1. Introduction
The severity of overpopulation and climate change has motivated the search for new sources of protein to combat food insecurity [
1]. In Western countries, food preferences are changing because of the increasing concerns about the environment and health and the growing demand for a reduction in animal protein consumption [
2,
3]. Alternative diets are emerging to meet this demand. However, the Western population is not ready to drastically change its diet or replace it with plant-based or alternative-protein-based foods. Even so, the need for affordable, appealing alternative products with familiar tastes is often voiced [
3].
The hybrid products, partially replacing animal protein with alternatives, might meet this dual demand: to reduce the environmental footprint of food manufacture and offer affordable, familiar, healthy and tasty products [
3,
4]. Hybrid foods containing dairy or egg ingredients might emerge during the transition to an alternative-protein-based diet; using these animal protein sources contributes less to climate change than meat products [
3].
The market for plant-based dairy alternatives is growing fast [
5]. However, the reformulation of conventional dairy products to substitute animal fats and proteins remains challenging. Among dairy products, cheese is a widely consumed food. It has a high content of protein, saturated fats and calories [
2], which makes it a good candidate for reformulation. Consequently, the market for cheese analogues is booming. These substitutes could have nutritional and economic advantages over conventional cheese. However, most commercial plant-based cheese analogues on the European market consist of a mixture of water, oils rich in saturated fats, starch and stabilisers. Accordingly, they are very low in protein [
6,
7,
8]. Consumers are aware of the poor nutritional quality of these starch-based analogues and generally believe that these alternatives fail to match the taste and texture of conventional cheese [
8].
To develop a hybrid cheese analogue similar to the conventional product, it is necessary partially to replace dairy protein with an alternative protein of similar nutritional quality and functionality. Pulses and insects seem to be interesting protein sources due to their high nutritional quality, techno-functional potential and reduced environmental impact [
9].
Faba bean is a pulse of great interest as it is a rich source of protein (25–40%), carbohydrate (47–68%) and fibre (11–30%), and it might afford better environmental performance than other legumes. Its cultivation is considered an important method for reducing the environmental footprint; it fixes biological nitrogen, increases the subsequent crop yields, and is easy to cultivate in crop rotation. Faba bean is one of four crops identified by the EU Smart Protein Project as a promising alternative to animal protein. Several studies have evaluated the substitution of animal protein with faba bean protein in food formulations (e.g., bread, pasta and meat and dairy analogues) [
9]. Despite its strong nutritional, techno-functional and environmental potential, this plant protein is industrially underexploited as an ingredient for human consumption [
10].
In contrast, insect proteins have recently received more attention in the food industry due to their high protein (40–70% dry basis), mineral and vitamin contents and their advantageous polyunsaturated to saturated fatty acid ratios. Insect farming has a reduced environmental footprint compared to livestock. Consumer acceptance is one of the biggest challenges for insect-based food since Western societies are often repulsed by the idea of consuming insects. However, when insect body parts are not evident, the idea seems to create less aversion. This suggests that insect-based products such as flours might be acceptable [
11,
12]. Moreover, Michel and Begho [
13] have noticed that consumers preferred insect flour when it was mixed with pork protein, demonstrating that it might be easier to introduce consumers to insect-based foods through hybrid products.
Tenebrio molitor larvae are now more likely to be included in the Western diet after the EFSA declared them safe for human consumption [
11]. Research on the techno-functional properties of mealworm proteins and their inclusion in food products has been quite extensive in recent years (e.g., meat products, snacks and bakery) [
11,
12].
To the best of our knowledge, only one study has examined the introduction of insect protein in dairy substitutes [
5]. Another group has evaluated the use of faba bean flour in making plant-based cheese analogues [
8]. There is little data on utilising faba bean protein, insect protein or their combination to produce plant-based spreadable cheese analogues or to develop other dairy alternatives.
The mixture design methodology is used to determine the optimal relative proportion of ingredients using effective information from a relatively small number of experiments. Earlier studies by Talens, Llorente [
14] and Talens, Lago [
15] have applied this approach to protein blend design for modelling the food product and ingredients. The desirability-based design helps achieve the balance between the various desirable properties. It is more efficient than traditional methods using the simultaneous optimisation of several responses; fewer formulations have to be evaluated, and it results in more desirable property levels [
16].
The main aim of this study was to develop a hybrid spreadable cheese with nutritional value, texture and flavour similar to those of conventional spreadable cheese. The study also evaluates the changes in the textural, sensory and nutritional properties of a spreadable cheese analogue (SCA) with dairy protein replaced by the insect (Tenebrio molitor) and faba bean flours. It employs a desirability-based mixture design approach. The desirability function is used to optimise the properties of the product and choose the best protein combination.
2. Materials and Methods
2.1. Raw Materials
Vegetable margarine (70% fat) and salt were purchased at a Makro store (Erandio, Spain). Insect flour was obtained from Insekt Label Biotech, S.L. (Bilbao, Spain). The mealworm (Tenebrio molitor) flour composition was 52.2% protein, 33.0% fat (8.0% saturated fatty acids, SFA, and 25.0% unsaturated fatty acids, UFA), 3.7% fibre, 0.4% salt and 10.7% moisture. Faba bean protein concentrate was obtained from AGT Foods (Regina, Canada). It contained 60% protein, 3.2% fat (0.6% SFA and 2.6% UFA), 14.4% dietary fibre, 5.3% starch and 10% moisture. Milk protein concentrate (70%) and a mixture of stabilisers were provided by Blendhub S.L. (Murcia, Spain). The MPC contained 70% protein, 1.75% fat (1.25% SFA and 0.5% UFA), and < 6% moisture. The stabiliser mix was based on carboxymethylcellulose (E-466), carrageenan (E-407), locust bean gum (E-410), sugar and maltodextrin, and it contained < 12% of moisture. Citric acid and sodium citrate were obtained from APASA (Renteria, Spain). Cylindrical glass jars of 128 ml and white lids were acquired from Berlin Packaging Juvasa (Sevilla, Spain).
2.2. Pre-Treatment of Insect Flour
As the large particles in the insect flour, reported in previous studies by Talens, Lago [
15] and Talens, Llorente et al. (2022), can affect the technical properties during hydration and the texture and appearance of the spreadable cheese, the mealworm powder was milled using an ultracentrifuge mill (ZM 100, Retsch, Haan, Germany) to reduce the particle size to ≤ 200 µm.
2.3. Physicochemical Analysis of Protein Ingredients
The particle size distribution of MPC, FBP and IF was obtained employing a Static Light Scattering Instrument Master-Sizer 3000 (Malvern Instruments Ltd., Malvern, UK) using bi-distilled water as a dispersion agent (refractive index = 1.33), following other studies by Talens, Lago [
15] and Talens, Llorente [
14]. The d50 (µm) or median particle size by volume (maximum particle diameter of 50% of the sample volume) was obtained. The most common percentiles found were the d10 (µm), d50 (µm) and d90 (µm). The volume moment mean or De Brouckere mean diameter (D [
3,
4]) was also obtained as it reflected the size of the particles that constitute the bulk of the sample volume. This parameter is most sensitive to large particulates in the size distribution.
The swelling capacity of the flours was determined following Talens, Lago [
15] and Talens, Llorente [
14]. Water solubility index (WSI), water-holding capacity (WHC) and oil-holding capacity (OHC) were calculated using equations 1, 2 and 3, respectively:
The colour of the ingredients was measured using a colourimeter (CR-400 Chroma Meter, Konica Minolta Inc., Tokyo, Japan) in the CIE L*a*b* system. The colourimetric parameters L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) were determined.
