1. Introduction
Viruses, bacteria and parasites are among those pathogens which play an immense role in developing infectious diseases. Infections that are caused by pathogens are important to be diagnosed at their earliest stage as they are transmitted between animals and human carriers by means of inoculation from different media like water and air. [
1,
2] On the other hand, pathogen detection is not only important in the health care sector but is also important regarding safety issues in areas such as food, water or production facilities.[
3] Therefore, it is important to detect them so that treatment can be initiated properly. Along with treatment, its prevention is also necessary, which calls for a low cost, rapid, on-site and accurate diagnosis, especially in areas which are devoid of resources and have severe and prevalent infections.[
4]
In this perspective, microfluidic technology which combines material science, nanotechnology, micro electromechanical system for specified fluidic manipulations provide opportunities for Point of Care Testing (POCT) devices for detection of pathogens and diseases. In comparison to conventional diagnostic methods, portable microfluidic devices employ miniaturized devices that range from large laboratory analyzers to small disease specific screening strips such as lateral flow assays by which test can be performed near the sampling site without any need of a laboratory. The reason behind fast expansion of microfluidics is the development of small-scale components that allow handling of materials at a microscale level.[
5,
6] Among the multiple methods for disease diagnosis, molecular diagnosis is the most reliable, precise and sensitive method.[
7]
The first and most important advantage of microfluidic technology is low consumption of reagents, higher surface to volume ratio, high throughput applications, high spatial temporal resolution, portable systems and rapid prototyping.[
8] Conventional methods used for direct detection of nucleic acid or specific antigen consist of Polymerase Chain Reaction (PCR), microarray, Enzyme Linked Immunosorbent Assay (ELISA), isothermal amplification, Clustered Regulatory Interspaced Short Palindromic Repeats (CRISPR) and aptamer-based detection. Whereas indirect methods of nucleic acid detection involve the detection of antibodies generated by patients in response to infection. The indirect approach has a wide range of applications with some limitations including low specificity and accuracy.[
9,
10] The four main players that require accurate detection of nucleic acid are healthcare, agriculture, bio defense and environmental monitoring.[
11]
Here we have discussed the role of microfluidic technology in detection of nucleic acid with respect to health and safety. Then we discussed ways for amplification of nucleic acid in microfluidic system and use of microfluidics integrated lateral flow assays. Furthermore, the prospects and challenges faced by microfluidic technology in diagnostics have been debated at the end.
2. Microfluidic technology and POCT
2.1. Significance of POCT in diagnostics
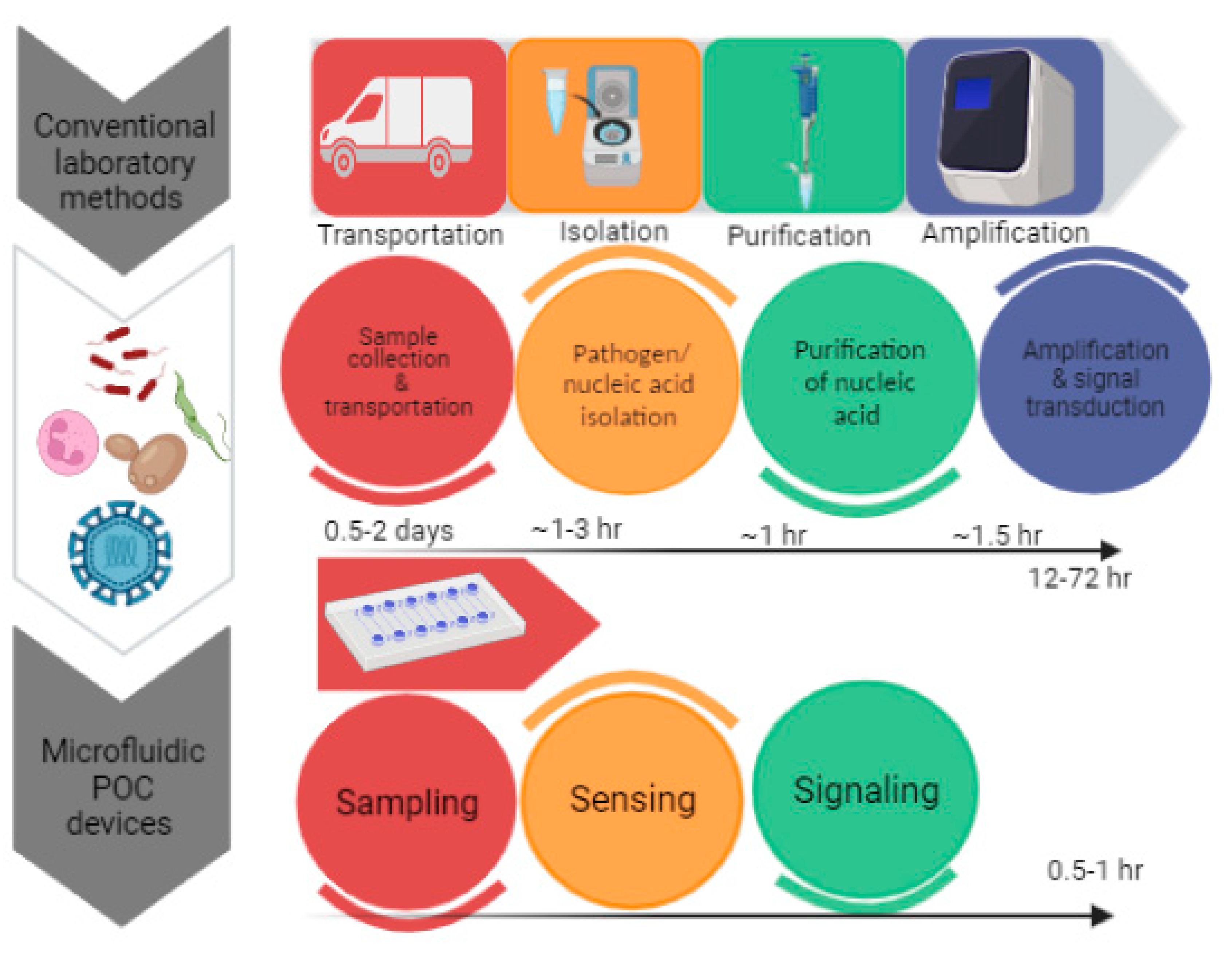
In comparison to traditional diagnostics, the POCT devices regulated by microfluidics utilize portable and diminutive devices for performing tests near to the sampling sites. The advancements have led to the development of rapid amplification of signals with enhanced sensitivity by excluding laborious sample preparation steps. In the field of molecular diagnostics, recent advancements have been made for the development of a nucleic acid-based diagnosis of disease. The three main steps in nucleic acid testing involve sample pre-processing, amplification of nucleic acid and signal read out.[
12]
POCT devices are not a replacement for sensitive laboratory tests but provide an initial screening of diseases in a non-laboratory set-up. By recognizing the significance of POCT devices, the World Health Organization has set a criterion for evaluation of medical diagnosis. According to WHO a POCT device has been given an acronym of ASSURED which means that test must be ‘Affordable, Sensitive, Specific, User friendly, Rapid and robust, Equipment free & Deliverable’. [
13]
Figure 1.
