reyes M, Wiest R, McKinley R. Surface-Based Brain Morphometry for the Prediction of Fluid Intelligence in the Neurocognitive Prediction Challenge 2019. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:26-34.
- Valverde JM, Imani V, Lewis JD, Tohka J. Predicting intelligence based on cortical WM/GM contrast, cortical thickness and volumetry. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:57-65.
- Pölsterl S, Gutiérrez-Becker B, Sarasua I, Roy AG, Wachinger C. Prediction of Fluid Intelligence from T1-Weighted Magnetic Resonance Images. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:35-46.
- Pölsterl S, Gutiérrez-Becker B, Sarasua I, Roy AG, Wachinger C. An AutoML Approach for the Prediction of Fluid Intelligence from MRI-Derived Features. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:99-107.
- Guerdan L, Sun P, Rowland C, et al. Deep learning vs. classical machine learning: A comparison of methods for fluid intelligence prediction. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:17-25.
- Li T, McCorkle GS, Williams DK, Badger TM, Ou X. Cortical Morphometry is Associated with Neuropsychological Function in Healthy 8-Year-Old Children. J Neuroimaging. 2020, 30, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Tadayon E, Pascual-Leone A, Santarnecchi E. Differential contribution of cortical thickness, surface area, and gyrification to fluid and crystallized intelligence. Cereb Cortex. 2020, 30, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Bajaj S, Raikes A, Smith R, et al. The relationship between general intelligence and cortical structure in healthy individuals. Neuroscience. 2018, 388, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Girault JB, Cornea E, Goldman BD, et al. Cortical structure and cognition in infants and toddlers. Cereb Cortex. 2020, 30, 786–800. [Google Scholar] [CrossRef]
- Adeli E, Meng Y, Li G, Lin W, Shen D. Multi-task prediction of infant cognitive scores from longitudinal incomplete neuroimaging data. NeuroImage. 2019, 185, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang C, Adeli E, Wu Z, Li G, Lin W, Shen D. Infant brain development prediction with latent partial multi-view representation learning. IEEE Trans Med Imaging. 2018, 38, 909–918. [Google Scholar]
- Zhang X, Cheng J, Ni H, et al. Infant Cognitive Scores Prediction with Multi-stream Attention-Based Temporal Path Signature Features. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. Springer; 2020:134-144.
- Squeglia LM, Jacobus J, Sorg SF, Jernigan TL, Tapert SF. Early adolescent cortical thinning is related to better neuropsychological performance. J Int Neuropsychol Soc. 2013, 19, 962–970. [Google Scholar] [CrossRef]
- Yang JJ, Yoon U, Yun HJ, et al. Prediction for human intelligence using morphometric characteristics of cortical surface: partial least square analysis. Neuroscience 2013, 246, 351–361. [Google Scholar] [CrossRef]
- Wang L, Wee CY, Suk HI, Tang X, Shen D. MRI-based intelligence quotient (IQ) estimation with sparse learning. PloS One. 2015, 10, e0117295. [Google Scholar]
- Choi YY, Shamosh NA, Cho SH, et al. Multiple bases of human intelligence revealed by cortical thickness and neural activation. J Neurosci. 2008, 28, 10323–10329. [Google Scholar] [CrossRef]
- Wright IC, McGuire PK, Poline JB, et al. A voxel-based method for the statistical analysis of gray and white matter density applied to schizophrenia. Neuroimage. 1995, 2, 244–252. [Google Scholar] [CrossRef]
- Kim H, Kim J hoon, Possin KL, et al. Surface-based morphometry reveals caudate subnuclear structural damage in patients with premotor Huntington disease. Brain Imaging Behav. 2017, 11, 1365–1372. [Google Scholar] [CrossRef]
- Whitwell, JL. Voxel-based morphometry: an automated technique for assessing structural changes in the brain. J Neurosci. 2009, 29, 9661–9664. [Google Scholar] [CrossRef] [PubMed]
- Hidese S, Ota M, Matsuo J, et al. Correlation Between the Wechsler Adult Intelligence Scale-3 (rd) Edition Metrics and Brain Structure in Healthy Individuals: A Whole-Brain Magnetic Resonance Imaging Study. Front Hum Neurosci 2020, 14. [Google Scholar]
- McDermott CL, Seidlitz J, Nadig A, et al. Longitudinally mapping childhood socioeconomic status associations with cortical and subcortical morphology. J Neurosci. 2019, 39, 1365–1373. [Google Scholar] [CrossRef]
- Ramsden S, Richardson FM, Josse G, et al. Verbal and non-verbal intelligence changes in the teenage brain. Nature. 2011, 479, 113–116. [Google Scholar] [CrossRef]
- Vang YS, Cao Y, Xie X. A Combined Deep Learning-Gradient Boosting Machine Framework for Fluid Intelligence Prediction. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:1-8.
