Submitted:
16 February 2023
Posted:
20 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
Study design
Study inclusion and exclusion criteria
Study protocol
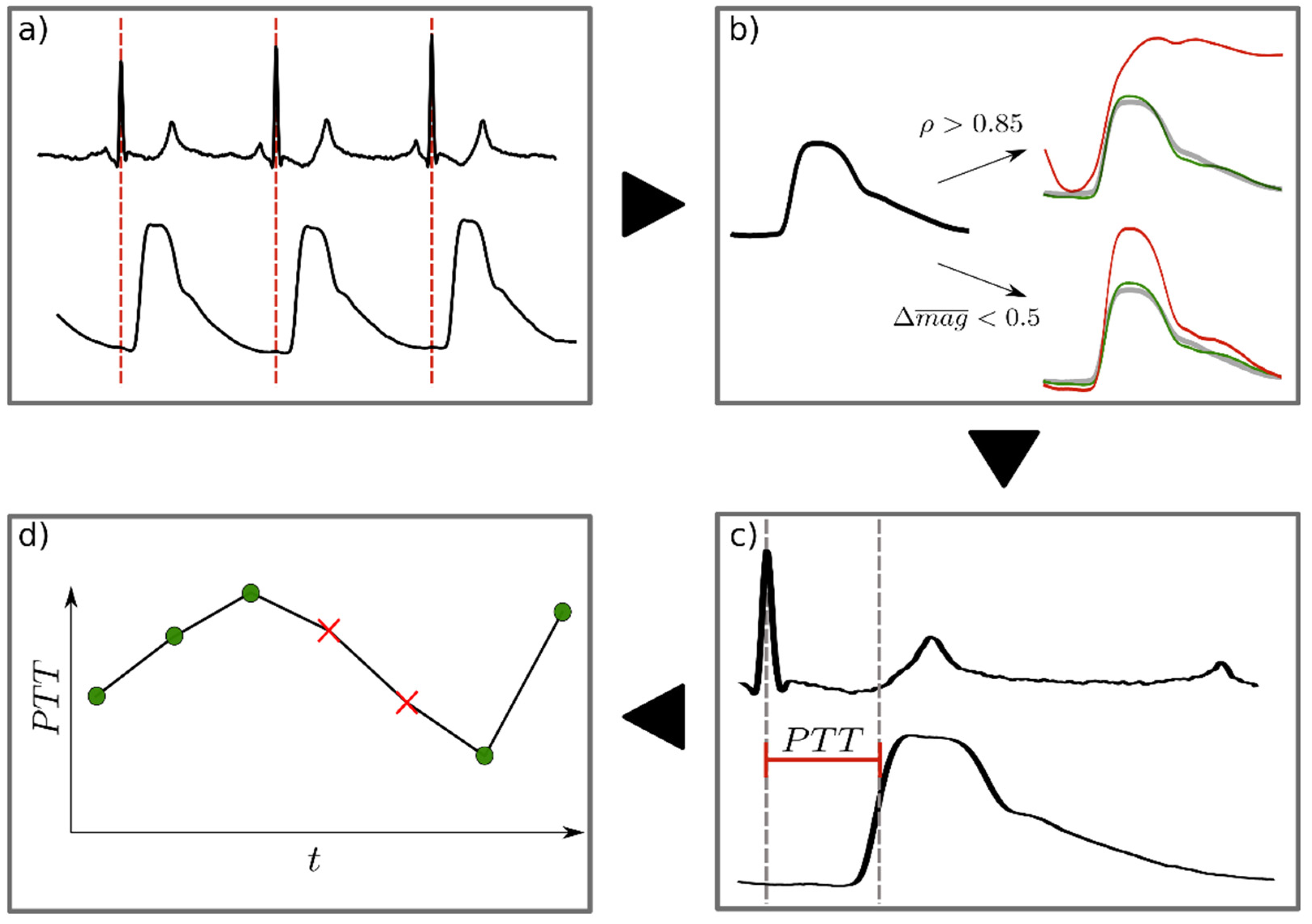
Data Analysis
- ▪ ρ > 0.85
- ▪ The absolute relative difference in magnitude to the mean PW (Δmag) is below 0.5
Statistical Analysis
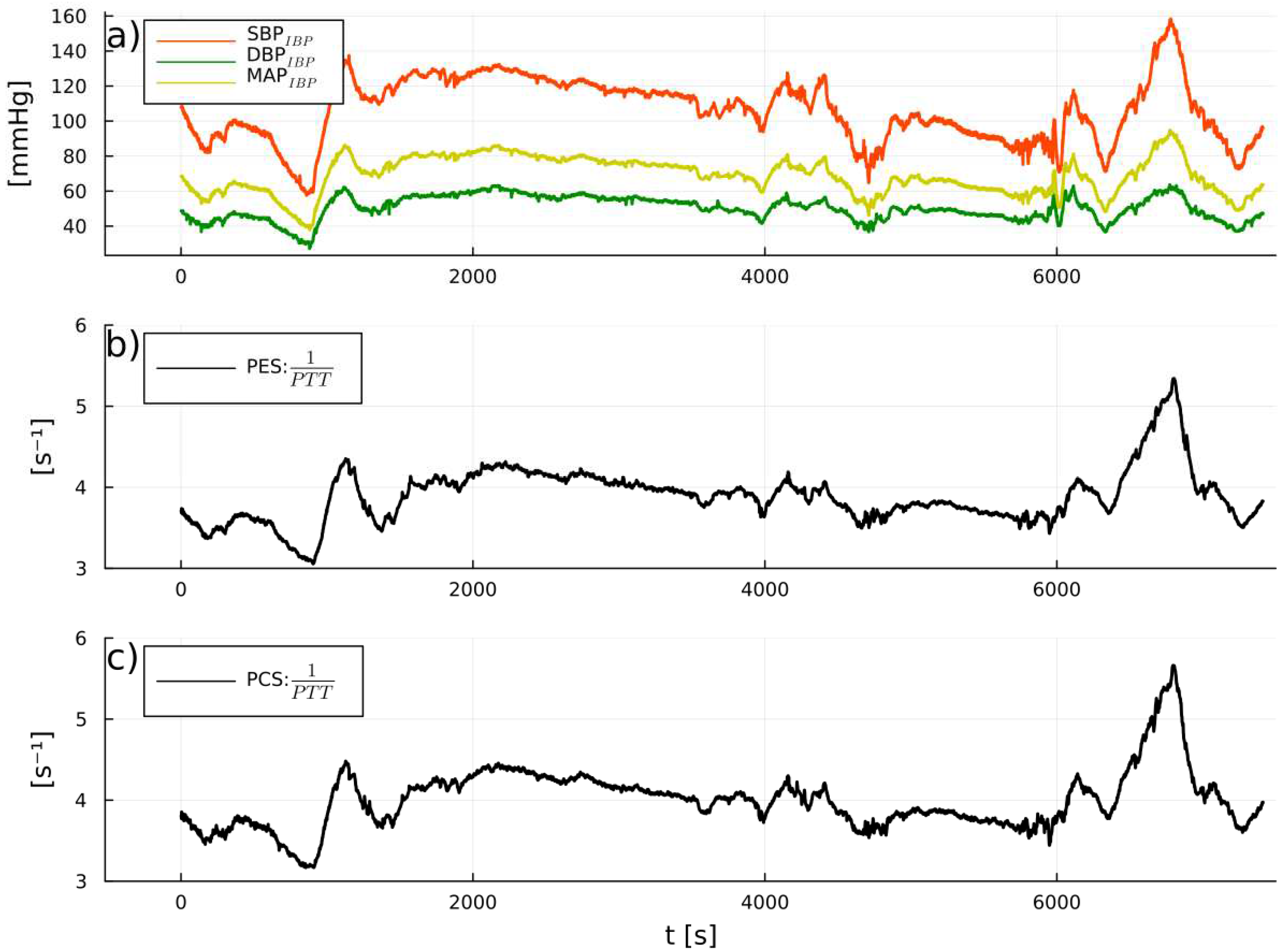
3. Results
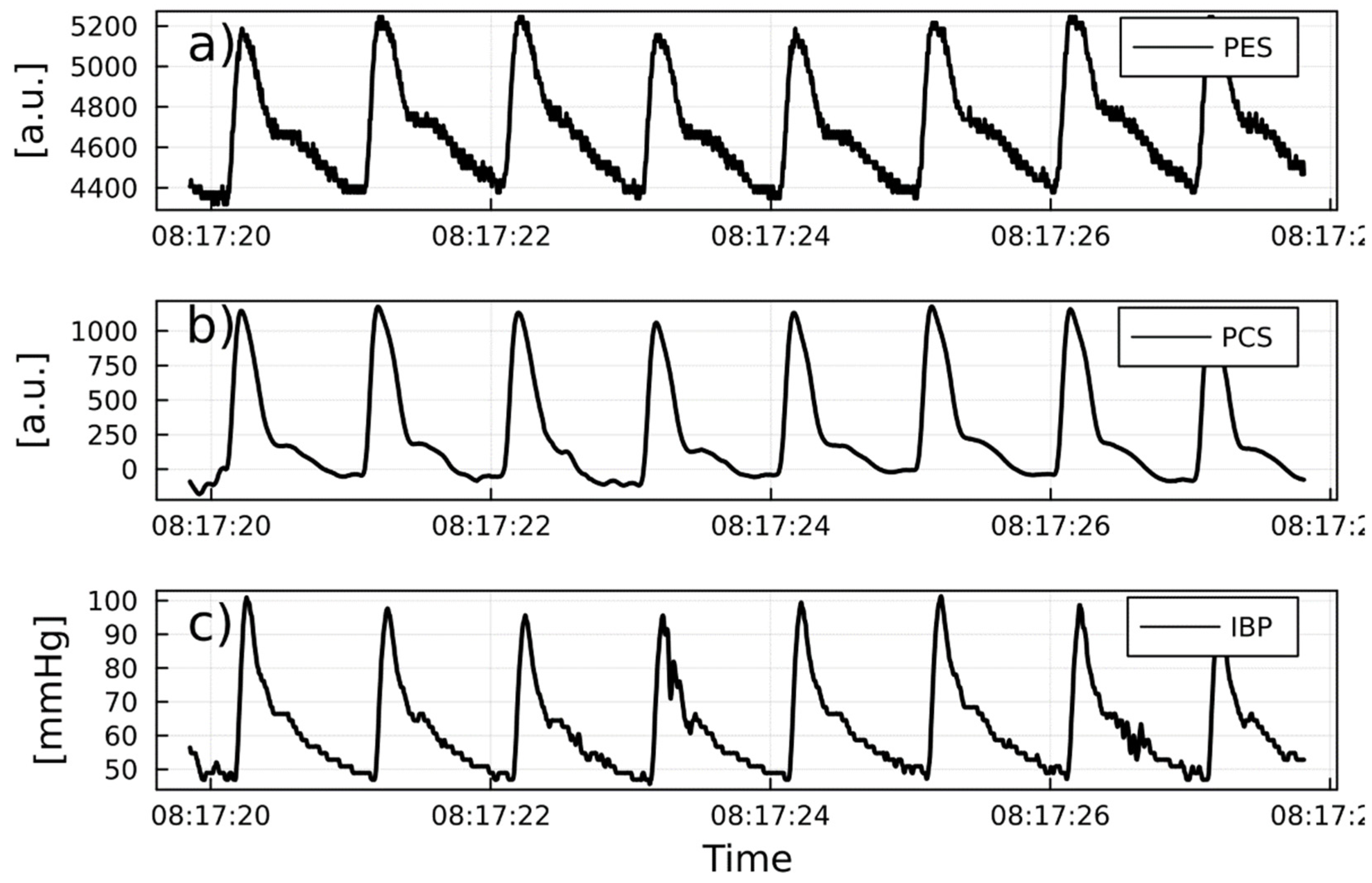
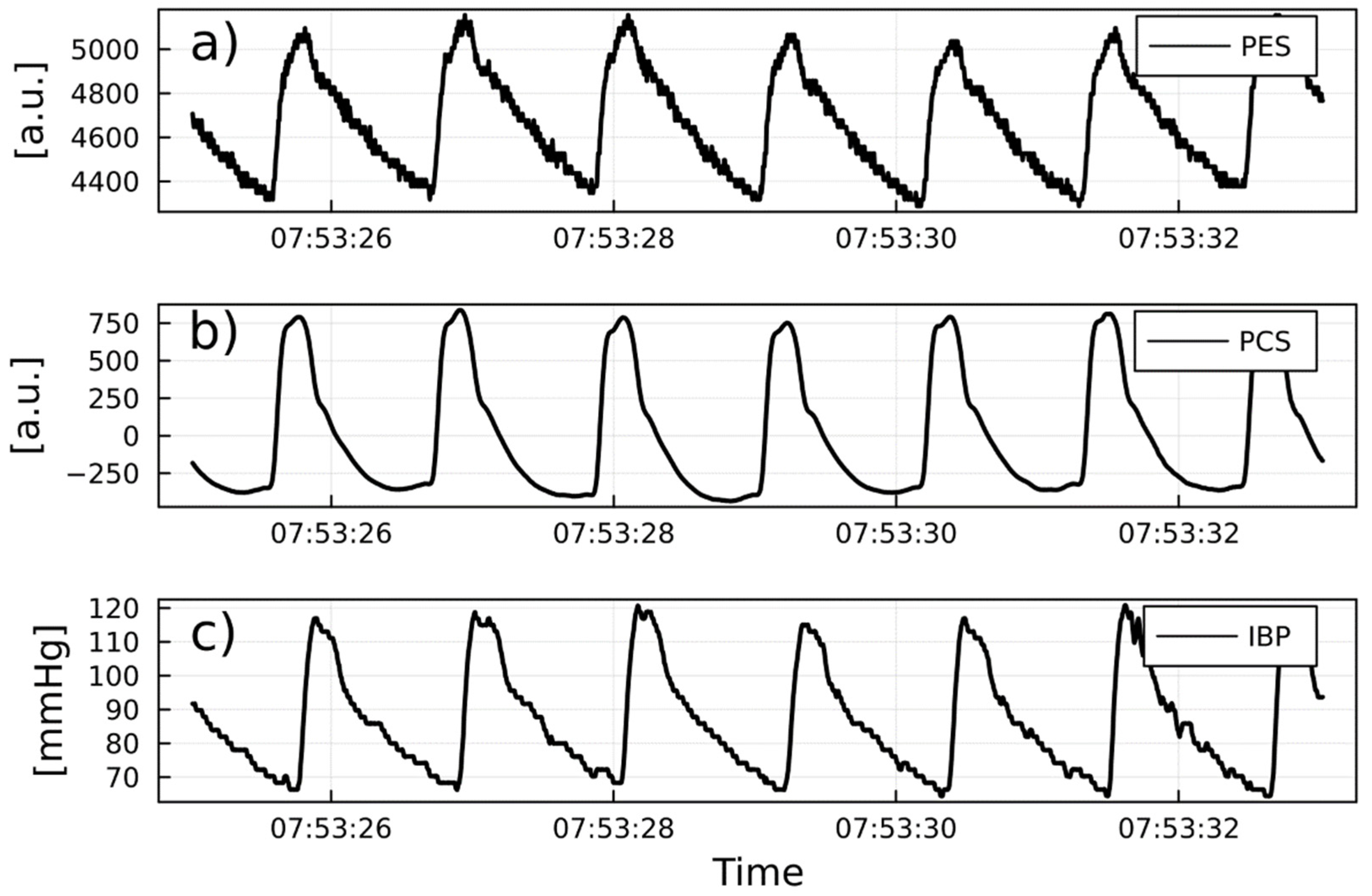
PW reliability detection
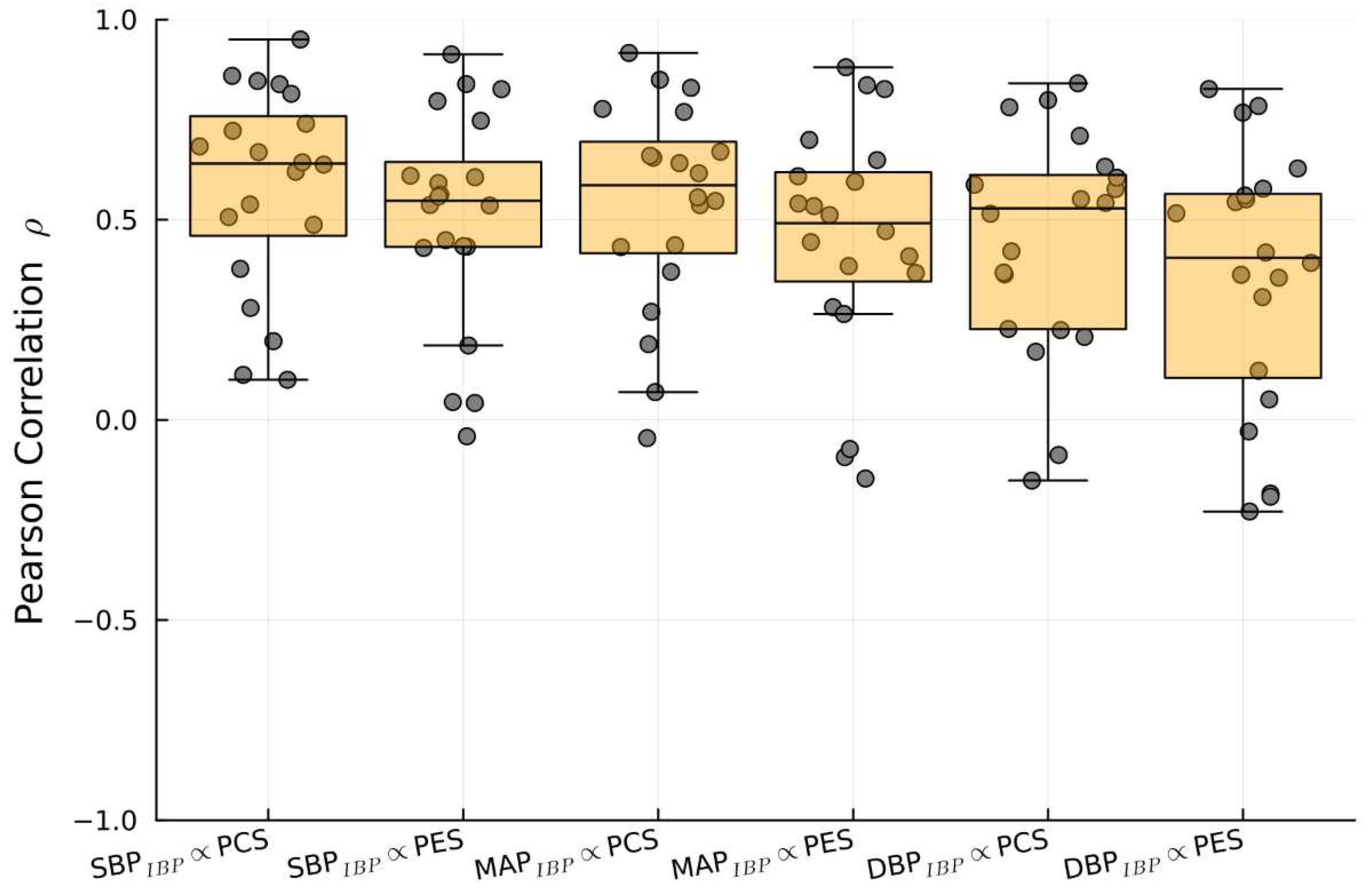
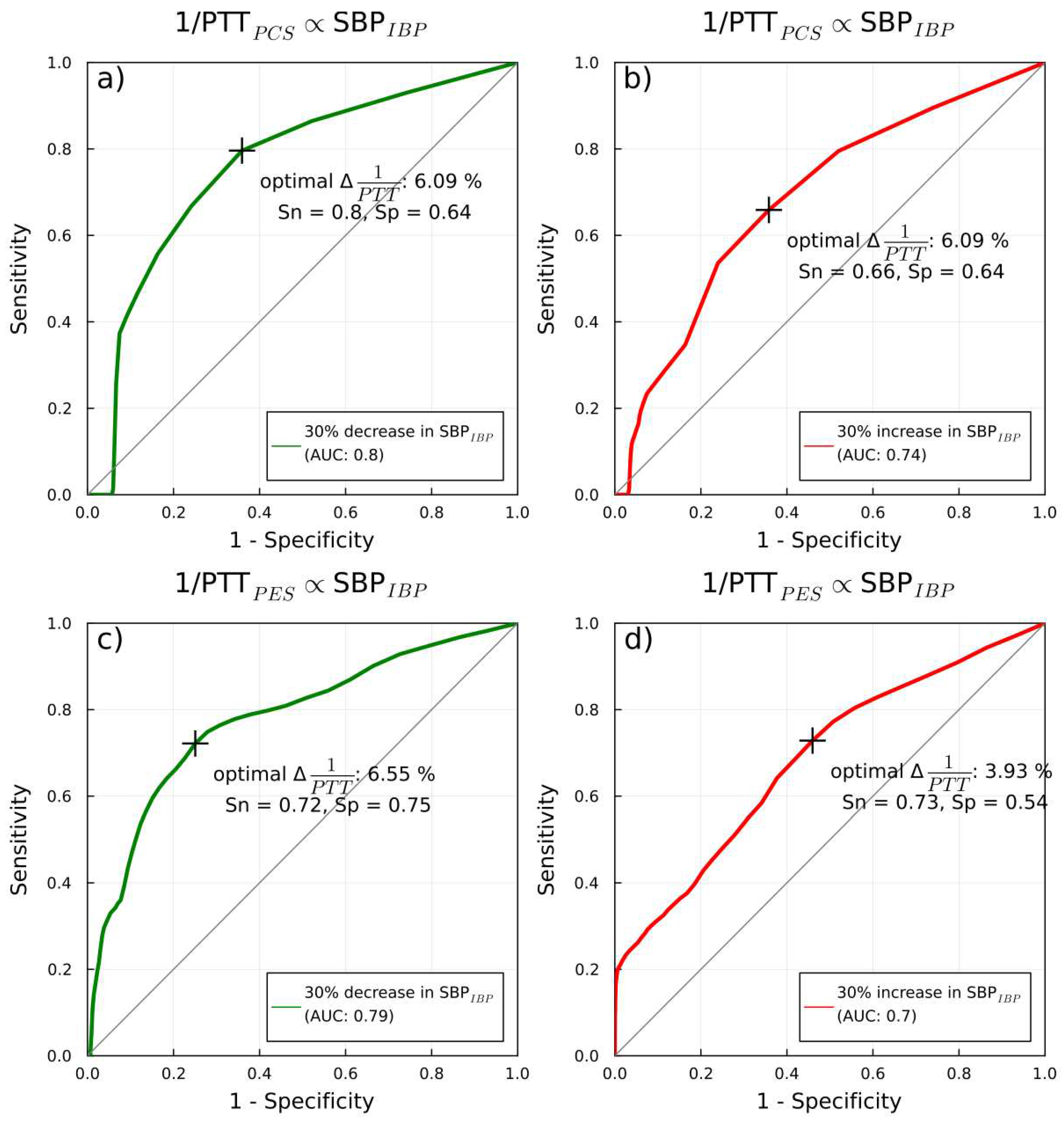
1/PTT and IBP: correlations and predicitive capabilities
4. Discussion
Prior PES/PEC research
PTT: correlation and predictive capabilities
Limitations
Summary and future applications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- L. Lonjaret, O. Lairez, T. Geeraerts, and V. Minville, “Optimal perioperative management of arterial blood pressure,” Integr. Blood Press. Control, p. 49, Sep. 2014. [CrossRef]
- L. Meng, W. L. Meng, W. Yu, T. Wang, L. Zhang, P. M. Heerdt, and A. W. Gelb, “Blood Pressure Targets in Perioperative Care: Provisional Considerations Based on a Comprehensive Literature Review,” Hypertension, vol. 72, no. 4, pp. 806–817, Oct. 2018. [CrossRef]
- S. Packiasabapathy K and B. Subramaniam, “Optimal Perioperative Blood Pressure Management,” Adv. Anesth., vol. 36, no. 1, pp. 67–79, Dec. 2018. [CrossRef]