Significance of POCT methods in diagnostics.
Figure 1.
Significance of POCT methods in diagnostics.
2.2. Microfluidic-POCT devices
The first and most important step in sample preparation for nucleic acid testing is cell lysis. Chemical and mechanical cell lysis methods are commonly deployed for this purpose.[
14] For the on-chip testing of nucleic acid, chips can be divided into three parts for sampling, sensing and signaling. Because of the stability, biocompatibility and modifiable characteristics, silicon proves to be a suitable substance for nucleic acid extraction.[
15] Porous materials have been used in POCT devices for diagnostic purposes for extraction of nucleic acid. Use of Finders Technology Associates (FTA) cards is a commercially available solid phase nucleic acid extraction technology used to fabricate POCT devices coupled with amplification. FTA cards can be coupled with Loop-mediated isothermal AMPlification (LAMP) cassette for detection of Human Immunodeficiency Virus (HIV).[
14] Microfluidic devices require sample volume less than a 100 µl for downstream detection purposes. [
16] Three types of reactors have been used for microfluidics PCR. For a microfluidic PCR, the thermal cycling time is calculated in minutes and size of reactor is required in μL, whereas a microfluidic PCR is defined as γ.[
17] The first type of reactor is termed as a stationary reactor, forced continuous flow Polymerase Chain Reaction is the second type of reactor and third type of reactor is composed of a free heat deportation system.[
17,
18,
19,
20]
2.3. Materials for microfluidic devices
Mainly three types of materials are used for developing microfluidic devices. These include paper, polymeric and inorganic materials. Initially glass and silicon materials were used. Polymeric substances that were introduced later have been further categorized into two fields including thermoplastics and elastomers. Inorganic materials include cofired low temperature ceramics and Vitro ceramics. Paper microfluidics have been termed a somewhat different technology than polymeric and inorganic microfluidics. Application, level of integration and function are the three factors that must be analysed before selection of material type used for microfluidic system. Some physical characteristics that must be considered before material selection are air permeability, flexibility, non-specific adsorption, electrical conductivity, cellular compatibility, optical transparency and solvent compatibility.[
21]
2.4. Nucleic acid-based detection strategies
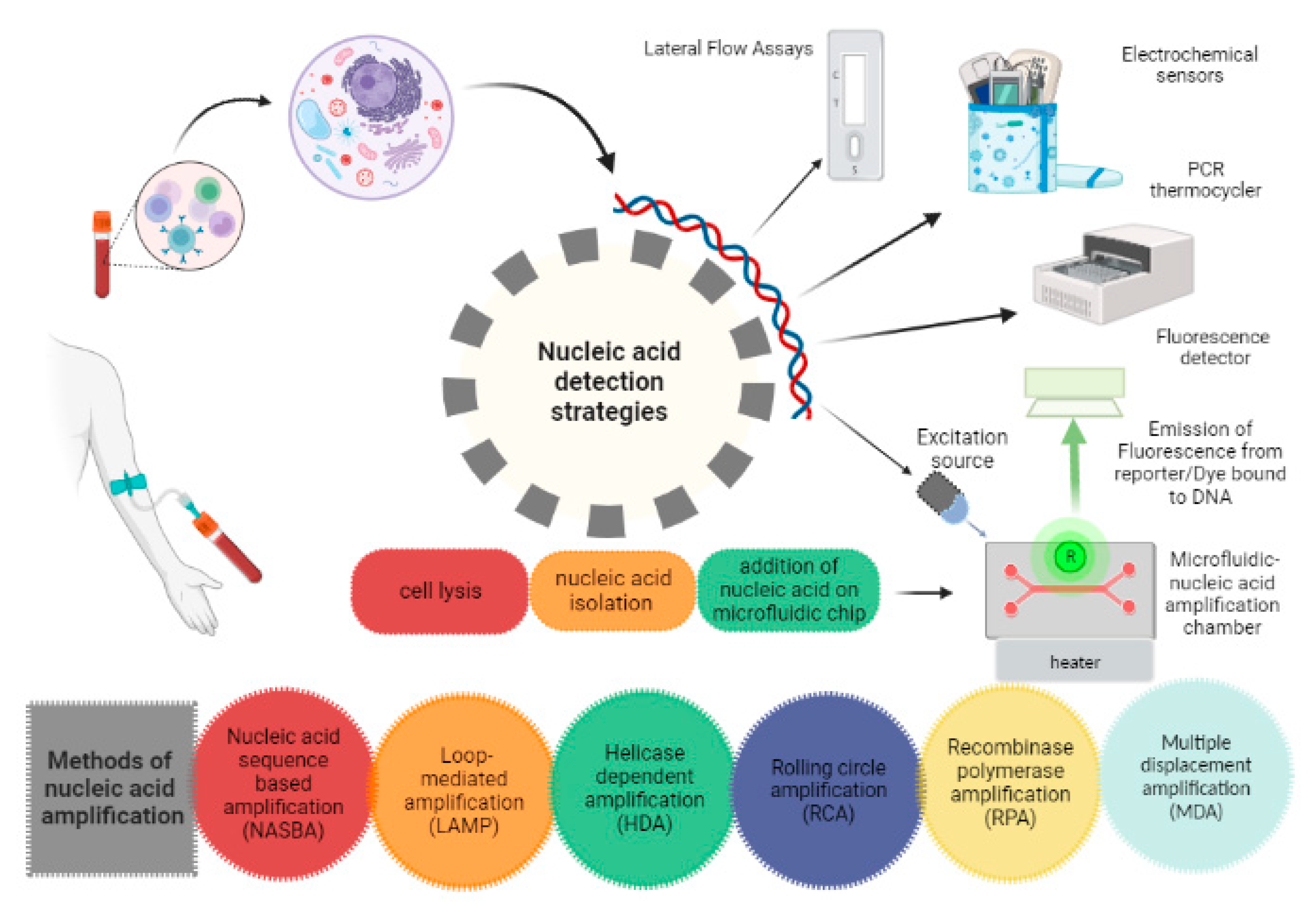
Nucleic acid testing (NAT) offers many advantages over conventional immunoassays due to better sensitivity and specificity. The most used laboratory techniques for nucleic acid detection include PCR, Real-time PCR and Reverse-transcription PCR. A membrane embedded in microfluidic chip binds to DNA isolated from the cell. The nucleic acid is further amplified at a constant temperature and the amplified product containing fluorophore reporter is detected by a detector (CCD detector or photodiode) initially after excitation with Light Emitting Diode (LED) based light source.[
22]To carry out NAT on microfluidic POCT, the device must be fabricated when it is in its initial manufacturing phase. The continually used fabrication techniques for microfluidic systems are printing and cutting, photolithography and moulding.[
23] Typical problems of DNA amplification in microchannels such as bubble generation, cross contamination and reagent evaporation, which can be minimized by sealing with Polydimethylsiloxane (PDMS).[
24]
Figure 2.