2.4. Spreadable Cheese Analogues
2.4.1. Experimental design and spreadable cheese analogues preparation
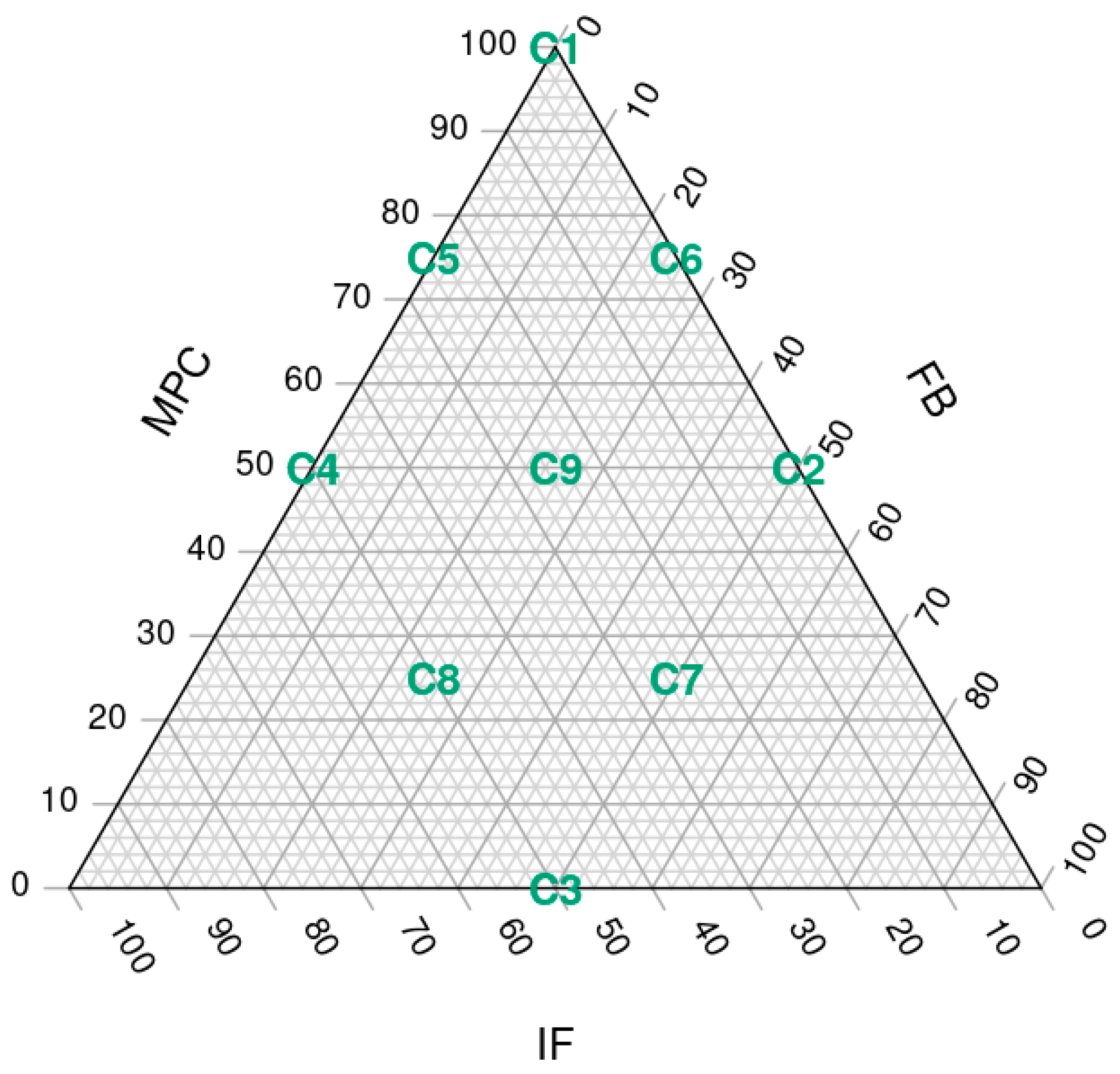
A three-factor simplex lattice design with three levels was used to formulate all the possible mixtures (9 assays) containing milk protein concentrate (MPC), faba bean protein concentrate (FBP) and insect flour (IF), adding up to 7.1% of the total weight of the ingredients. Within this 7.1%, the maximum content of each FBP and IF was 50%, and MPC a maximum of 100%. For the three ingredients, the minimum representation possible was 0% (
Figure 1).
The nine different SCA formulations, labelled from C1 to C9 (
Figure 2), contained different percentages of MPC, FBPC and IF: C1 (7.1, 0, 0), C2 (3.55, 3.55, 0), C3 (0, 3.55, 3.55), C4 (3.55, 0, 3.55), C5 (5.33, 0, 1.77), C6 (5.33, 1.77, 0), C7 (1.77, 3.55, 1.77), C8 (1.77, 1.77, 3.55) and C9 (3.55, 1.77, 1.77). However, for convenience, throughout the article, we will refer to the MPC/FBP/IF percentage ratio of each SCA: C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
All nine formulations contained 49% water, 42.8% vegetable margarine, 0.4% stabiliser mix, 0.6% salt and 0.1% trisodium citrate. The remaining 7.1% was composed of a mixture of MPC, FBP and/or IF in different proportions. All ingredients were weighed using high-precision (±0.0001 g) scales AB304-S (Mettler Toledo, Greifensee, Switzerland) and then mixed in a Thermomix food processor (Thermomix TM31; Vorwerk, Wuppertal, Germany). The fat and water were first blended for 3.5 min at 250 rpm and 50 °C. Then, all powdered ingredients, previously blended, were added to the mixture and blended again for 3 min at 2000 rpm. This method was expected to stimulate the emulsification process. The mixture was pasteurised at 90 °C for 1 min. The pH was adjusted to 4.6─4.8 using citric acid. Then, the mixture was homogenised for 30 s at 300 rpm to give it the right texture. Finally, the cheese analogues were deposited in cylindrical glass jars, covered with lids, labelled and refrigerated at 4 °C for further characterisation. Two batches were elaborated for further analysis.
2.4.2. Physicochemical Characterisation of Spreadable Hybrid Cheeses
The theoretical nutritional content of the SCAs (protein, crude fat, saturated fat, unsaturated fat, total dietary fibre, carbohydrates, sugar and salt) was calculated using the nutritional content provided by the product data sheets, following Equation 4.
where m = g of nutrient per 100 g of SCA, mi = g of ingredient per 100 g of SCA, qi = (g of nutrient per 100 g of ingredient)/100 and n = the number of ingredients.
Energy values were calculated using the Atwater general factors (4 kcal/g for protein and carbohydrates, 9 kcal/g for fat and 2 kcal/g for fibre). The protein-source ingredients constituted 7.1% of the formula and contained similar percentages of protein (between 52 and 70%). The differences between the powdered ingredients were "diluted" in the final product, and changing the ratios of these ingredients did not significantly affect the nutritional profile of the SCAs. As the nutritional profiles of the samples obtained by theoretical estimation were similar, experimental nutritional characterisation was omitted to save resources and reduce costs.
The moisture content of SCAs was determined by drying an approximately 3-g sample at 105 °C in a drying oven to a constant weight [
17] and using Equation 5.
The pH of cheese samples was determined during the processing and after refrigeration using a pH Meter (pH Meter GLP 21+, Crison Instruments, Alella, Spain).
The colour of cheese samples was measured using the colourimeter in the CIE L*a*b* system, determining the values of L* (lightness), a* (redness/greenness) and b* (yellowness/blueness) colourimetric parameters.
2.4.3. Textural Properties
The texture properties of SCAs were examined using the Spreadability Test, employing a TA.HDplus texturometer (Stable Micro System, Godalming, Surrey, UK) equipped with a Texture Technologies Corporation (TCC) Spreadability Rig and a 5-kg load cell, following the method described by Brighenti, Govindasamy-Lucey [
18], with some modifications.