- B. Saugel and D. I. Sessler, “Perioperative Blood Pressure Management,” Anesthesiology, vol. 134, no. 2, pp. 250–261, Feb. 2021. [CrossRef]
- T. Yamada, S. Vacas, Y. Gricourt, and M. Cannesson, “Improving Perioperative Outcomes Through Minimally Invasive and Non-invasive Hemodynamic Monitoring Techniques,” Front. Med., vol. 5, p. 144, May 2018. [CrossRef]
- S. G. Schaanning and N. K. Skjaervold, “Rapid declines in systolic blood pressure are associated with an increase in pulse transit time,” PLOS ONE, vol. 15, no. 10, p. e0240126, Oct. 2020. [CrossRef]
- N. Pilz, A. N. Pilz, A. Patzak, and T. L. Bothe, “Continuous cuffless and non-invasive measurement of arterial blood pressure—concepts and future perspectives,” Blood Press., vol. 31, no. 1, pp. 254–269, Dec. 2022. [CrossRef]
- R. Mukherjee, S. Ghosh, B. Gupta, and T. Chakravarty, “A Literature Review on Current and Proposed Technologies of Noninvasive Blood Pressure Measurement,” Telemed. E-Health, vol. 24, no. 3, pp. 185–193, Mar. 2018. [CrossRef]
- T. Athaya and S. Choi, “A Review of Noninvasive Methodologies to Estimate the Blood Pressure Waveform,” Sensors, vol. 22, no. 10, p. 3953, May 2022. [CrossRef]
- T. Katsuura, S. Izumi, M. Yoshimoto, H. Kawaguchi, S. Yoshimoto, and T. Sekitani, “Wearable pulse wave velocity sensor using flexible piezoelectric film array,” in 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Torino, Oct. 2017, pp. 1–4. [CrossRef]
