1. Introduction
Aspartylglucosaminuria (AGU, OMIM 208400) is a neurodevelopmental disease caused by the deficiency of a lysosomal hydrolase, aspartylglucosaminidase (AGA, EC 3.5.1.26). AGU patients show a progressive but relatively slow cognitive decline, which may prevent an early diagnosis. Beyond the age of 20 to 25, AGU patients are usually severely cognitively impaired and may also exhibit physical deterioration and profound handicap. The life expectancy of the patients is higher than 50 years of age, and the oldest AGU patients currently alive are over 60 (reviewed in [
1,
2]).
AGU is enriched in the Finnish population, and due to a founder effect, almost all Finnish patients carry a specific double missense variant, termed AGU
Fin-major, in at least one allele [
3,
4]. This variant consists of a neutral amino acid exchange, Arg161Gln, combined with a pathogenic Cys163Ser exchange that prevents the formation of a disulfide bond and results in a folding defect [
5]. Currently, there are no approved therapies for AGU, although several potential treatments have been assessed in preclinical studies [
6,
7,
8,
9].
In Finland, AGU is usually diagnosed after analysis of urine oligosaccharides, especially N-acetylglucosamine-asparagine (GlcNAc-Asn), and the diagnosis is verified by means of gene sequencing [
10,
11]. The patients outside of Finland, who often exhibit their own, family-specific pathogenic variants, are identified either by gene/exome sequencing, or urine analysis. Measurement of AGA activity in white blood cells has also been used for diagnostic means or for the verification of previous findings [
12,
13,
14,
15], but this method is rarely available in routine diagnostic laboratories. Nevertheless, findings of a genetic analysis or urine glycoasparagine measurement should always be verified by a second method, especially if a novel, potentially pathogenic variant of the
AGA gene is identified [
10]. Furthermore, glycoasparagines can also be present in the urine of patients with
NGLY1 deficiency, which is a related neurodevelopmental disorder [
11,
16,
17,
18].
In the era of emerging treatment strategies, such as pharmacological chaperones, gene therapy and enzyme replacement therapy for rare genetic disorders, including AGU, there is a high need for validated biomarkers that can be used to measure the effect of the potential treatments. Optimally, such biomarkers can be obtained in a minimally invasive fashion. For AGU, accurate measurement of AGA activity in serum or plasma samples would be highly desirable, in addition to analysis of urine GlcNAc-Asn.
The purpose of this study was to establish and validate an AGA enzyme activity assay for human serum samples. We here provide a validated method for the measurement of AGA activity from human serum samples, for use as a biomarker for diagnostic purposes and, potentially, for the assessment of treatment efficiency.
2. Results
2.1. Validation of the AGA Enzyme Activity Assay
The development of an optimized method for the determination of AGA activity in AGU patient sera was based on previously published methods [
6,
7,
8,
11,
15]. The measurement of AGA enzyme activity in human fibroblasts and leucocytes using a fluorometric substrate Asp-AMC (L-Aspartic acid β-(7-amido-4-methylcoumarin) was originally proposed by Mononen et al. and Voznyi et al. [
13,
14,
15,
19]. The suitability of Asp-AMC as an AGA substrate for the measurement of AGA activity in clinical samples from AGU patients (e.g. serum, plasma and leucocytes) has also been described by Mononen et al. [
13]. In addition, we have previously used this activity assay for biological samples, such as cell lysates [
6,
7,
8,
20]. However, the method for a quantitative measurement of AGA activity has not been validated for clinical use for human serum.
Due to these reasons, it was necessary to further optimize and validate the AGA activity assay for human serum samples. According to the European Medicines Agency (EMA) guidelines on bioanalytical method validation, the imprecision and inaccuracy of an analytical method should be lower than ±15%, with the only exception of the lower limit of quantification (LLOQ), for which a deviation of ±20% is allowed [
21]. The validation of the AGA activity measurement from human serum samples (healthy and AGU patients) was done by testing various parameters, as described in the following sections.