Nucleic acid-based detection strategies.
Figure 2.
Nucleic acid-based detection strategies.
2.5. Methods of isothermal amplification
2.5.1. Loop-mediated isothermal AMPlification (LAMP)
Two sets of specially designed inner and outer primers are utilized in this method called forward inner primer (FIP) and backward inner primer (BIP) along with a DNA polymerase having the activity of strand displacement. Moreover, four other primers are also used for enhancing specificity of amplification. After target sequence recognition, amplification is performed within one hour. In LAMP, 60 to 65°C temperate is required to carry out amplification and the amplified product is simply detected by naked eye because of the formation of white precipitates which are by-products of pyrophosphate.[
25] One such device has been developed for amplification as well as nucleic acid detection of Hepatitis B Virus (HBV). [
26,
27] However this technique is not suitable for amplification of short genes. [
28] Contributions has been made in this area for reducing the reaction volume to 5µl in a microfluidic integrated LAMP device termed as microLAMP.[
26]
2.5.2. Helicase Dependent Amplification (HDA)
By understanding the properties of helicases that were discovered in 1976 in Escherichia coli. Researchers have combined polymerase with helicases along with some other additional proteins for nucleic acid amplification procedure. HDA utilizes the activity of DNA helicase for separation of double stranded DNA (dsDNA) thus mimicking the denaturation step required in conventional PCR. Which is followed by replication by DNA Polymerase. Chemical energy is used in the formation of a replication fork which enables initiation of replication. Two target sequence specific primers are used which anneal to 3’ end of both strands of single stranded DNA (ssDNA).[
29] Microfluidic chip for HDA (isothermal amplification) has been integrated with a small-scale solid phase extraction column for isolation of DNA.[
30] A new method was adopted for detection of ruminant faecal contamination source by HDA integrated on strip. [
31]
2.5.3. Rolling Circle Amplification (RCA)
This approach utilizes amplification of Circular DNA by using a unique DNA polymerase having activity of strand-displacement. Constant temperature is required for carrying out amplification and ssDNA containing tandem repeats of circular template is produced. Both linear and exponential methods are used to perform RCA. Amplification efficiency of this method depends upon the requirement of circular and small ssDNA as a template. However, most of the DNA templates required for diagnosis are single stranded DNA. To avoid this problem, a special type of probe has been designed called padlock probes which circularizes after hybridization with the target sequence.[
32] Single molecular amplification of DNA by droplet microfluidic hyperbranched-rolling circle amplification has been developed. Nowadays, droplet digital RCA systems have been developed for sensitive and rapid detection of cancerous cells derived extracellular vesicles.[
33] An electrochemical aptasensor (ultrasensitive) was developed for multiple exosome biomarker detection in breast cancer which was based on dual rolling circle amplification.[
34]
2.5.4. Multiple Displacement Amplification (MDA)
This amplification approach is used for amplification of whole genome by generating many amplicons from a small number of molecules of DNA. Primers are resistant random exonuclease and there is strand displacement activity due to which no thermal cycling is needed for the reaction. Specificity of MDA has been increased by utilization of microfluidic reactors having nano litre size and a cell sorting chamber for isolation of individual selected cells. Non-specific amplification is reduced by reducing the reaction volumes from µl to nl. [
35,
36,
37] The technique has been expanding rapidly because of its ability to examine and differentiate defective DNA. MDA has been used in diagnosis of Tuberculosis by integrating MDA specific PCR for amplification of DNA in a two-step process. [
38]
2.5.6. Recombinase Polymerase Amplification (RPA)
DNA is amplified at a low temperature i.e., 37 °C by using a DNA polymerase, DNA binding proteins and a recombinase. The binding of primer with DNA template is carried out by using complexes of nucleoproteins consisting of oligonucleotide primers and recombinase. This complex is used for scanning of dsDNA followed by binding of primer at target sequence of dsDNA and non-template strand displacement. Single stranded DNA binding proteins (SSBP) stabilize the displaced strand and recombinase make the 3’ end accessible for DNA Polymerase.
A simple and low-cost method was used for development of microfluidic chip for RPA by combining dry film resist technique and direct wafer bonding.[
39] As RPA reaction is carried out at or near to room temperature range, premixing of sample with initial reagent may proceed the reaction without compartmentalization. Inspite of its little applications in clinical sectors, authentications have been made regarding its use in detection of virus, bacteria, protozoa and human pathogens. [
40,
41]
2.5.7. Nucleic Acid Sequence based Amplification (NASBA)
An amplification method performed at 41°C, especially designed for amplification of ssDNA or RNA sequences. This method is not suitable for dsDNA templates as there is no denaturation step. Preferable templates for carrying out NASBA are mRNA, genomic RNA and rRNA. two RNA sequence specific primers and three enzymes are used in this technique. These enzymes are T7 DNA dependent RNA polymerase (DdRp), avian myeloblastosis virus reverse transcriptase and RNase H. A microfluidic device has been developed for immuno NASBA detection of waterborne pathogens. This lab-on-chip device relies on the utilization of different antibodies for recognition of various targets in a single step procedure.[
42]
Table 1.