- Bijender and, A. Kumar, “One-Rupee Ultrasensitive Wearable Flexible Low-Pressure Sensor,” ACS Omega, vol. 5, no. 27, pp. 16944–16950, Jul. 2020. [CrossRef]
- B. You, C. J. Han, Y. Kim, B.-K. Ju, and J.-W. Kim, “A wearable piezocapacitive pressure sensor with a single layer of silver nanowire-based elastomeric composite electrodes,” J. Mater. Chem. A, vol. 4, no. 27, pp. 10435–10443, 2016. [CrossRef]
- T.-W. Wang and S.-F. Lin, “Wearable Piezoelectric-Based System for Continuous Beat-to-Beat Blood Pressure Measurement,” Sensors, vol. 20, no. 3, p. 851, Feb. 2020. [CrossRef]
- D. Benitez, P. A. Gaydecki, A. Zaidi, and A. P. Fitzpatrick, “The use of the Hilbert transform in ECG signal analysis,” Comput. Biol. Med., vol. 31, no. 5, pp. 399–406, Sep. 2001. [CrossRef]
- G. Janett, O. Steiner, E. Alsina Ballester, L. Belluzzi, and S. Mishra, “A novel fourth-order WENO interpolation technique: A possible new tool designed for radiative transfer,” Astron. Astrophys., vol. 624, p. A104, Apr. 2019. [CrossRef]
- J. Bezanson, A. Edelman, S. Karpinski, and V. B. Shah, “Julia: A Fresh Approach to Numerical Computing,” SIAM Rev., vol. 59, no. 1, pp. 65–98, Jan. 2017. [CrossRef]
- S.-H. Kim, J.-G. Song, J.-H. Park, J.-W. Kim, Y.-S. Park, and G.-S. Hwang, “Beat-to-Beat Tracking of Systolic Blood Pressure Using Noninvasive Pulse Transit Time During Anesthesia Induction in Hypertensive Patients,” Anesth. Analg., vol. 116, no. 1, pp. 94–100, Jan. 2013. [CrossRef]