The TTC spreadability fixture is a set of precisely matched male and female Perspex 45° cones. The instrument was calibrated at the test speed of 1 mm/s, post-test speed of 1 mm/s, trigger force of 2 g, and a penetration depth of 23 mm. SCA samples were placed inside the female cone, which was then pressed down, with minimal structural disruption, to eliminate air pockets. Any excess sample was removed with a spatula to leave a flat test surface. Measurements were performed at 18.5 ± 0.5 °C (after 48 h of cold storage), considered the serving temperature. During the test, the product was squeezed out between the cones and force-time curves were recorded.
The maximum value of the force recorded by the device when the probe was at its maximum penetration depth was recorded as spreadability firmness (Hs, g). The area under the curve corresponding to the total force required to carry out the first phase of the test is referred to as spreadability (work of shear, g.mm). During the second phase of the test, the male cone moved in the opposite direction. The maximum force recorded during this phase is called stickiness (g), whereas the area under the curve corresponds to the work of adhesion (g.mm).
Each batch was examined in quadruplicate.
2.4.4. Sensory Analysis
A quantitative descriptive analysis (QDA) was performed to assess the sensory characteristics of the SCAs.
A preliminary recall training was preceded by three sessions with the members of a semi-trained panel of 8 assessors (5 women and 3 men). They were re-trained to identify and evaluate sensory characteristics of a commercial spreadable dairy cheese and a commercial plant-based SCA from a Spanish supermarket. They were also re-trained in the use of a 0–5 intensity scale. The panellists generated the descriptors that they considered important for the descriptive evaluation of spreadable cheese and formulated their definitions in open discussion. The nine attributes generated are described in
Table 1. After re-training, a statistical analysis was conducted to confirm the discrimination capacity, repeatability and reproducibility of the panel.
In QDA evaluation, each panellist examined 5 samples presented in random order and labelled with 3-digit random numbers. The panellists rated the attributes of each sample on a 1–5 intensity scale. Three tasting sessions were carried out as replicates.
2.5. Statistical Analysis, Modelling and Optimisation of Experimental Data
The experimental design, modelling and optimisation were conducted employing the R-project software v 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria). The package used for the experimental design was "Ternary". For modelling, the packages employed were "readxl", "tidyverse", "mixexp", "rsm" and "Ternary", and the polynomial equation used was:
where Y is the estimated response and β1, β2, β3, β12, β13, β23 and β123 are constant coefficients for each linear and nonlinear (interaction) term produced for the prediction models of processing components. The model for each response was obtained based on the fitting quality, the coefficient of determination (R2) and the significant level of regression (p < 0.05).
The "desirability" package was used for the optimisation of the experimental design to obtain the maximum firmness and spreadability (considering closeness to the texture of spreadable dairy cheese) and maximum L* (considering closeness to the colour of dairy cheese).
The packages used to analyse the QDA results were "readxl", "tidyverse", "ggplot2", "agricolae" and "fmsb". For the physicochemical characterisation of protein ingredients and physicochemical, texture and sensory analysis of SCAs, a one-way analysis of variance (ANOVA) and a Tukey HDS test were used to determine pairwise differences between groups. A 5% significance level (p < 0.05) was used in all cases.The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
Research manuscripts reporting large datasets that are deposited in a publicly available database should specify where the data have been deposited and provide the relevant accession numbers. If the accession numbers have not yet been obtained at the time of submission, please state that they will be provided during review. They must be provided prior to publication.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
3. Results
3.1. Physicochemical Characterisation of Protein Ingredients
Table 2 summarises the functional properties, colour parameters and particle sizes of MPC, FBP and IF.
The moisture content of MPC, FBP and IF was 5.22, 6.89 and 1.41%, respectively, with significant differences between the three ingredients (p < 0.05). This might be due to the different moisture of raw ingredients and the differences in the production processes for powdered ingredients. The water content of MPC coincided with the value given by the manufacturer (< 6%). For FBP, the moisture content was slightly lower (10%) and for IF, much lower than reported by the manufacturer (10.74%). In the last case, the moisture might have evaporated during the milling pre-treatment of insect flour.
Swelling capacity was significantly lower for MPC (2.88 mL/g) than for FBP and IF (4.01 and 3.64 mL/g, respectively). This might be due to particle size differences since MPC mainly contained particles of average diameter (d50) of 94.17 µm, significantly larger than in FBP and IF (35.68 and 56.20 µm, respectively). Moreover, d90 (306.50 µm) for MPC was significantly larger than for FBP and IF (130.33 and 161.17 µm, respectively). This means that the MPC ingredient contained coarse particles larger than 300 µm, which could affect its swelling properties [
19]. The coarse particles (D[4.3]) in MPC (135.0 µm) were significantly larger than in FBP (54.63 µm) and IF (77.33 µm). The differences in particle size might be due to the different milling procedures used to obtain the powdered ingredients.
The water solubility index was significantly lower for IF (7.62 g/100g) than for MPC (31.08 g/100g) and FBP (24.23 g/100g). The highest value for water-holding capacity was found for MPC (2.69 g/g), followed by IF and FBP (1.64 and 1.35 g/g, respectively). The oil-holding capacity also significantly differed between the ingredients; the highest OHC value was observed for FBP (2.31 cm3/g), followed by MPC (1.77 cm3/g) and IF (0.92 cm3/g). The variation in WHC and OHC values may be due to the differences in protein concentration, conformational characteristics and the degree of interaction with water. An increase in the content of non-polar amino acids strengthens hydrophobic interactions with lipids (and the OHC) and weakens the hydrophilic interactions with water (and the WHC) [
19].
The colours of the ingredients differed significantly (p < 0.05). L* values closer to 100 indicate light colours, and values closer to 0, dark colours. Positive a* values are associated with a colour close to red, whilst negative values indicate closeness to green. Large positive b* values are observed for colours close to yellow, and small positive b* values indicate closeness to blue. FBP had the highest L* (69.29) and b* (15.13) values and the lowest a* value (-1.92). IF had the lowest L* value (21.53) and the highest a* value (2.44), and the MPC had the lowest b* value (9.63).
3.2. Physicochemical Characterisation of the Spreadable Cheese Analogues
Table 3 summarises the colour parameters, the moisture content and the pH of the SCAs.
No significant differences in the moisture content of various SCAs were observed except for samples C3 and C4.
Similarly, there were no significant differences between pH values, except for sample C1, whose pH increased from 4.60 to 5.20 after packaging and refrigeration. The pH remained within the range of 4.6─4.8 for all the other samples. Significant colour differences were detected between some sample groups. The IF content negatively affected the lightness; the samples with 50% IF (C3 and C4) had the lowest L* values. The samples with 25% IF (C5, C7 and C9) had lower L* values than those without IF (C1, C2, C6). In contrast, raising the FBP content increased the L* values of the cheese; samples C2 and C6 had the highest L*. Sample C1, containing only MPC, had an intermediate L* value. Both the MPC and FBP contributed positively to the yellowness (b*) of the SCAs. Sample C1 showed the highest degree of yellowness, and C2 and C6 had higher b* values than the remaining samples. However, IF seemed to counteract the effect of MPC (C4 and C5) and FBP (C3) on yellowness, contributing to redness. C3, C4 and C8 had colours closer to red (a*) because of their high content (50%) of IF. C7 and C5 (25% IF) displayed had lower redness values (they were closer to green). C1 was greener than C2 and C6 because of its 100% MPC content. Samples C2 and C6 contained FBP (50% and 25%, respectively) and had higher greenness values than MPC but also higher levels of yellowness and lightness. C9 had more redness than C7 because (despite the same amount of IF) it contained more MPC and less FBP than C7 (see
Table 3).
3.3. Nutritional Estimates for the Spreadable Cheese Analogues
The theoretical estimation of the nutritional content of SCAs, presented in
Table 4, showed that the nine formulations had very similar nutritional profiles. This is probably because the combined protein ingredients represented only 7.1% of the formula, and they contained similar percentages of protein (between 52 and 70%). Thus, the differences between the powdered ingredients were diluted in the final product and changing the ratios of protein ingredients did not significantly affect the nutritional profile of the SCAs.