- Pominova M, Kuzina A, Kondrateva E, et al. Ensemble of 3D CNN regressors with data fusion for fluid intelligence prediction. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:158-166.
- Zou Y, Jang I, Reese TG, Yao J, Zhu W, Rispoli JV. Cortical and Subcortical Contributions to Predicting Intelligence Using 3D ConvNets. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:176-185.
- Liu L, Yu L, Wang S, Heng PA. Predicting Fluid Intelligence from MRI Images with Encoder-Decoder Regularization. In: Challenge in Adolescent Brain Cognitive Development Neurocognitive Prediction. Springer; 2019:108-113.
- Arrieta AB, Díaz-Rodríguez N, Del Ser J, et al. Explainable Artificial Intelligence (XAI): Concepts, taxonomies, opportunities and challenges toward responsible AI. Inf Fusion. 2020, 58, 82–115. [Google Scholar] [CrossRef]
- Gunning D, Stefik M, Choi J, Miller T, Stumpf S, Yang GZ. XAI—Explainable artificial intelligence. Sci Robot. 2019, 4, eaay7120. [Google Scholar] [CrossRef]
- Speith, T. A review of taxonomies of explainable artificial intelligence (XAI) methods. In: 2022 ACM Conference on Fairness, Accountability, and Transparency; 2022:2239-2250.
- Malpas CB, Genc S, Saling MM, Velakoulis D, Desmond PM, O’Brien TJ. MRI correlates of general intelligence in neurotypical adults. J Clin Neurosci. 2016, 24, 128–134. [Google Scholar] [CrossRef]
- Feng K, Rowell AC, Andres A, et al. Diffusion tensor MRI of white matter of healthy full-term newborns: relationship to neurodevelopmental outcomes. Radiology. 2019, 292, 179–187. [Google Scholar] [CrossRef]
- Konrad A, Vucurevic G, Musso F, Winterer G. VBM–DTI correlates of verbal intelligence: a potential link to Broca’s Area. J Cogn Neurosci. 2012, 24, 888–895. [Google Scholar] [CrossRef]
- Casson IR, Viano DC, Haacke EM, Kou Z, LeStrange DG. Is there chronic brain damage in retired NFL players? Neuroradiology, neuropsychology, and neurology examinations of 45 retired players. Sports Health. 2014, 6, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Lee SJ, Steiner RJ, Yu Y, et al. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc Natl Acad Sci. 2017, 114, 148–153. [Google Scholar] [CrossRef]
- Zhang Z, Allen GI, Zhu H, Dunson D. Tensor network factorizations: Relationships between brain structural connectomes and traits. Neuroimage. 2019, 197, 330–343. [Google Scholar] [CrossRef]
- Gore JC, Li M, Gao Y, et al. Functional MRI and resting state connectivity in white matter-a mini-review. Magn Reson Imaging. 2019, 63, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012, 22, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Song M, Zhou Y, Li J, et al. Brain spontaneous functional connectivity and intelligence. Neuroimage. 2008, 41, 1168–1176. [Google Scholar] [CrossRef]
- Kwak S, Kim H, Kim H, Youm Y, Chey J. Distributed functional connectivity predicts neuropsychological test performance among older adults. Hum Brain Mapp. Published online 2021.