- Z. Yi et al., “Piezoelectric Dynamics of Arterial Pulse for Wearable Continuous Blood Pressure Monitoring,” Adv. Mater., vol. 34, no. 16, p. 2110291, Apr. 2022. [CrossRef]
- C.-Y. Guo, K.-J. Wang, and T.-L. Hsieh, “Piezoelectric Sensor for the Monitoring of Arterial Pulse Wave: Detection of Arrhythmia Occurring in PAC/PVC Patients,” Sensors, vol. 21, no. 20, p. 6915, Oct. 2021. [CrossRef]
- F. Clemente, P. Arpaia, and P. Cimmino, “A piezo-film-based measurement system for global haemodynamic assessment,” Physiol. Meas., vol. 31, no. 5, pp. 697–714, May 2010. [CrossRef]
- J. McLaughlin, M. McNeill, B. Braun, and P. D. McCormack, “Piezoelectric sensor determination of arterial pulse wave velocity,” Physiol. Meas., vol. 24, no. 3, pp. 693–702, Aug. 2003. [CrossRef]
- J. C. Murphy, K. Morrison, J. McLaughlin, G. Manoharan, and A. J. Adgey, “An Innovative Piezoelectric-Based Method for Measuring Pulse Wave Velocity in Patients With Hypertension: Measuring Pulse Wave Velocity Based on Piezoelectric Method,” J. Clin. Hypertens., vol. 13, no. 7, pp. 497–505, Jul. 2011. [CrossRef]
- S.-K. Xu et al., “Validation of a Piezoelectric Sensor Array-Based Device for Measurement of Carotid-Femoral Pulse Wave Velocity: The Philips Prototype,” Pulse, vol. 5, no. 1–4, pp. 161–168, 2017. [CrossRef]
- E. Finnegan et al., “Pulse arrival time as a surrogate of blood pressure,” Sci. Rep., vol. 11, no. 1, p. 22767, Dec. 2021. [CrossRef]