Comparison of isothermal methods for nucleic acid amplification.
Table 1.
Comparison of isothermal methods for nucleic acid amplification.
| Isothermal method |
Template |
Time |
Primers |
Tm (◦C) |
Ref. |
| LAMP |
DNA/RNA |
15-60 min |
3 pairs |
60-65 |
[43,44] |
| HDA |
DNA, rRNA |
1-1.5 h |
1 pair |
60-65 |
[45,46] |
| RCA |
cssDNA, RNA, miRNA |
1h |
1 single primer, 1 padlock probe |
25-37 |
[47,48] |
| MDA |
dsDNA |
2 h |
Random hexamer |
30 |
[49,50] |
| RPA |
DNA/RNA |
5-7 min |
1 pair |
37-42 |
[44,45,46] |
| NASBA |
SsRNA, tmRNA, rRNA1
|
1.5 h |
1 pair |
41 |
[47,48] |
2.6. Nucleic Acid Lateral Flow (NALF) Assay
The nucleic acid lateral flow assay has drawn attention in point of care testing fields due to its ease of implementation, rapidity and less requirement of equipment which makes them a well-suited candidate in environmental monitoring, food authentication and diagnosis in resource limited areas. Integration of isothermal amplification on LFA increases sensitivity of assay. The two frequently used isothermal amplification strategies include RPA and LAMP.[
51] A sensitive NALFA was developed, which at lower concentration of target, gave a little difference in capturing efficiency between the capture and detector probe conjugated AuNPs. And when concentration was increased, the capturing efficiency of capture and detector probe conjugated gold nanoparticles (AuNPs) lagged behind the AuNP–detector probe conjugates.[
52] Bio-barcode designing was another approach adopted for the development of NALFA. [
53]
One approach which can be adopted to prevent the spreading of reagents during deposition of narrow microfluidic control and test line is the utilization of inkjet printers. Filter paper based LFAs fabricated with inkjet printing technology offer a confined control and test line. Despite their challenges inkjet printed micro PADs are still considered attractive. Formulation of ink is the main key to printing which require various compositions of inks. [
54]
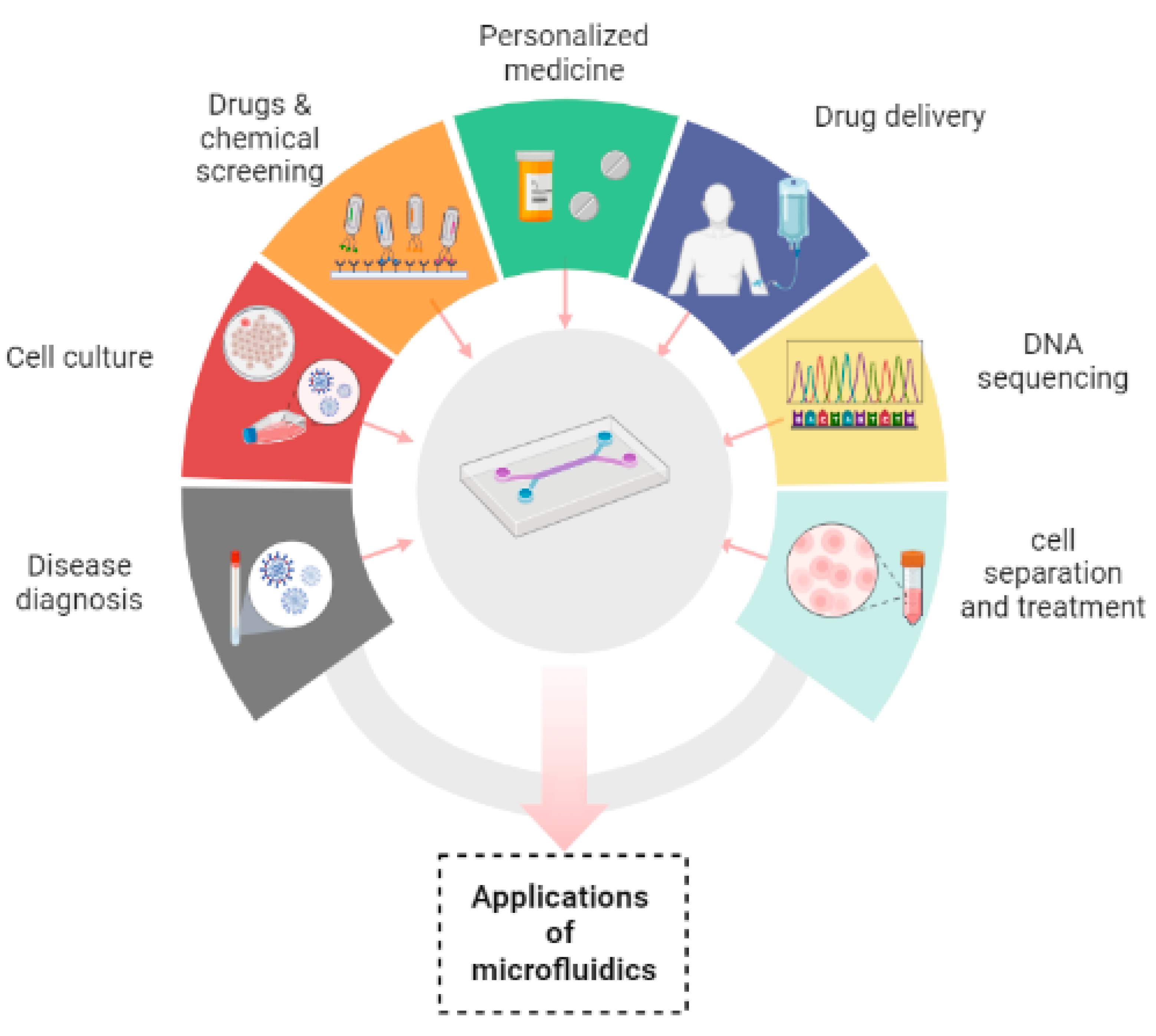
2.7. Applications of microfluidics
Microfluidic devices have made their contribution in innumerable applications by overcoming the difficulties of conventional assays. It has been manifested that the devices that are based on microfluidic systems contain great potential in disease diagnosis, personalized medicine, cell culture, chemical screening, cell separation, drug screening, cell treatment, drug delivery and DNA sequencing.[
55,
56]
In disease diagnosis, microfluidic devices play a great and robust role in analysis of numerous samples which include saliva, blood, cell tissue for the purpose of specific diagnosis.[
57] These devices have been developed for extraction of microbes in combination with many analytical systems for detection of pathogenic microbes. As a microfluidic device can easily detain airborne pathogen, therefore by simply conversion of laminar flow into twisted airflow the probability of contact between microfluidic channels and microbe can be increased. Moreover, hundreds of microbes are collected by the device in some microliters of the solution which proves to be sufficient for nucleic acid or immune analysis. There are many substances which can be detected by microfluidic systems such as creatinine. [
58,
59] Hormones are also detected by these devices; whose most common example is diagnosis of human chorionic gonadotropin hormone by a paper based microfluidic device. Recent advancements have been made in this area for not only detecting but quantifying hormone levels by displaying digitally the number of weeks of pregnancy.[
60,
61]
Microfluidics has played a promising role in studying growth of tissue and its renewal by controlling fluid flows and ensuring specific biophysical and biochemical cues to the cells that are cultured in a reproducible and good manner. Nowadays, a lot of interest has been evolving in the development of organs/ tissues on chips. There are two main reasons to do this; Firstly, experiments cannot be directly performed on humans and secondly, human physiology cannot be imitated by animal models. Furthermore, timing required for testing of drugs and their discovery can also be reduced along with investment. [
57,
62,
63]
In drug delivery microfluidic systems, precise dosage, controlled and sustained release of drug, targeted delivery, likelihood of multiple dosing and emergence of very little side effects have proved to be of great advantageous than traditional ways of drug delivery systems. Microfluidic systems have been shifted towards advanced delivery systems with 100% encapsulation efficiency (theoretically). Three main types of microfluidic systems for drug delivery have been used which include drug carrier integrated with the microfluidic Lab-on-Chip system, drug carrier free microfluidic system and microneedle-based drug delivery systems. [