2.1.1. Linearity and Lower Limit of Quantification
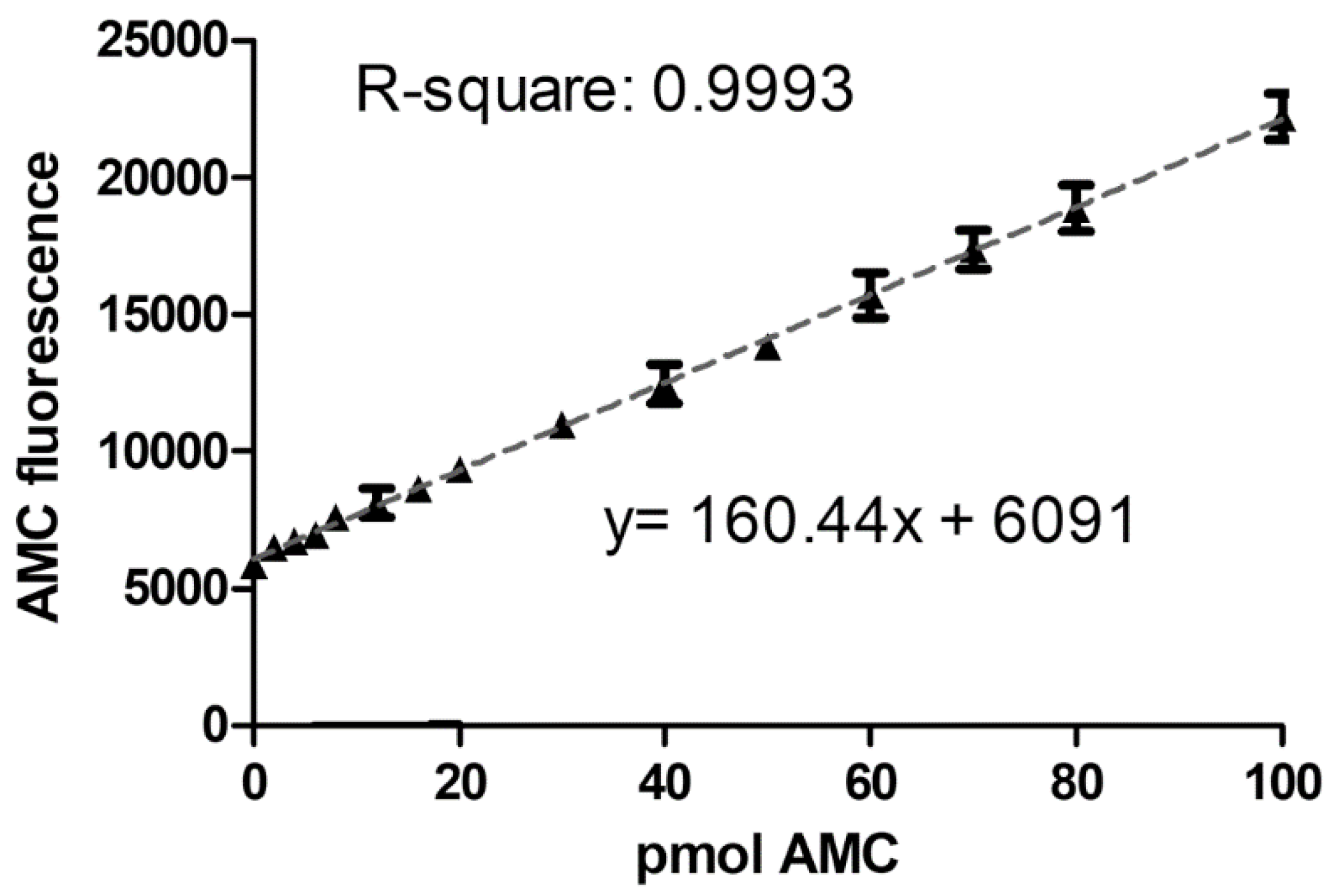
For the calibration standards, 7-Amino-4-methylcoumarin (AMC), the fluorescent product that arises from the substrate Asp-AMC in the AGA enzymatic reaction, was used. According to the guideline [
21], the calibration standards should be prepared in the same matrix as the matrix of the samples of interest. Therefore, 14 calibration samples with different AMC amounts were prepared in a commercially available, artificial serum matrix. The total amount of AMC in the calibration standards ranged from 0–100 pmol. Five separate runs were performed with the calibration standards, and each calibration curve was fitted by linear regression. The measured concentrations of the calibration standards were back-calculated to the expected nominal value. The five individual calibration curves (
Supplementary Figure S1) were averaged (
Figure 1), with the average equation represented as: y = 160.44x + 6091, R-square = 0.99. For each of the five calibration curves, at least 11 standards (i.e., more than 75%) fulfilled the criteria of back-calculation within the ±15% of the nominal value range. The average LLOQ was calculated to be 4.8 pmol AMC (corresponding to 0.18 mU/L AGA activity), but the lowest AMC-containing standard with 2 pmol AMC (0.09 mU/L) could also be detected. However, the back-calculation of this standard was not within the required ±20% range, indicating that measuring samples with enzyme activities as low as 0.09 mU/L is still possible, but less precise.