- M. Gao, N. B. Olivier, and R. Mukkamala, “Comparison of noninvasive pulse transit time estimates as markers of blood pressure using invasive pulse transit time measurements as a reference,” Physiol. Rep., vol. 4, no. 10, p. e12768, May 2016. [CrossRef]
- P. A. Obrist, K. C. Light, J. A. McCubbin, J. S. Hutcheson, and J. L. Hoffer, “Pulse transit time: Relationship to blood pressure,” Behav. Res. Methods Instrum., vol. 10, no. 5, pp. 623–626, Sep. 1978. [CrossRef]
- R. A. Payne, C. N. Symeonides, D. J. Webb, and S. R. J. Maxwell, “Pulse transit time measured from the ECG: an unreliable marker of beat-to-beat blood pressure,” J. Appl. Physiol., vol. 100, no. 1, pp. 136–141, Jan. 2006. [CrossRef]
- G. Sharwood-Smith, J. Bruce, and G. Drummond, “Assessment of pulse transit time to indicate cardiovascular changes during obstetric spinal anaesthesia,” Br. J. Anaesth., vol. 96, no. 1, pp. 100–105, Jan. 2006. [CrossRef]
- J. G. Kips et al., “Evaluation of Noninvasive Methods to Assess Wave Reflection and Pulse Transit Time From the Pressure Waveform Alone,” Hypertension, vol. 53, no. 2, pp. 142–149, Feb. 2009. [CrossRef]
- D. R. Wagner et al., “Relationship between pulse transit time and blood pressure is impaired in patients with chronic heart failure,” Clin. Res. Cardiol., vol. 99, no. 10, pp. 657–664, Oct. 2010. [CrossRef]
- C. Ahlstrom, A. Johansson, F. Uhlin, T. Länne, and P. Ask, “Noninvasive investigation of blood pressure changes using the pulse wave transit time: a novel approach in the monitoring of hemodialysis patients,” J. Artif. Organs, vol. 8, no. 3, pp. 192–197, Oct. 2005. [CrossRef]