64,
65] The role of nanotechnology cannot be denied in revolutionizing many aspects of medicine, more specifically in biosensors, drug delivery and therapeutics. Microfluidic devices have served nanotechnology in plethora of applications such as it has been used in synthesis of nanoparticles. Due to uniform shape, narrow size distribution, improved reproducibility and higher efficiency of encapsulation have made microfluidic devices an excellent platform for their synthesis. The microfluidic synthetic products that can be served as biosensors are AuNPs that differentiate the variable concentrations of E. coli and indicate color change of nanoparticles through an application installed in smart phone.[
51] Lipid nanoparticles such as liposomes synthesized by microfluidic systems have been widely used in drug delivery systems because of their prolonged drug delivery and enhanced stability.[
66]
In addition to already available microfluidic applications, there are some distinct fields in which microfluidics has been playing its part. These are template stickers manufacturing, contact lens sensors for assessment of physiological parameters of astronauts, devices with low sample requirement from pediatric patients, microfluidic machine learning based approach for processing data and making accurate predictions in results optimization. This approach can be of great advantage in next generation drug discovery, organ modelling and developmental biology.[
67,
68]
Figure 3.
Applications of microfluidics.
Figure 3.
Applications of microfluidics.
3. Limits, challenges and policy recommendations
Microfluidic POCT devices offer a rapid, easy to use, cost effective approach of nucleic acid testing. But at the same time the device must be sensitive, specific and reliable in clinical settings. It must be fully enclosed to avoid any contamination and evaporation of reactants. Ideally, all three steps of sample preparation, amplification of nucleic acid and signal detection must be integrated on a single chip to enhance detection efficiency. But to achieve enough detection sensitivity, sample pre-treatment is required in most of the cases. As there are limited sample preparation techniques, an efficient system must be integrated on the chip for on-site sample preparation without any need of extra steps. Furthermore, false-negative results may be obtained due to non-specific adsorption of target biomolecule to microfluidic channel walls especially when there is a very low concentration of target analyte in sample. For that, there is a need for specific modification of microfluidic channels. These modifications also play an important role in reproducibility of results and ensures sensitivity of the system. By designing different sets of primers for various targets, multiplex DNA analysis of different targets can be performed on a single chip. Developing a single chip based multiplexed microfluidic system has a wide range of applications but amplification of multiple targets in a single reactor or in multiple chambers will make the system more complex. However, there is a possibility that a better sensitivity and specificity may be obtained in this regard. Lastly, development of a user friendly, sensitive, specific, portable and low-cost detection method for multiplex detection will be a point of interest for research in the future.
4. Conclusion
The micro-structured microfluidic devices for carrying out isothermal amplification of the nucleic acid render a great capability for higher speed, cost-effective, automation of steps ranging from the preparation of sample to signal detection. Reduced sample volume requirement is of great usefulness especially where limited amount of sample is available. By integrating sample preparation and one of the several isothermal methods of amplification on microfluidic chip, nucleic acid can be isolated and amplified within minutes to hours on the chip without any need of traditional laborious sample preparation and amplification steps. Signal detection is also performed on-chip which makes it convenient and an ideal POCT device. Due to better sensitivity and specificity, NAT assays have prevailed over the use of conventional immunoassays having problems of sensitivity and selectivity. Selection of amplification type for microfluidic NAT entirely depends upon the nature of target. Despite its inception, the field of microfluidics has attained attention from researchers present all over the world. However, it is expected that the field will inflate knowledge of biomedicine and synthesis of nanoparticles and will point towards disruptions by solving many healthcare related problems.