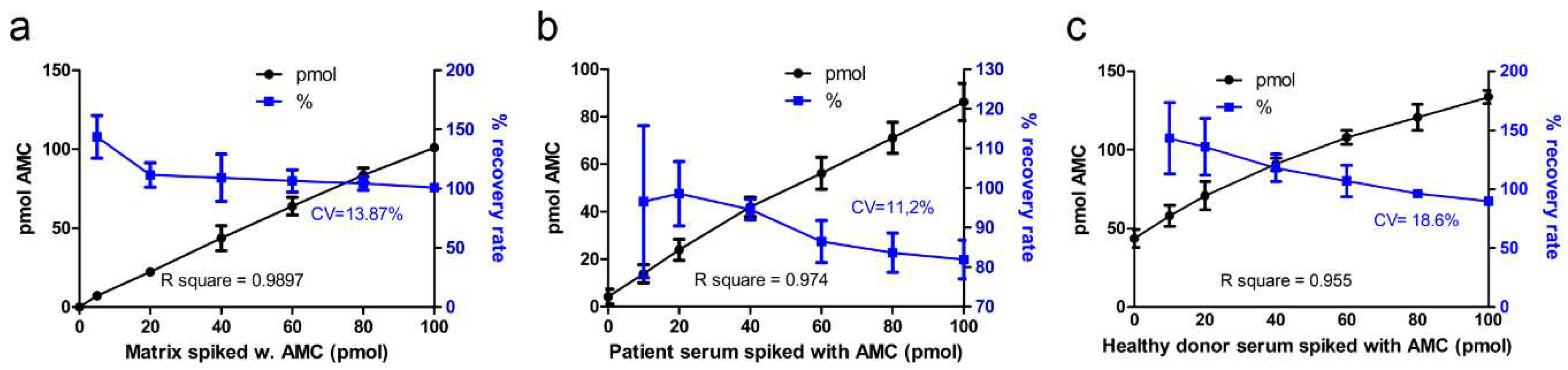
2.1.2. Accuracy
Inter-assay accuracy was determined by spiking of samples (i.e. artificial serum, patient serum, healthy donor serum) with known amounts of AMC. Artificial serum matrix was spiked with 5 to 100 pmol AMC, and the resulting fluorescence was measured (
Figure 2a). Recovery rate for most of the AMC standards spiked in the artificial serum was on average 106%, with coefficient of variation (CV) of 13.9%. Only for the lowest spiked amount of AMC (5 pmol), the recovery rate (140%) was not within the allowed 15% range. The R-square for all AMC amounts was 0.9897. The recovery rate for AMC spiked in patient sera was on average 93%, with CV 11.2% (
Figure 2b). However, AMC spiked in healthy donor serum samples (
Figure 2c) showed a higher variation (CV 18.6%) of the recovery rate (115%), which was mainly due to the low accuracy of the lowest amount of spiked AMC (10 pmol). Since the AGA activity in healthy donors is already in the upper range of the calibration curve (see
Figure 3), detection of spiked AMC in such samples would require an extrapolation of the calibration curve. However, since samples close to the LLOQ (i.e. AGU patient samples) are more relevant for diagnostics, the lower accuracy of the simulated high activity samples is acceptable.