- J. Bolea et al., “Pulse Rate and Transit Time Analysis to Predict Hypotension Events After Spinal Anesthesia During Programmed Cesarean Labor,” Ann. Biomed. Eng., vol. 45, no. 9, pp. 2253–2263, Sep. 2017. [CrossRef]
- K. Kouz, P. Hoppe, L. Briesenick, and B. Saugel, “Intraoperative hypotension: Pathophysiology, clinical relevance, and therapeutic approaches,” Indian J. Anaesth., vol. 64, no. 2, p. 90, 2020. [CrossRef]
- D. I. Sessler et al., “Perioperative Quality Initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery,” Br. J. Anaesth., vol. 122, no. 5, pp. 563–574, May 2019. [CrossRef]
- L. Brunaud et al., “Predictive factors for postoperative morbidity after laparoscopic adrenalectomy for pheochromocytoma: a multicenter retrospective analysis in 225 patients,” Surg. Endosc., vol. 30, no. 3, pp. 1051–1059, Mar. 2016. [CrossRef]
- Y.-Y. Hsieh, C.-D. Wu, S.-S. Lu, and Y. Tsao, “A linear regression model with dynamic pulse transit time features for noninvasive blood pressure prediction,” in 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, Oct. 2016, pp. 604–607. [CrossRef]
- S. Singham, L. Voss, J. Barnard, and J. Sleigh, “Nociceptive and anaesthetic-induced changes in pulse transit time during general anaesthesia,” Br. J. Anaesth., vol. 91, no. 5, pp. 662–666, Nov. 2003. [CrossRef]