However, it should be noted that adding the FBP affected the fibre, carbohydrate and sugar levels of the samples; FBP contained 14.4% of dietary fibre, 5.3% of starch and 2.1% of sugars. The addition of IF had a slight effect on the fat content since this powder contained 33% of fat (25% UFA and 8% SFA).
3.4. Texture Characterisation of the Spreadable Cheese Analogues
The mean values for the textural properties of the different SCAs can be found in
Table 5.
Spreadability (work of shear) is defined as the work required to spread a product on an immobile surface; it also reflects the structural breakdown of the product during oral processing [
20]. This parameter is considered a good instrumental measure of the spreadability of cream cheeses [
21] and other spreadable products [
22]. It must be noted that the higher the value of spreadability (work of shear), the lower the spreadability of the product.
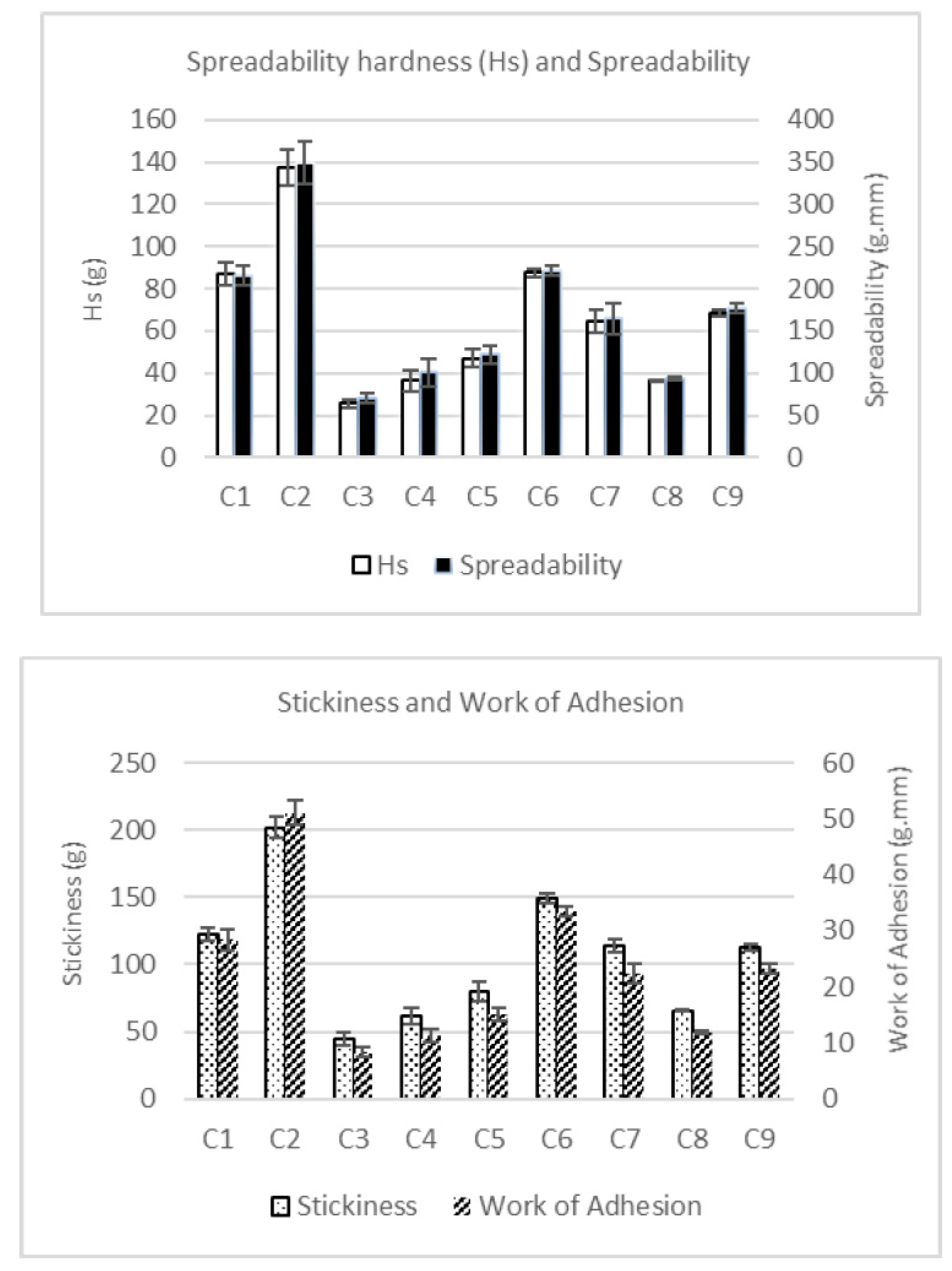
All the texture parameters showed similar patterns in all the samples (see
Figure 3). Moreover, the spreadability (work of shear) was directly proportional to spreadability firmness (Hs), and the stickiness was directly proportional to work of adhesion.
The addition of IF had a significant (p < 0.05) effect on the textural properties of SCAs. It caused a reduction in the values of Hs (firmness), spreadability, stickiness and work of adhesion. SCAs with 50% substitution of MCP by IF (C3, C4 and C8) had the lowest firmness (25.5, 36.4 and 36.4 g, respectively), spreadability (70.6, 100.4 and 93.6 g.mm), stickiness (44.7, 61.3 and 65.4 g) and work of adhesion (8.4,11.3 and 11.9 g.mm) values. Furthermore, sample C3 (without MPC) showed significantly lower values than samples C4 and C8 (containing MPC). This indicates that, on its own, MPC increases the firmness of the spreadable cheese more than FBP. However, SCAs with 25% MPC substituted by IF (C5, C7 and C9) showed lower values of firmness, spreadability, stickiness and work of adhesion (46.9, 64.9 and 68.9 g; 121.8, 164.8 and 177.3 g.mm; 79.7, 114.3 and 112.9 g; and 15.0, 22.3 and 23.3 g.mm) than SCAs with no IF, i.e., C1, C2 and C6 (87.1, 137.6 and 87.5; 216.0, 349.9 and 221.3 g.mm; 122.5, 202.1 and 148.9 g; and 28.4, 51.1 and 33.5 g.mm), but higher than for SCAs with 50% IF. Therefore, as the proportion of IF rose, the firmness and stickiness of the SCA decreased, and the spreadability increased.
Within the 0%-IF and 25%-IF sample groups, a synergy effect between MPC and FBP can be observed in samples containing both ingredients. Generally, these samples showed higher firmness, spreadability, stickiness and work of adhesion than their counterparts containing MPC but not FBP (C2 and C6 compared with C1, and C7 and C9 compared with C5). This indicates that FBP only had a positive effect on texture when it was combined with MPC. The synergy was observed when the MPC was combined with FBP. However, as we mentioned before, the IF combined with MPC and FBP (C7, C8 and C9) had an opposite effect on all the texture properties of the SCAs.
3.5. Modelling and Optimisation
Multiple linear regression analysis was performed to model and predict the responses to various ingredient combinations.
Table 6 shows the results. The effect of the three different protein sources and their combinations is reflected by the F-value and the corresponding p-value.