- Finn ES, Shen X, Scheinost D, et al. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci. 2015, 18, 1664–1671. [Google Scholar] [CrossRef]
- Powell MA, Garcia JO, Yeh FC, Vettel JM, Verstynen T. Local connectome phenotypes predict social, health, and cognitive factors. Netw Neurosci. 2018, 2, 86–105. [Google Scholar] [CrossRef]
- Sripada C, Rutherford S, Angstadt M, et al. Prediction of neurocognition in youth from resting state fMRI. Mol Psychiatry. 2020, 25, 3413–3421. [Google Scholar] [CrossRef]
- Jiang R, Qi S, Du Y, et al. Predicting individualized intelligence quotient scores using brainnetome-atlas based functional connectivity. In: 2017 IEEE 27th International Workshop on Machine Learning for Signal Processing (MLSP). IEEE; 2017:1-6.
- Hart SJ, Davenport ML, Hooper SR, Belger A. Visuospatial executive function in Turner syndrome: functional MRI and neurocognitive findings. Brain. 2006, 129, 1125–1136. [Google Scholar] [CrossRef]
- Greene AS, Gao S, Scheinost D, Constable RT. Task-induced brain state manipulation improves prediction of individual traits. Nat Commun. 2018, 9, 1–13. [Google Scholar]
- Elliott ML, Knodt AR, Cooke M, et al. General functional connectivity: Shared features of resting-state and task fMRI drive reliable and heritable individual differences in functional brain networks. Neuroimage. 2019, 189, 516–532. [Google Scholar] [CrossRef] [PubMed]
- He T, Kong R, Holmes A, et al. Do deep neural networks outperform kernel regression for functional connectivity prediction of behavior. BioRxiv. Published online 2018:473603. =, 4: 2018.
- Li C, Yang G, Li M, Li B. Fluid intelligence relates to the resting state amplitude of low-frequency fluctuation and functional connectivity: a multivariate pattern analysis. NeuroReport. 2018, 29, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dubois J, Galdi P, Han Y, Paul LK, Adolphs R. Resting-state functional brain connectivity best predicts the personality dimension of openness to experience. Personal Neurosci. 2018, 1.
- Yoo K, Rosenberg MD, Noble S, Scheinost D, Constable RT, Chun MM. Multivariate approaches improve the reliability and validity of functional connectivity and prediction of individual behaviors. Neuroimage. 2019, 197, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Noble S, Spann MN, Tokoglu F, Shen X, Constable RT, Scheinost D. Influences on the test–retest reliability of functional connectivity MRI and its relationship with behavioral utility. Cereb Cortex. 2017, 27, 5415–5429. [Google Scholar] [CrossRef] [PubMed]
- Kanaya AM, Grady D, Barrett-Connor E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: a meta-analysis. Arch Intern Med. 2002, 162, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry. 2007, 62, 847–855. [Google Scholar] [CrossRef] [PubMed]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004, 24, 8223–8231. [Google Scholar] [CrossRef] [PubMed]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Herting MM, Johnson C, Mills KL, et al. Development of subcortical volumes across adolescence in males and females: A multisample study of longitudinal changes. NeuroImage. 2018, 172, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Nagel BJ, Herting MM, Maxwell EC, Bruno R, Fair D. Hemispheric lateralization of verbal and spatial working memory during adolescence. Brain Cogn. 2013, 82, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Vendetti MS, Johnson EL, Lemos CJ, Bunge SA. Hemispheric differences in relational reasoning: novel insights based on an old technique. Front Hum Neurosci. 2015, 9, 55. [Google Scholar]
- Steffener J, Habeck C, O’Shea D, Razlighi Q, Bherer L, Stern Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol Aging. 2016, 40, 138–144. [Google Scholar] [CrossRef]
- Hackman DA, Cserbik D, Chen JC, et al. Association of Local Variation in Neighborhood Disadvantage in Metropolitan Areas With Youth Neurocognition and Brain Structure. JAMA Pediatr. Published online 2021:e210426-e210426.