- M. C. Kortekaas, S. P. Niehof, M. H. N. Van Velzen, E. M. Galvin, F. J. P. M. Huygen, and R. J. Stolker, “Pulse transit time as a quick predictor of a successful axillary brachial plexus block: PTT as a predictor of an axillary block,” Acta Anaesthesiol. Scand., vol. 56, no. 10, pp. 1228–1233, Nov. 2012. [CrossRef]
- Q. Qananwah, A. Dagamseh, H. Alquran, K. S. Ibrahim, M. Alodat, and O. Hayden, “A comparative study of photoplethysmogram and piezoelectric plethysmogram signals,” Phys. Eng. Sci. Med., vol. 43, no. 4, pp. 1207–1217, Dec. 2020. [CrossRef]
- A. M. Youn, Y. S. Shin, and S. I. Park, “Changes in pulse transit time according to target controlled infusion of propofol versus sevoflurane inhalation induction,” Anesth. Pain Med., vol. 9, no. 1, pp. 48–53, Jan. 2014. [CrossRef]
- A. Mol, C. G. M. Meskers, S. P. Niehof, A. B. Maier, and R. J. A. van Wezel, “Pulse transit time as a proxy for vasoconstriction in younger and older adults,” Exp. Gerontol., vol. 135, p. 110938, Jul. 2020. [CrossRef]
- S. Dash, K. H. Shelley, D. G. Silverman, and K. H. Chon, “Estimation of Respiratory Rate From ECG, Photoplethysmogram, and Piezoelectric Pulse Transducer Signals: A Comparative Study of Time–Frequency Methods,” IEEE Trans. Biomed. Eng., vol. 57, no. 5, pp. 1099–1107, May 2010. [CrossRef]
- P. Samartkit, S. Pullteap, and O. Bernal, “A non-invasive heart rate and blood pressure monitoring system using piezoelectric and photoplethysmographic sensors,” Measurement, vol. 196, p. 111211, Jun. 2022. [CrossRef]