References
- Stockmaier, S.; Stroeymeyt, N.; Shattuck, E.C.; Hawley, D.M.; Meyers, L.A.; Bolnick, D.I. Infectious Diseases and Social Distancing in Nature. Science 2021, 371, eabc8881. [Google Scholar] [CrossRef] [PubMed]
- Funk, S.; Salathé, M.; Jansen, V.A.A. Modelling the Influence of Human Behaviour on the Spread of Infectious Diseases: A Review. J. R. Soc. Interface. 2010, 7, 1247–1256. [Google Scholar] [CrossRef] [PubMed]
- Lazcka, O.; Campo, F.J.D.; Muñoz, F.X. Pathogen Detection: A Perspective of Traditional Methods and Biosensors. Biosensors and Bioelectronics 2007, 22, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Rajakaruna, S.J.; Liu, W.-B.; Ding, Y.-B.; Cao, G.-W. Strategy and Technology to Prevent Hospital-Acquired Infections: Lessons from SARS, Ebola, and MERS in Asia and West Africa. Military Med Res 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.A.; Stroock, A.D.; Ajdari, A. Engineering Flows in Small Devices: Microfluidics Toward a Lab-on-a-Chip. Annu. Rev. Fluid Mech. 2004, 36, 381–411. [Google Scholar] [CrossRef]
- Cavaniol, C.; Cesar, W.; Descroix, S.; Viovy, J.-L. Flowmetering for Microfluidics. Lab Chip 2022, 22, 3603–3617. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Xia, A.; Wang, D.; Zhang, Y.; Deng, S.; Lu, W.; Luo, J.; Zhong, Q.; Zhang, F.; Zhou, L.; et al. An Ultraportable and Versatile Point-of-Care DNA Testing Platform. Sci. Adv. 2020, 6, eaaz7445. [Google Scholar] [CrossRef] [PubMed]
- Ortseifen, V.; Viefhues, M.; Wobbe, L.; Grünberger, A. Microfluidics for Biotechnology: Bridging Gaps to Foster Microfluidic Applications. Front. Bioeng. Biotechnol. 2020, 8, 589074. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hong, X.-Z.; Li, Y.-W.; Li, Y.; Wang, J.; Chen, P.; Liu, B.-F. Microfluidics-Based Strategies for Molecular Diagnostics of Infectious Diseases. Military Med Res 2022, 9, 11. [Google Scholar] [CrossRef]
- Tarim, E.A.; Karakuzu, B.; Oksuz, C.; Sarigil, O.; Kizilkaya, M.; Al-Ruweidi, M.K.A.A.; Yalcin, H.C.; Ozcivici, E.; Tekin, H.C. Microfluidic-Based Virus Detection Methods for Respiratory Diseases. emergent mater. 2021, 4, 143–168. [Google Scholar] [CrossRef]
- Lui, C.; Cady, N.; Batt, C. Nucleic Acid-Based Detection of Bacterial Pathogens Using Integrated Microfluidic Platform Systems. Sensors 2009, 9, 3713–3744. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.-C.; Fu, C.-C.; Hu, L.; Thakur, R.; Feng, J.; Lee, L.P. Self-Powered Integrated Microfluidic Point-of-Care Low-Cost Enabling (SIMPLE) Chip. Sci. Adv. 2017, 3, e1501645. [Google Scholar] [CrossRef] [PubMed]
- Carrell, C.; Kava, A.; Nguyen, M.; Menger, R.; Munshi, Z.; Call, Z.; Nussbaum, M.; Henry, C. Beyond the Lateral Flow Assay: A Review of Paper-Based Microfluidics. Microelectronic Engineering 2019, 206, 45–54. [Google Scholar] [CrossRef]
- Ye, X.; Xu, J.; Lu, L.; Li, X.; Fang, X.; Kong, J. Equipment-Free Nucleic Acid Extraction and Amplification on a Simple Paper Disc for Point-of-Care Diagnosis of Rotavirus A. Analytica Chimica Acta 2018, 1018, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Urbaniak, J.; Janowski, D.; Jacewski, B. Isolation of Nucleic Acids Using Silicon Dioxide Powder as a Tool for Environmental Monitoring. Environ Monit Assess 2019, 191, 732. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Asai, S.; Kakizoe, H.; Saeki, H.; Masukawa, A.; Miyazawa, M.; Ohtagawa, K.; Ravzanaaadii, M.-A.; Doi, M.; Atsumi, H.; et al. Evaluation of the Basic Assay Performance of the GeneSoc® Rapid PCR Testing System for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. PLoS ONE 2021, 16, e0248397. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, L.; Tu, Y.; Zhang, J.; Miao, G.; Zhang, L.; Ge, S.; Xia, N.; Yu, D.; Qiu, X. Rapid PCR Powered by Microfluidics: A Quick Review under the Background of COVID-19 Pandemic. TrAC Trends in Analytical Chemistry 2021, 143, 116377. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Gallegos, R.A.; Rios, A.; Garcia-Cordero, J.L. An Affordable and Portable Thermocycler for Real-Time PCR Made of 3D-Printed Parts and Off-the-Shelf Electronics. Anal. Chem. 2018, 90, 5563–5568. [Google Scholar] [CrossRef]
- Jafek, A.R.; Harbertson, S.; Brady, H.; Samuel, R.; Gale, B.K. Instrumentation for XPCR Incorporating QPCR and HRMA. Anal. Chem. 2018, 90, 7190–7196. [Google Scholar] [CrossRef]
- Espulgar, W.V.; Saito, M.; Takahashi, K.; Ushiro, S.; Yamamoto, N.; Akeda, Y.; Hamaguchi, S.; Tomono, K.; Tamiya, E. Deskilled and Rapid Drug-Resistant Gene Detection by Centrifugal Force-Assisted Thermal Convection PCR Device. Sensors 2021, 21, 1225. [Google Scholar] [CrossRef]
- Nge, P.N.; Rogers, C.I.; Woolley, A.T. Advances in Microfluidic Materials, Functions, Integration, and Applications. Chem. Rev. 2013, 113, 2550–2583. [Google Scholar] [CrossRef] [PubMed]
- Mauk, M.G.; Liu, C.; Sadik, M.; Bau, H.H. Microfluidic Devices for Nucleic Acid (NA) Isolation, Isothermal NA Amplification, and Real-Time Detection. In Mobile Health Technologies; Rasooly, A., Herold, K.E., Eds.; Methods in Molecular Biology; Springer New York: New York, NY, 2015; Vol. 1256, pp. 15–40. ISBN 978-1-4939-2171-3. [Google Scholar]
- Berkenbrock, J.A.; Grecco-Machado, R.; Achenbach, S. Microfluidic Devices for the Detection of Viruses: Aspects of Emergency Fabrication during the COVID-19 Pandemic and Other Outbreaks. Proc. R. Soc. A. 2020, 476, 20200398. [Google Scholar] [CrossRef]