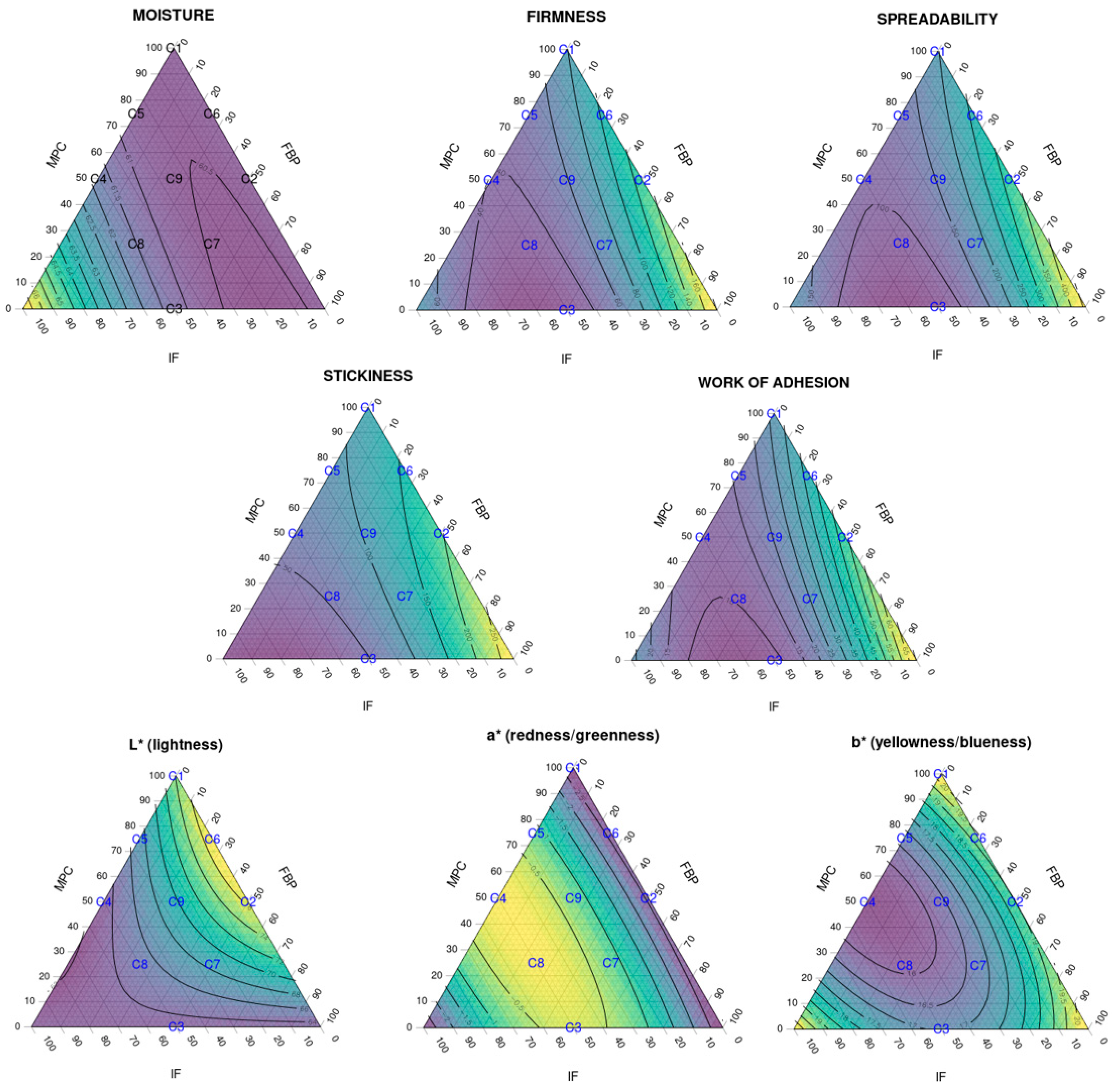
Figure 4 shows ternary diagrams for each response.
Data showed no correlations for the moisture parameter (R2 = 0.744); the effect of IF had a low significance even if itwas assumed (p = 0.028).
For firmness and spreadability, low significance (p = 0.001) was observed for the FBP–IF interaction. The effect of this ingredient combination was probably more due to the IF than the FBP content or the interaction between the two, as seen in the texture analysis. Further studies are necessary to examine the individual effect of each ingredient on its own (particularly for the FBP and IF). The loss of stability observed in SCA texture analysis of non-dairy protein sources (FBP and IF) was probably due to the lack of specific interactions between milk proteins and stabilisers such as carrageenan, which affected the final texture of the cream cheese [
23,
24,
25].
A strongly significant correlation was detected between stickiness and MPC content (p=0.001), IF content (p < 0.0001) and FBP–IF interaction (p=0.004), and between work of adhesion and FBP content (p=0.002), MPC-FBP interaction (0=0.009 and FBP-IF interaction (p<0.001)
FBP and MPC showed opposite effects to IF, as shown in the texture analysis and
Figure 4. The FBP increased the stickiness (and, consistently, the work of adhesion) when combined with MPC. The IF significantly reduced the stickiness and work of adhesion when combined with FBP and/or MPC. Therefore, the effect of FBP–IF and MPC–IF interactions on these parameters was probably more related to the IF than to the FBP content, MPC content or the interactions. Further studies are necessary to define the individual effect of each ingredient. This diversity in the functionality of different protein sources could be an opportunity to expand the range of solutions for creating new dairy-free cheese alternatives or reducing their dairy protein content.
Colour parameters also showed correlations with the different protein combinations. MPC content was clearly correlated with the lightness of the SCA (p < 0.001). A simultaneous addition of IF increased the redness (p < 0.0001) and reduced the yellowness (p < 0.001) of the SCA. These changes could be related to the colour of the protein ingredients and the SCA production process.
Finally, the "desirability" function was used to select the samples to be evaluated by the trained panel. This mathematical function allows optimisation of the models for each parameter, selecting the maximum, minimum or target desired values. In the present study, the desirability function was applied to choose the samples with the maximum firmness, spreadability and L* (lightness) values. These three parameters were selected considering the significance of the regression coefficients obtained and the relevance of the analysed properties to a spreadable cheese-like product. The desirability values were obtained for all samples: C1 (0.821), C2 (0.871), C3 (0.168), C4 (0), C5 (0.410), C6 (0.911), C7 (0.588), C8 (0.084) and C9 (0.5). The aim of this study was to develop a hybrid spreadable cheese (50% or less animal protein) with nutritional value, texture, and flavour similar to those of conventional spreadable cheese. Therefore, the SCAs with more than 50% MPC were not included in the sensory analysis. Accordingly, only the desirability values of SCAs with 50% or less MPC (C2, C3, C4, C7, C8, C9) were considered. The number of samples that a trained panel can assess simultaneously is usually no more than five to six per session. A commercial dairy spreadable cheese and a plant-based spreadable cheese analogue were used as QDA references (the former as texture and appearance reference and the latter as flavour reference). Therefore, only three hybrid SCAs with the highest desirability were chosen to be assessed by the trained panel (samples C2, C7 and C9).
3.6. Quantitative Descriptive Analysis of Spreadable Cheese Analogues
Table 7 presents the results of the Quantitative Descriptive Analysis performed by the trained panel.
Figure 5 shows the sensory profiles of SCAs C2, C7, C9 and the QDA reference for the chosen attributes.
Means with different superscript letters in the same row are significantly different according to Tukey's test (p < 0.05). Significance codes for p-value: '***' (p ≈ 0), '**' (p < 0.001).
No significant differences in granularity were detected between samples C2 (1) and C9 (1.35) and between C7 (1.6) and C9. However, the panel reported higher granularity scores for C7 than C2 (p = 0.00157). This could be explained by undissolved insect particles in samples C7 and C9.
Significant differences (p ≈ 0) were detected in the creaminess, firmness and adherence of the three analogues. C2 was the creamiest analogue (2.5), followed by C9 (2) and C7 (1.3). C2 was also the sample with the highest firmness (3), with C9 in the second place (2) and C7 (1.25) in the third. Similarly, C2 showed stronger adherence (3.35) than C9 (2.9) and C7 (the weakest adherence, 2.35). These results indicate that these three texture parameters are positively correlated in a directly proportional manner. Sample C2 was significantly less spreadable (4) than samples C7 and C9 (4.85 and 4.7, respectively) (p ≈ 0). The spreadability tended to correlate with creaminess, firmness and adherence.