2.1.3. Precision
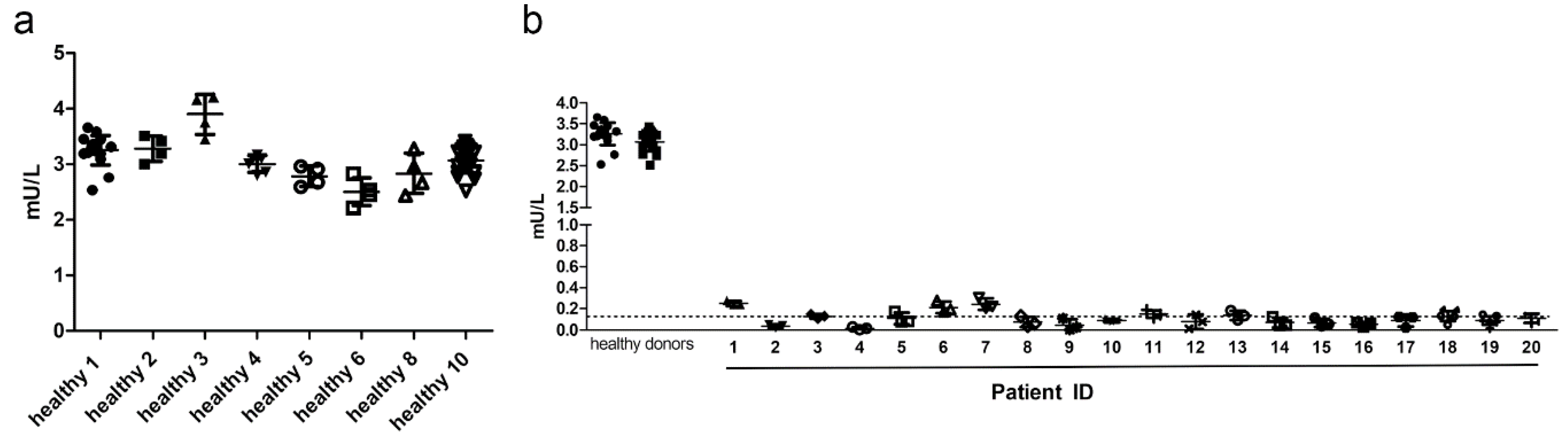
Next, the precision of the AGA activity assay was determined using clinical samples of healthy volunteers and AGU patients, and the AGA activity was measured using the substrate Asp-AMC. Precision describes the degree of scatter of repeated measurements and is expressed as CV. Both accuracy and precision should optimally be within ±15%, except for the LLOQ, for which ±20% is considered acceptable [
21]. Within-run precision was determined using serum samples from 6 healthy donors, representing the “high” quality control (QC) samples, and 6 AGU patients, representing the “low” QC samples, each measured in triplicates (
Figure 3). Average CV was 4.96%, and all subjects were within the required ±15% precision range, demonstrating that the AGA activity assay is suitable for measuring the AGA activity even in patient samples (
Supplementary Table S1).
Figure 3.
Within-run precision. AGA activity in three technical replicates with 10 µl serum from 6 healthy donors or 20 µl of serum from 6 AGU patients was measured following the optimized AGA activity assay protocol. Data show one representative run out of at least 3 runs.
Figure 3.
Within-run precision. AGA activity in three technical replicates with 10 µl serum from 6 healthy donors or 20 µl of serum from 6 AGU patients was measured following the optimized AGA activity assay protocol. Data show one representative run out of at least 3 runs.
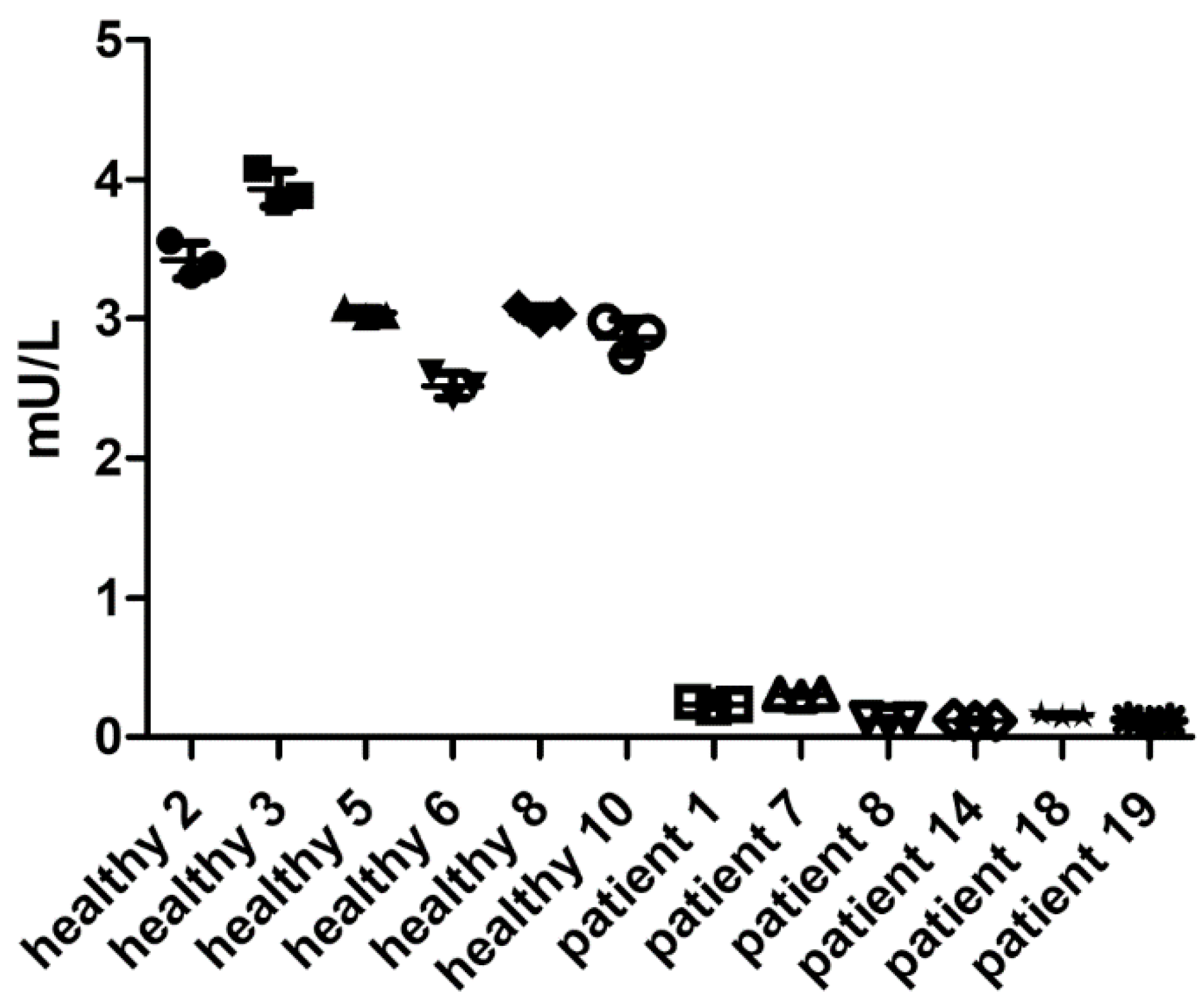
Between-run precision of at least 3 individual runs was assessed with serum samples of 8 healthy donors (