- Fang, X.; Liu, Y.; Kong, J.; Jiang, X. Loop-Mediated Isothermal Amplification Integrated on Microfluidic Chips for Point-of-Care Quantitative Detection of Pathogens. Anal. Chem. 2010, 82, 3002–3006. [Google Scholar] [CrossRef]
- Zanoli, L.; Spoto, G. Isothermal Amplification Methods for the Detection of Nucleic Acids in Microfluidic Devices. Biosensors 2012, 3, 18–43. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Huang, J.-G.; Chuang, T.-L.; Sheu, J.-C.; Chuang, Y.-K.; Holl, M.; Meldrum, D.R.; Lee, C.-N.; Lin, C.-W. Compact Optical Diagnostic Device for Isothermal Nucleic Acids Amplification. Sensors and Actuators B: Chemical 2008, 133, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Lee, C.-N.; Mark, H.; Meldrum, D.R.; Lin, C.-W. Efficient, Specific, Compact Hepatitis B Diagnostic Device: Optical Detection of the Hepatitis B Virus by Isothermal Amplification. Sensors and Actuators B: Chemical 2007, 127, 598–605. [Google Scholar] [CrossRef]
- Ding, X.; Wang, G.; Mu, Y. Single Enzyme-Based Stem-Loop and Linear Primers Co-Mediated Exponential Amplification of Short Gene Sequences. Analytica Chimica Acta 2019, 1081, 193–199. [Google Scholar] [CrossRef]
- Jeong, Y.-J.; Park, K.; Kim, D.-E. Isothermal DNA Amplification in Vitro: The Helicase-Dependent Amplification System. Cell. Mol. Life Sci. 2009, 66, 3325–3336. [Google Scholar] [CrossRef]
- Mahalanabis, M.; Do, J.; ALMuayad, H.; Zhang, J.Y.; Klapperich, C.M. An Integrated Disposable Device for DNA Extraction and Helicase Dependent Amplification. Biomed Microdevices 2010, 12, 353–359. [Google Scholar] [CrossRef]
- Kolm, C.; Martzy, R.; Führer, M.; Mach, R.L.; Krska, R.; Baumgartner, S.; Farnleitner, A.H.; Reischer, G.H. Detection of a Microbial Source Tracking Marker by Isothermal Helicase-Dependent Amplification and a Nucleic Acid Lateral-Flow Strip Test. Sci Rep 2019, 9, 393. [Google Scholar] [CrossRef]
- Mothershed, E.A.; Whitney, A.M. Nucleic Acid-Based Methods for the Detection of Bacterial Pathogens: Present and Future Considerations for the Clinical Laboratory. Clinica Chimica Acta 2006, 363, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Di, K.; Adeel, K.; Fan, B.; Gu, X.; Xu, H.; Shen, H.; He, N.; Li, Z. The Exploration of Droplet Digital Branched Rolling Circle Amplification Based Ultrasensitive Biosensor for Gastric Cancer Cell-Derived Extracellular Vesicles Detection. Materials Today Advances 2022, 16, 100296. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Cha, B.S.; Lee, E.S.; Park, K.S. Dual Rolling Circle Amplification-Enabled Ultrasensitive Multiplex Detection of Exosome Biomarkers Using Electrochemical Aptasensors. Analytica Chimica Acta 2022, 1205, 339762. [Google Scholar] [CrossRef]
- Marcy, Y.; Ishoey, T.; Lasken, R.S.; Stockwell, T.B.; Walenz, B.P.; Halpern, A.L.; Beeson, K.Y.; Goldberg, S.M.D.; Quake, S.R. Nanoliter Reactors Improve Multiple Displacement Amplification of Genomes from Single Cells. PLoS Genet 2007, 3, e155. [Google Scholar] [CrossRef] [PubMed]
- Dean, F.B.; Hosono, S.; Fang, L.; Wu, X.; Faruqi, A.F.; Bray-Ward, P.; Sun, Z.; Zong, Q.; Du, Y.; Du, J.; et al. Comprehensive Human Genome Amplification Using Multiple Displacement Amplification. Proc. Natl. Acad. Sci. U.S.A. 2002, 99, 5261–5266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Martiny, A.C.; Reppas, N.B.; Barry, K.W.; Malek, J.; Chisholm, S.W.; Church, G.M. Sequencing Genomes from Single Cells by Polymerase Cloning. Nat Biotechnol 2006, 24, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, Y.; Fu, J.; Zhang, R.; Feng, L.; Hu, Y.; Li, X.; Lu, N.; Zhao, X.; Pan, Y.; et al. Performance Assessment of a Novel Two-Step Multiple Displacement Amplification-PCR Assay for Detection of Mycobacterium Tuberculosis Complex in Sputum Specimens. J Clin Microbiol 2012, 50, 1443–1445. [Google Scholar] [CrossRef] [PubMed]
- Hakenberg, S.; Hügle, M.; Weidmann, M.; Hufert, F.; Dame, G.; Urban, G.A. A Phaseguided Passive Batch Microfluidic Mixing Chamber for Isothermal Amplification. Lab Chip 2012, 12, 4576. [Google Scholar] [CrossRef] [PubMed]
- Kersting, S.; Rausch, V.; Bier, F.F.; von Nickisch-Rosenegk, M. Multiplex Isothermal Solid-Phase Recombinase Polymerase Amplification for the Specific and Fast DNA-Based Detection of Three Bacterial Pathogens. Microchim Acta 2014, 181, 1715–1723. [Google Scholar] [CrossRef]
- Alamolhoda, S.Z.; Zarghami, N.; Kahroba, H.; Mehdipour, A.; Pourhassan-Moghaddam, M.; Jahanban-Esfahlan, R.; Milani, M. Isothermal Amplification of Nucleic Acids Coupled with Nanotechnology and Microfluidic Platforms for Detecting Antimicrobial Drug Resistance and Beyond. Adv Pharm Bull 2021, 1. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, T.; Yang, Z.; Pires, N.; Høivik, N. Compatible Immuno-NASBA LOC Device for Quantitative Detection of Waterborne Pathogens: Design and Validation. Lab Chip 2012, 12, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Hardinge, P.; Murray, J.A.H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification Using Quenched Fluorescent Primers. Sci Rep 2019, 9, 7400. [Google Scholar] [CrossRef] [PubMed]