The panel observed an augmented cheesy flavour in the analogue with the addition of IF; C7 and C9 obtained cheese flavour scores of 3 and 3.4, respectively. Sample C2 showed a significantly lower flavour intensity (2.6) (p ≈ 0). Similarly, the uncharacteristic flavour decreased significantly with the addition of IF (p ≈ 0), with an intensity of 3 for sample C2. Samples C7 and C9 had an intensity of 2.65 and 2.35, respectively. Sourness was significantly augmented by adding the IF to the formulation (p ≈ 0). Sourness intensity scores were 3.7 and 3.85 for C7 and C9, respectively, and 3.1 for C2. Thus, the resemblance to the characteristic cheesy and sour flavour of the dairy cheese increased after adding IF to the formulation.
Sample C2 showed more cheese-characteristic yellowness (score of 4) than samples C7 and C9 (2.95 and 3) (p ≈ 0). Thus, the resemblance to the characteristic colour of the dairy cheese decreased in the formulations containing the IF.
The QDA reference was divided in two. For texture (granularity, creamy, firmness, spreadability and adherence) and appearance (characteristic yellow colour) attributes, the developed SCAs were compared to a commercial dairy cheese reference, with the aim of observing if the texture and appearance of the analogues were similar to what would be the ideal of a spreadable cheese. For flavour attributes (cheesy flavour, uncharacteristic flavour and sourness), the developed SCAs were compared to a commercial plant-based cheese analogue reference, with the aim of seeing if the developed analogues had an improved flavour profile compared to that of the commercial plant-based analogues (see
Figure 5).
Samples C2 and C9 showed granularity values (1 and 1.35) similar to the dairy reference (1); only sample C7 had a slightly higher granularity (1.6) (p = 0.00157). All the SCAs showed lower creaminess, firmness and adherence (2.5, 1.3 and 2; 3, 1.25 and 2; 3.35, 2.35 and 2.9, respectively) than the reference sample (5, 4 and 5, respectively). However, their spreadability values were higher (4, 4.85 and 4.7 vs 3) (p ≈ 0). The colours of SCAs differed slightly (4, 2.95 and 3) from the characteristic yellow colour of the QDA reference (5) (p ≈ 0).
The flavour profiles showed that the SCAs were more sour (3.1, 3.7 and 3.85 vs 1), had more cheesy flavour (2.6, 3 and 3.4 vs 2) and less uncharacteristic flavour (3, 2.65 and 2.35 vs 4) (p ≈ 0) than the commercial plant-based reference. These differences demonstrated the sensory superiority of the new analogues over their commercial counterpart.
Figure 1.
Simplex lattice design plot of ingredient proportions representing 7.1% of spreadable cheese analogue formula, with MPC replacements adding up to 100%. The samples, labelled from C1 to C9, contain different ratios of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 1.
Simplex lattice design plot of ingredient proportions representing 7.1% of spreadable cheese analogue formula, with MPC replacements adding up to 100%. The samples, labelled from C1 to C9, contain different ratios of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 2.
Images of the nine spreadable cheese analogues used in the experiments. The samples, labelled from C1 to C9, contain different ratios of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 2.
Images of the nine spreadable cheese analogues used in the experiments. The samples, labelled from C1 to C9, contain different ratios of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 3.
Instrumental texture analysis of the nine spreadable cheese analogues. The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 3.
Instrumental texture analysis of the nine spreadable cheese analogues. The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 4.
Ternary diagrams showing the effect of the three different protein sources and their combinations to the analysed responses: spreadability, stickiness, work of adhesion and L*a*b* colour parameters (R2 > 90% for all responses except for moisture, R2 = 0.744). The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 4.
Ternary diagrams showing the effect of the three different protein sources and their combinations to the analysed responses: spreadability, stickiness, work of adhesion and L*a*b* colour parameters (R2 > 90% for all responses except for moisture, R2 = 0.744). The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Figure 5.
Spider charts showing the sensory results obtained by the trained panel for each attribute of the 3 spreadable cheese analogues and the QDA reference (a commercial dairy cheese and plant-based cheese). MPC/FBP/IF ratio: C2 (50/50/0), C7 (25/50/25) and C9 (50/25/25).
Figure 5.
Spider charts showing the sensory results obtained by the trained panel for each attribute of the 3 spreadable cheese analogues and the QDA reference (a commercial dairy cheese and plant-based cheese). MPC/FBP/IF ratio: C2 (50/50/0), C7 (25/50/25) and C9 (50/25/25).
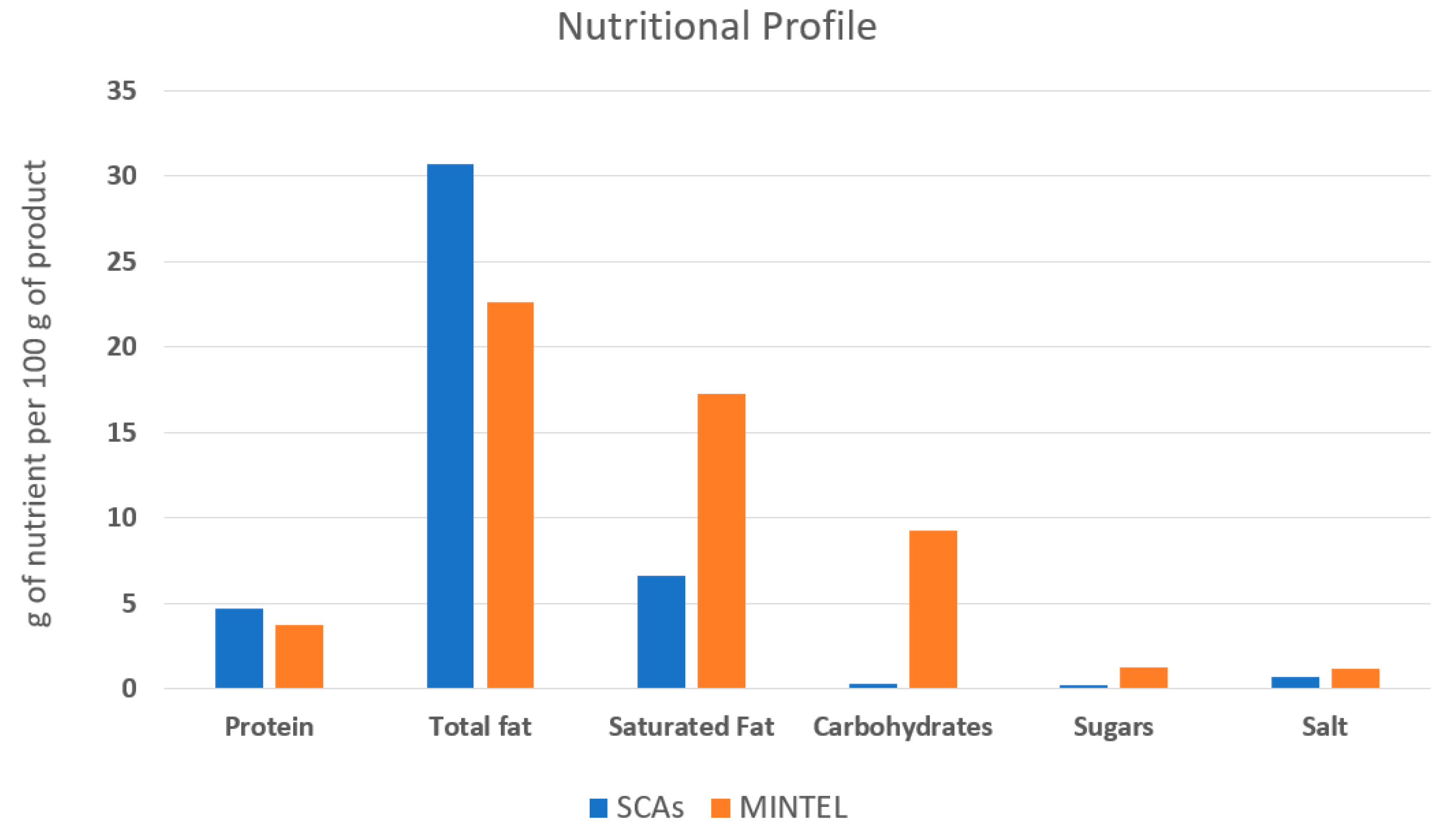
Figure 6.