Figure 4a) and 20 AGU-patients (
Figure 4b). Average CV was 8.25% for healthy donors, and all healthy donors passed the quality criteria. Most patient samples showed CV higher than 20%, which is caused by the very low AGA activity, often being even below the determined LLOQ (dotted line in
Figure 4b).
Supplementary Table S1 shows a summary of the individual coefficients of variation.
All 20 patient samples displayed severely reduced AGA activities that were on average 0.11 mU/L (range 0.0123 – 0.251 mU/L). Healthy donors typically had AGA activities of around 3 mU/L. With our assay, the diagnosis of an AGU patient is thus unambiguous, and a misdiagnosis is very unlikely.
Figure 4.
Between-run precision. a) Between-run precision of the AGA activity assay was assessed with serum samples of 8 healthy donors. Each symbol represents one independent measurement out of at least 4 experiments. b) Between-run precision of the optimized AGA activity assay in AGU patient sera. Samples from 20 AGU patients were assessed by three independent experiments. The data for the healthy donors from panel a) are shown for comparison.
Figure 4.
Between-run precision. a) Between-run precision of the AGA activity assay was assessed with serum samples of 8 healthy donors. Each symbol represents one independent measurement out of at least 4 experiments. b) Between-run precision of the optimized AGA activity assay in AGU patient sera. Samples from 20 AGU patients were assessed by three independent experiments. The data for the healthy donors from panel a) are shown for comparison.