- Dolka, B.; Cisek, A.A.; Szeleszczuk, P. The Application of the Loop-Mediated Isothermal Amplification (LAMP) Method for Diagnosing Enterococcus Hirae-Associated Endocarditis Outbreaks in Chickens. BMC Microbiol 2019, 19, 48. [Google Scholar] [CrossRef] [PubMed]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Helicase-Dependent Isothermal Amplification: A Novel Tool in the Development of Molecular-Based Analytical Systems for Rapid Pathogen Detection. Anal Bioanal Chem 2018, 410, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Kim, H.; Li, Y.; Kong, H.; Lemieux, B. Helicase-Dependent Amplification of Nucleic Acids. Current Protocols in Molecular Biology 2013, 104. [Google Scholar] [CrossRef]
- Murakami, T.; Sumaoka, J.; Komiyama, M. Sensitive Isothermal Detection of Nucleic-Acid Sequence by Primer Generation–Rolling Circle Amplification. Nucleic Acids Research 2009, 37, e19–e19. [Google Scholar] [CrossRef] [PubMed]
- Pumford, E.A.; Lu, J.; Spaczai, I.; Prasetyo, M.E.; Zheng, E.M.; Zhang, H.; Kamei, D.T. Developments in Integrating Nucleic Acid Isothermal Amplification and Detection Systems for Point-of-Care Diagnostics. Biosensors and Bioelectronics 2020, 170, 112674. [Google Scholar] [CrossRef] [PubMed]
- Spits, C.; Le Caignec, C.; De Rycke, M.; Van Haute, L.; Van Steirteghem, A.; Liebaers, I.; Sermon, K. Whole-Genome Multiple Displacement Amplification from Single Cells. Nat Protoc 2006, 1, 1965–1970. [Google Scholar] [CrossRef] [PubMed]
- Bleier, S.; Maier, P.; Allgayer, H.; Wenz, F.; Zeller, W.J.; Fruehauf, S.; Laufs, S. Multiple Displacement Amplification Enables Large-Scale Clonal Analysis Following Retroviral Gene Therapy. J Virol 2008, 82, 2448–2455. [Google Scholar] [CrossRef]
- Zheng, C.; Wang, K.; Zheng, W.; Cheng, Y.; Li, T.; Cao, B.; Jin, Q.; Cui, D. Rapid Developments in Lateral Flow Immunoassay for Nucleic Acid Detection. Analyst 2021, 146, 1514–1528. [Google Scholar] [CrossRef]
- Hu, J.; Wang, L.; Li, F.; Han, Y.L.; Lin, M.; Lu, T.J.; Xu, F. Oligonucleotide-Linked Gold Nanoparticle Aggregates for Enhanced Sensitivity in Lateral Flow Assays. Lab Chip 2013, 13, 4352. [Google Scholar] [CrossRef] [PubMed]
- Hill, H.D.; Mirkin, C.A. The Bio-Barcode Assay for the Detection of Protein and Nucleic Acid Targets Using DTT-Induced Ligand Exchange. Nat Protoc 2006, 1, 324–336. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Henares, T.G.; Suzuki, K.; Citterio, D. Paper-Based Inkjet-Printed Microfluidic Analytical Devices. Angew. Chem. Int. Ed. 2015, 54, 5294–5310. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Khanra, K.; Chowdhury, A.R.; Datta, P. Lab-on-a-Chip Sensing Devices for Biomedical Applications. In Bioelectronics and Medical Devices; Elsevier, 2019; pp. 47–95. ISBN 978-0-08-102420-1. [Google Scholar]
- Nielsen, J.B.; Hanson, R.L.; Almughamsi, H.M.; Pang, C.; Fish, T.R.; Woolley, A.T. Microfluidics: Innovations in Materials and Their Fabrication and Functionalization. Anal. Chem. 2020, 92, 150–168. [Google Scholar] [CrossRef] [PubMed]
- Merrin, J. Frontiers in Microfluidics, a Teaching Resource Review. Bioengineering 2019, 6, 109. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Sui, G. Bioanalysis within Microfluidics: A Review. In ACS Symposium Series; Wang, C., Leblanc, R.M., Eds.; American Chemical Society: Washington, DC, 2015; Vol. 1215, pp. 245–268. ISBN 978-0-8412-3120-7. [Google Scholar]
- Narimani, R.; Azizi, M.; Esmaeili, M.; Rasta, S.H.; Khosroshahi, H.T. An Optimal Method for Measuring Biomarkers: Colorimetric Optical Image Processing for Determination of Creatinine Concentration Using Silver Nanoparticles. 3 Biotech 2020, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Walgama, C.; Nguyen, M.P.; Boatner, L.M.; Richards, I.; Crooks, R.M. Hybrid Paper and 3D-Printed Microfluidic Device for Electrochemical Detection of Ag Nanoparticle Labels. Lab Chip 2020, 20, 1648–1657. [Google Scholar] [CrossRef] [PubMed]
- Gnoth, C.; Johnson, S. Strips of Hope: Accuracy of Home Pregnancy Tests and New Developments. Geburtshilfe Frauenheilkd 2014, 74, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.W. Microfluidic Devices for Biomedical Applications: Biomedical Microfluidic Devices 2019. Micromachines 2020, 11, 370. [Google Scholar] [CrossRef]
- Williams, M.J.; Lee, N.K.; Mylott, J.A.; Mazzola, N.; Ahmed, A.; Abhyankar, V.V. A Low-Cost, Rapidly Integrated Debubbler (RID) Module for Microfluidic Cell Culture Applications. Micromachines 2019, 10, 360. [Google Scholar] [CrossRef]
- Sanjay, S.T.; Zhou, W.; Dou, M.; Tavakoli, H.; Ma, L.; Xu, F.; Li, X. Recent Advances of Controlled Drug Delivery Using Microfluidic Platforms. Advanced Drug Delivery Reviews 2018, 128, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.P.; Torrieri, G.; Santos, H.A. The Importance of Microfluidics for the Preparation of Nanoparticles as Advanced Drug Delivery Systems. Expert Opinion on Drug Delivery 2018, 15, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Arduino, I.; Liu, Z.; Rahikkala, A.; Figueiredo, P.; Correia, A.; Cutrignelli, A.; Denora, N.; Santos, H.A. Preparation of Cetyl Palmitate-Based PEGylated Solid Lipid Nanoparticles by Microfluidic Technique. Acta Biomaterialia 2021, 121, 566–578. [Google Scholar] [CrossRef]
- Galan, E.A.; Zhao, H.; Wang, X.; Dai, Q.; Huck, W.T.S.; Ma, S. Intelligent Microfluidics: The Convergence of Machine Learning and Microfluidics in Materials Science and Biomedicine. Matter 2020, 3, 1893–1922. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and Applications of Microfluidic Devices: A Review. IJMS 2021, 22, 2011. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).