The average nutritional profile of the commercial vegan spreadable cheese analogues on the European market between 2017 and 2022 (based on a search of the Mintel database) and the spreadable cheese analogues developed in the present study (SCAs).
Figure 6.
The average nutritional profile of the commercial vegan spreadable cheese analogues on the European market between 2017 and 2022 (based on a search of the Mintel database) and the spreadable cheese analogues developed in the present study (SCAs).
Table 1.
Sensory descriptors for the spreadable cheese analogues.
Table 1.
Sensory descriptors for the spreadable cheese analogues.
| |
SENSORIAL ATTRIBUTE |
DEFINITION |
| Texture by mouthfeel |
Granularity |
Presence of fine particles or granules. 1 = Low-as smooth cheese; 5 = High-as grainy cheese. |
| |
Creaminess |
Creamy feeling of fullness in the mouth. 1 = not creamy; 5 = Very creamy. |
| |
Firmness |
Effort needed to compress and break down the cheese when pressed to the roof of the mouth. 1 = Smooth; 5 = Firm. |
| Texture by hand |
Spreadability |
Ease with which cheese can be spread on a cracker. 1 = little spreadable, it required much effort to spread; 5 = very spreadable, less effort is required to spread. |
| |
Adhesion |
Ease with which cheese sticks to bread or knife. 1 = Low adhesion 5 = High adhesion. |
| Taste |
Sourness |
The basic taste, perceived on the tongue, stimulated by acids such as critic acid. Pungent acidic aroma/flavour resembling sour cream. 1 = little sour; 5 = very sour. |
| |
Cheesy flavour |
Characteristic flavour of the cheese. 1 = little cheesy flavour; 5 = lots of cheese flavour. |
| |
Uncharacteristic flavour |
Beany flavour or buttery flavour. 1 = non uncharacteristic flavours; 5 = strong uncharacteristic flavours. |
| Appearance |
Characteristic yellow colour
|
Pale yellow, such as cheese colour. 1 = very different colour; 5 = characteristic pale yellow colour. |
Table 2.
Physicochemical characteristics of milk protein concentrate (MPC), faba bean protein concentrate (FBP) and insect flour (IF). The entries show means ± standard deviations.
Table 2.
Physicochemical characteristics of milk protein concentrate (MPC), faba bean protein concentrate (FBP) and insect flour (IF). The entries show means ± standard deviations.
| Functional Properties |
MPC |
FBP |
IF |
p- Value |
| Moisture (%) |
5.22a ± 0.25 |
6.89b ± 0.03 |
1.41c ± 0.03 |
< 0.001*** |
| Swelling capacity (mL/g) |
2.88a ± 0.09 |
4.01b ± 0.11 |
3.64b ± 0.37 |
0.0338* |
| Water solubility index (%) |
31.08a ± 7.05 |
24.23a ± 0.83 |
7.62b ± 1.26 |
0.0234* |
| Water-holding capacity (g/g) |
2.69a ± 0.09 |
1.35b ± 0.08 |
1.64c ± 0.08 |
0.0011* |
| Oil-holding capacity (cm3/g) |
1.77a ± 0.11 |
2.31b ± 0.0 |
0.92c ± 0.0 |
< 0.001*** |
| Colour parameters |
|
|
|
|
| L* |
69.29a ± 0.02 |
79.92b ± 0.01 |
21.53c ± 0.01 |
< 0.001*** |
| a* |
─ 1.80a ± 0.02 |
─ 1.92b ± 0.02 |
2.44c ± 0.06 |
< 0.001*** |
| b* |
9.63a ± 0.04 |
15.13b ± 0.02 |
12.70c ± 0.01 |
< 0.001*** |
| Particle size characteristics |
|
|
|
|
| d10 (µm) |
25.90a ± 0.31 |
6.45b ± 0.19 |
7.11b ± 0.98 |
< 0.001*** |
| d50 (µm) |
94.17a ± 3.44 |
35.68b ± 2.40 |
56.20c ± 7.45 |
< 0.001*** |
| d90 (µm) |
306.50a ± 24.12 |
130.33b ± 7.39 |
161.17c ± 27.33 |
< 0.001*** |
| D[4.3] (µm) |
135.0a ± 8.22 |
54.63b ± 3.16 |
77.33c ± 13.24 |
< 0.001*** |
| D[3.2] (µm) |
52.18a ± 0.73 |
14.95b ± 0.47 |
14.72b ± 1.56 |
< 0.001*** |
| Specific Surface area |
115.0a ± 1.57 |
401.88b ± 12.49 |
411.42b ± 43.54 |
< 0.001*** |
Table 3.
Physicochemical and nutritional characterisation of the spreadable cheese analogues. Values show means ± standard deviations.
Table 3.
Physicochemical and nutritional characterisation of the spreadable cheese analogues. Values show means ± standard deviations.
| |
Ratio MPC/FBP/IF |
Moisture (%) |
pH |
L* |
a* |
b* |
| C1 |
100/0/0 |
60.72a ± 0.36 |
5.20a ± 0.20 |
74.42a ± 0.26 |
-2.99a ± 0.03 |
20.32a ± 0.69 |
| C2 |
50/50/0 |
60.72a ± 0.16 |
4.60b ± 0.28 |
76.81b ± 0.17 |
-2.57b ± 0.06 |
19.48b ± 0.19 |
| C3 |
0/50/50 |
61.47b ± 0.05 |
4.41b ± 0.06 |
62.57c ± 0.43 |
-0.25c ± 0.19 |
17.23c ± 0.79 |
| C4 |
50/0/50 |
62.15c ± 0.28 |
4.55b ± 0.13 |
63.46c ± 0.61 |
-0.15c ± 0.17 |
15.93e ± 0.60 |
| C5 |
75/0/25 |
60.55a ± 0.16 |
4.63b ± 0.09 |
67.43d ± 0.22 |
-0.86de ± 0.14 |
16.68cd ± 0.21 |
| C6 |
75/25/0 |
60.66a ± 0.06 |
4.56b ± 0.12 |
76.84b ± 0.28 |
-2.55b ± 0.06 |
19.18b ± 0.38 |
| C7 |
25/50/25 |
60.61a ± 0.07 |
4.43b ± 0.04 |
70.06e ± 0.98 |
-1.04d ± 0.12 |
16.40de ± 0.14 |
| C8 |
25/25/50 |
60.93a ± 0.02 |
4.46b ± 0.13 |
65.27f ± 0.29 |
-0.21c ± 0.11 |
15.92e ± 0.07 |
| C9 |
50/25/25 |
60.59a ± 0.01 |
4.55b ± 0.07 |
68.08d ± 1.81 |
-0.79e ± 0.32 |
15.95e ± 0.69 |
Table 4.
Theoretical nutritional profiles of the nine spreadable cheese analogues. The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
Table 4.