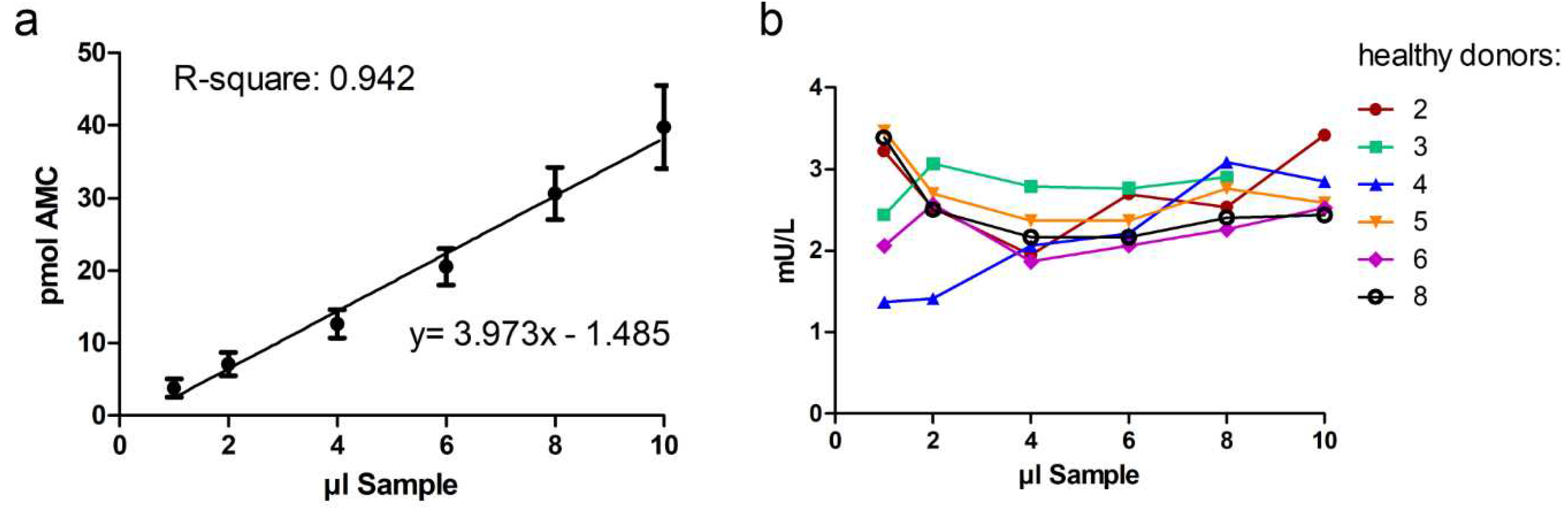
2.1.4. Dilution Integrity
Dilution of the samples should not affect the accuracy and precision of a method. Here, dilution integrity was demonstrated by measuring the AGA activity from 1-10 µl of serum from 6 healthy donors. The volume of the samples was adjusted to 20 µl with isotonic NaCl or the artificial serum matrix. Dilution of patient samples was not carried out, since the activity with 20 µl of patient serum is already at the detection limit (see
Figure 4b). Dilution of sera from healthy donors showed that the assay was precise even for serum volumes as low as 4 µl (
Figure 5a and 5b). With volumes less than 4 µl serum, the precision decreased (
Figure 5b)
3. Discussion
The main purpose of this study was to establish and validate a method for a quantitative measurement of AGA activity in human serum samples. The fluorometric method that was used as a starting point was originally developed and then further refined by Mononen et al. and Voznyi et al. [
13,
14,
15]. Before that, a colorimetric assay was used that is less sensitive and more error-prone than the fluorometric method [
22,
23]. Voznyi et al. have used the fluorometric method for the assessment of AGA activity also from amniotic fluid, amniocytes, chorionic villi biopsies and cultured trophoblasts for prenatal diagnostics [
15]. However, the methods have so far not been validated for a precise measurement of AGA activity in human samples, and they mainly allow the distinction of AGU patients from healthy individuals or carriers. For example, Mononen et al. showed that the identification of carriers by a measurement of AGA activity in serum samples was not possible with the previous methods [
13]. Furthermore, as AGA activity in the AGU patients is very low, the activity measurement needs to be carefully validated for the low activity range so that effect of potential treatments such as gene therapy can be accurately assessed in clinical trials. Therefore, we here validated the AGA activity measurement for human serum samples, using both healthy volunteers and AGU patients.