- Z. Zhang, S. Ansari, L. Wang, K. D. Aaronson, J. R. Golbus, and K. R. Oldham, “Noninvasive Systemic Vascular Resistance Estimation using a Photoplethysmogram and a Piezoelectric Sensor,” IFAC-Pap., vol. 54, no. 20, pp. 298–303, 2021. [CrossRef]

| Patient characteristics | |
| Gender (F/M) | 12/8 |
| Age (years) | 62 ± 11.5 |
| Height (cm) | 170 ± 0.15 |
| Weight (kg) | 78 ± 18 |
| BMI (kg/m2) | 25 ± 8.2 |
| BSA (m2) | 1.93 ± 0.3 |
| ASA classification II III |
10 10 |
| Co-morbidities Hypertension Coronary artery disease Diabetes Mellitus Heart Failure Chronic kidney disease Hypothyroidism Asthma COPD |
10 5 4 2 2 2 2 1 |
| Type of surgery Cardiac (Coronary artery bypass grafting [CAB]) Abdominal (Whipple) (Pancreas resection) (Intestinal resection) (Hepatic resection) Urological (nephritic) (cholecystectomy) |
7 7 6 |
| Average length of surgery (minutes) Cardiac Abdominal Urological |
213 ± 30 214 ± 110 323 ± 23 |
| Inoperative vasoactive medications Noradrenaline (infusion) Dopamine (infusion) Enoximone (infusion) Caffeine/Theodrenaline (Bolus) Atropine (Bolus) |
17 2 2 14 6 |
| Sources compared | Pearson Correlation (median and IQR) |
| SBPIBP vs.1/PTTPES | 0.64 (0.32) |
| SBPIBP vs. 1//PTTPCS | 0.55 (0.28) |
| DBPIBP vs. 1/PTTPES | 0.55 (0.36) |
| DBPIBP vs. 1/PTTPCS | 0.45 (0.40) |
| MAPIBP vs. 1/PTTPES | 0.6 (0.31) |
| MAPIBP vs. 1/PTTPCS | 0.5 (0.27) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