Theoretical nutritional profiles of the nine spreadable cheese analogues. The samples, labelled from C1 to C9, contain different proportions of milk protein concentrate, faba bean protein concentrate and insect flour (MPC/FBP/IF): C1 (100/0/0), C2 (50/50/0), C3 (0/50/50), C4 (50/0/50), C5 (75/0/25), C6 (75/25/0), C7 (25/50/25), C8 (25/25/50) and C9 (50/25/25).
| |
Energy (kJ) |
Energy (Kcal) |
Protein (g) |
Total fat (g) |
SFA (g) |
UFA (g) |
CH (g) |
Sugars (g) |
Total fibre (g) |
Salt (g) |
| C1 |
1204.88 |
292.35 |
5.18 |
30.08 |
6.51 |
23.15 |
0.21 |
0.21 |
0.00 |
0.69 |
| C2 |
1208.05 |
293.17 |
4.83 |
30.14 |
6.49 |
23.20 |
0.40 |
0.29 |
0.51 |
0.69 |
| C3 |
1239.41 |
300.89 |
4.20 |
31.25 |
6.73 |
24.06 |
0.40 |
0.29 |
0.64 |
0.70 |
| C4 |
1236.24 |
300.07 |
4.55 |
31.19 |
6.75 |
24.01 |
0.21 |
0.21 |
0.13 |
0.70 |
| C5 |
1220.56 |
296.21 |
4.87 |
30.64 |
6.63 |
23.58 |
0.21 |
0.21 |
0.07 |
0.69 |
| C6 |
1206.47 |
292.76 |
5.01 |
30.11 |
6.50 |
23.17 |
0.31 |
0.25 |
0.26 |
0.69 |
| C7 |
1223.73 |
297.03 |
4.51 |
30.69 |
6.61 |
23.63 |
0.40 |
0.29 |
0.58 |
0.69 |
| C8 |
1237.82 |
300.48 |
4.37 |
31.22 |
6.74 |
24.04 |
0.31 |
0.25 |
0.39 |
0.70 |
| C9 |
1222.15 |
296.62 |
4.69 |
30.66 |
6.62 |
23.61 |
0.31 |
0.25 |
0.32 |
0.69 |
Table 5.
Texture properties of the nine spreadable cheese analogues. Values show means ± standard deviations.
Table 5.
Texture properties of the nine spreadable cheese analogues. Values show means ± standard deviations.
| |
Ratio MPC/FBP/IF |
Hs(g) |
Spreadability (g.mm) |
Stickiness(g) |
Work of Adhesion(g.mm) |
| C1 |
100/0/0 |
87.1b ± 5.2 |
216.0c ± 12.0 |
122.5b ± 4.9 |
28.4c ± 1.8 |
| C2 |
50/50/0 |
137.6a ± 8.4 |
349.9a ± 24.9 |
202.1a ± 7.3 |
51.1a ± 2.1 |
| C3 |
0/50/50 |
25.5e ± 2.1 |
70.6e ± 6.2 |
44.7e ± 4.6 |
8.4g ± 0.9 |
| C4 |
50/0/50 |
36.4d ± 5.0 |
100.4d ± 17.3 |
61.3d ± 6.3 |
11.3f ± 1.2 |
| C5 |
75/0/25 |
46.9d ± 4.2 |
121.8d ± 10.6 |
79.7c ± 7.1 |
15.0e ± 1.1 |
| C6 |
75/25/0 |
87.5b ± 2.2 |
221.3b ± 6.2 |
148.9a ± 3.4 |
33.5b ± 0.9 |
| C7 |
25/50/25 |
64.9c ± 5.5 |
164.8c ± 18.3 |
114.3b ± 4.8 |
22.3d ± 1.7 |
| C8 |
25/25/50 |
36.4d ± 0.3 |
93.6 de ± 1.2 |
65.4d ± 0.8 |
11.9f ± 0.2 |
| C9 |
50/25/25 |
68.4c ± 1.9 |
177.3b ± 5.5 |
112.9b ± 2.5 |
23.3d ± 0.9 |
Table 6.
Summary of statistics for the multiple linear regression, showing R-squared fit, F-value, and p-value for the model and each independent variable and their interactions.
Table 6.
Summary of statistics for the multiple linear regression, showing R-squared fit, F-value, and p-value for the model and each independent variable and their interactions.
| |
Moisture |
Firmness (g) |
Spreadability (g.mm) |
Stickiness (g) |
Work of adhesion (g.mm) |
L* |
a* |
b* |
| R² |
0.744 |
0.945 |
0.944 |
0.977 |
0.969 |
0.973 |
0.991 |
0.965 |
| F |
4.848 |
28.455 |
27.900 |
71.150 |
51.842 |
60.260 |
176.372 |
46.122 |
| Pr > F |
0.061 |
0.001 |
0.002 |
0.000 |
0.000 |
0.000 |
< 0.000 |
0.000 |
| MPC |
|
|
|
43.568 |
|
68.581 |
|
|
| |
|
|
0.001 |
|
0.000 |
|
|
| FBP |
|
15.134 |
16.434 |
|
32.981 |
|
|
|
| |
0.012 |
0.010 |
|
0.002 |
|
|
|
| IF |
9.387 |
|
|
148.689 |
|
|
|
|
| 0.028 |
|
|
< 0.0001 |
|
|
|
|
| MPC–FBP |
|
|
|
|
|
42.280 |
3.457 |
9.913 |
| |
|
|
|
|
0.001 |
0.122 |
0.025 |
| MPC–IF |
4.646 |
10.359 |
8.805 |
|
16.805 |
21.165 |
291.024 |
109.041 |
| 0.084 |
0.024 |
0.031 |
|
0.009 |
0.006 |
< 0.0001 |
0.000 |
| FBP–IF |
6.007 |
58.259 |
58.657 |
25.839 |
105.231 |
|
268.403 |
58.037 |
| 0.058 |
0.001 |
0.001 |
0.004 |
0.000 |
|
< 0.0001 |
0.001 |
| MPC–FBP–IF |
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
|
Table 7.
Sensory characterisation of the spreadable cheese analogues. Values are shown as means ± standard deviations. F-values and p-values for the product from one-way ANOVA on all descriptors are also indicted.
Table 7.
Sensory characterisation of the spreadable cheese analogues. Values are shown as means ± standard deviations. F-values and p-values for the product from one-way ANOVA on all descriptors are also indicted.
| Descriptors |
Reference |
C2 |
C7 |
C9 |
F-value |
P-value |
| Granularity |
1.0a ± 0.0 |
1.0a ± 0.0 |
1.60b ± 0.6 |
1.35ab ± 0.5 |
6.26 |
0.00157** |
| Creaminess |
5.0a ± 0.0 |
2.5b ± 0.5 |
1.3d ± 0.4 |
2.0c ± 0.0 |
259.30 |
< 2e-16 *** |
| Firmness |
4.0a ± 0.0 |
3.0b ± 0.0 |
1.25d ± 0.3 |
2.0c ± 0.0 |
825.0 |
< 2e-16 *** |
| Spreadability |
3.0a ± 0.0 |
4.0b ± 0.0 |
4.85c ± 0.3 |
4.7c ± 0.4 |
120.70 |
< 2e-16 *** |
| Adherence |
5.0a ± 0.0 |
3.35b ± 0.4 |
2.35d ± 0.4 |
2.9c ± 0.5 |
94.91 |
< 2e-16 *** |
| Cheesy flavour |
2.0a ± 0.0 |
2.6b ± 0.5 |
3.0bc ± 0.2 |
3.4c ± 0.5 |
26.75 |
2.82e-09*** |
| Uncharacteristic flavour |
4.0a ± 0.0 |
3.0b ± 0.0 |
2.65bc ± 0.3 |
2.35c ± 0.5 |
45.78 |
2.26e-12*** |
| Sourness |
1.0a ± 0.0 |
3.1b ± 0.3 |
3.7c ± 0.4 |
3.85c ± 0.2 |
246.70 |
< 2e-16 *** |
| Characteristic colour |
5.0a ± 0.0 |
4.0b ± 0.0 |
2.95c ± 0.2 |
3.0c ± 0.0 |
1508.0 |
< 2e-16 *** |