- Skotting MB, Eskildsen SF, Ovesen AS, et al. Infants with congenital heart defects have reduced brain volumes. Sci Rep. 2021, 11, 1–8. [Google Scholar]
- Bolduc ME, Lambert H, Ganeshamoorthy S, Brossard-Racine M. Structural brain abnormalities in adolescents and young adults with congenital heart defect: a systematic review. Dev Med Child Neurol. 2018, 60, 1209–1224. [Google Scholar] [CrossRef]
- Asschenfeldt B, Evald L, Heiberg J, et al. Neuropsychological status and structural brain imaging in adults with simple congenital heart defects closed in childhood. J Am Heart Assoc. 2020, 9, e015843. [Google Scholar] [CrossRef]
- Oster ME, Watkins S, Hill KD, Knight JH, Meyer RE. Academic outcomes in children with congenital heart defects: a population-based cohort study. Circ Cardiovasc Qual Outcomes. 2017, 10, e003074. [Google Scholar] [CrossRef] [PubMed]
- Savory K, Manivannan S, Zaben M, Uzun O, Syed YA. Impact of copy number variation on human neurocognitive deficits and congenital heart defects: a systematic review. Neurosci Biobehav Rev. 2020, 108, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Derridj N, Guedj R, Calderon J, et al. Long-term Neurodevelopmental Outcomes of Children with Congenital Heart Defects. J Pediatr. Published online 2021.
- Huang SC, Pareek A, Seyyedi S, Banerjee I, Lungren MP. Fusion of medical imaging and electronic health records using deep learning: a systematic review and implementation guidelines. NPJ Digit Med. 2020, 3, 1–9. [Google Scholar]
- Guyon I, Elisseeff A. An introduction to variable and feature selection. J Mach Learn Res 2003, 3, 1157–1182. [Google Scholar]
- Li J, Cheng K, Wang S, et al. Feature selection: A data perspective. ACM Comput Surv CSUR. 2017, 50, 1–45. [Google Scholar]
- Smith SM, Nichols TE. Statistical challenges in “big data” human neuroimaging. Neuron. 2018, 97, 263–268. [Google Scholar] [CrossRef]
- He S, Pereira D, Perez JD, et al. Multi-channel attention-fusion neural network for brain age estimation: Accuracy, generality, and interpretation with 16,705 healthy MRIs across lifespan. Med Image Anal. 2021, 72, 102091. [Google Scholar] [CrossRef]
- He S, Grant PE, Ou Y. Global-Local Transformer for Brain Age Estimation. IEEE Trans Med Imaging. Published online 2021.
- Brookes AJ, Robinson PN. Human genotype–phenotype databases: aims, challenges and opportunities. Nat Rev Genet. 2015, 16, 702–715. [Google Scholar] [CrossRef]
- Sterling LH, Liu A, Ganni E, et al. Neurocognitive disorders amongst patients with congenital heart disease undergoing procedures in childhood. Int J Cardiol. Published online 2021.
- Calderon J, Bellinger DC. Executive function deficits in congenital heart disease: why is intervention important? Cardiol Young. 2015, 25, 1238–1246. [Google Scholar] [CrossRef]
- Cole JH, Leech R, Sharp DJ, Initiative ADN. Prediction of brain age suggests accelerated atrophy after traumatic brain injury. Ann Neurol. 2015, 77, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Cole JH, Ritchie SJ, Bastin ME, et al. Brain age predicts mortality. Mol Psychiatry. 2018, 23, 1385. [Google Scholar] [CrossRef] [PubMed]
- Franke K, Ziegler G, Klöppel S, Gaser C, Initiative ADN. Estimating the age of healthy subjects from T1-weighted MRI scans using kernel methods: exploring the influence of various parameters. Neuroimage. 2010, 50, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Franke K, Gaser C, Manor B, Novak V. Advanced BrainAGE in older adults with type 2 diabetes mellitus. Front Aging Neurosci. 2013, 5, 90. [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004, 70, 117–145. [Google Scholar] [CrossRef] [PubMed]
- Berkelhammer LD, Williamson AL, Sanford SD, et al. Neurocognitive sequelae of pediatric sickle cell disease: a review of the literature. Child Neuropsychol. 2007, 13, 120–131. [Google Scholar] [CrossRef]
- Shields LB, Choucair AK. Management of low-grade gliomas: a review of patient-perceived quality of life and neurocognitive outcome. World Neurosurg. 2014, 82, e299–e309. [Google Scholar] [CrossRef]
- Subramaniyan S, Terrando N. Narrative review article: neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019, 128, 781. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