In the previous published protocols for cell lysates and serum, very high Asp-AMC concentrations (0.5 to 1.5 mM) have been used [
13,
14,
15]. However, these concentrations produced a high background fluorescence when measured with our plate reader (data not shown) and were thus not suitable for the measurement in the low activity range, i.e. in patient samples. Hence, already in our previous cell culture studies with AGU patient fibroblasts and loss- and gain-of-function cell lines, the Asp-AMC concentration was reduced to 50 µM [
6,
8,
13,
14,
15,
24]. However, even then, the method had limitations when comparing the AGA activity of samples with very low enzyme activity. Therefore, further optimization of the method for human serum samples that have AGA activities close to the LLOQ, as systematically defined here, was performed.
Our validated activity assay differs from the previously published assays in several ways. The buffer system we have used is similar to the one used by Voznyi et al. (Mc Ilvain’s phosphate-citrate buffer) [
15], but we used a commercially available, artificial serum matrix for the blanks, standards, and for sample dilution. As compared to the assay published by Mononen et al. [
14], we have used a longer incubation time (24 h vs. 1 h) to reduce the variation in the low activity range.
For the validation, we used AMC standards diluted in artificial serum, for which the LLOQ was determined to be 4.8 pmol AMC (corresponding to 0.18 mU/L AGA activity). However, also the lowest AMC-containing standard with 2 pmol AMC (0.09 mU/L) was detectable, albeit with a lower accuracy. In the patient samples, the AGA activity was within the range of 0.0123–0.251 mU/L (average 0.11 mU/L). Although some patients fall below the LLOQ, they can be clearly distinguished from healthy probands, who show an AGA activity of about 3 mU/L. Therefore, a diagnosis of AGU can unambiguously be made from serum samples using our validated assay.
All patients in our study were homozygous for the major Finnish variant. However, most AGU patients with variants other than AGU
Fin-major are also expected to fall within the same activity range and can thus be identified with our assay. So far, only few AGU patients have been identified who show an intermediate AGA activity that is higher than that in the Finnish patients, but lower than that in the carriers [
7]. However, our activity assay allows the identification of these patients, too, even if they fall in the range above LLOQ. Unfortunately, medium QC samples, such as samples from non-affected carriers (e.g. parents of AGU-patients), were not available for our study, but they were mimicked by dilution of high QC samples from healthy individuals. Our data show that expected carrier activities can also be precisely measured, and dilution of the samples did not affect the precision of the method, since an amount as little as 4 µl of healthy donor serum was sufficient for an accurate AGA activity measurement. However, due to the low activity in AGU patients, 20 µl of patient serum is required.
The within-run precision (triplicate measurements) for the AGA activity was accurate (below the 15% variation range), even for most of the AGU patient samples. However, for samples at or below LLOQ, ±20% variation is actually considered acceptable. The between-run variation of at least 3 individual measurements for healthy samples was very good (CV 8.25%). Most of our 20 AGU patients, however, were above the 20% variation limit between the runs. Thus, it can be concluded that the precision of the method in AGU patient samples is acceptable for intra-assay replicates, but not always for inter-assay analysis. Therefore, patient samples that need to be directly compared with each other, for example when treatment effects are longitudinally measured, should preferably be analyzed in the same run. Future data from clinical studies on AGU, such as our study registered under EudraCT number 2017-000645-48 [
25], will reveal if the activity assay described here will be capable of measuring the changes in AGA activity as a result of an interventional treatment.
4. Materials and Methods
4.1. Sample Collection
Blood was collected from healthy volunteers via venipuncture. After 15 minutes at room temperature, samples were centrifuged at 4°C for 10 min at 390x g. Serum samples from 20 AGU patients were obtained within the framework of an interventional clinical study (EudraCT number 2017-000645-48) [
25], before any intervention was started. All serum samples were divided in aliquots and stored at -80°C until analysis. Prior to use, the aliquots were thawed on ice and mixed well to dissolve any precipitated material.
4.2. Reagents
The AGA substrate Asp-AMC (Cat. sc-211699) was obtained from Santa Cruz Biotechnology (Heidelberg, Germany). The substrate was dissolved in 1 M HCl as 1 mM stock solution. Out of this stock solution, the 25 µM substrate solution was prepared in McIlvain’s phosphate-citrate buffer, pH 6.5. McIlvain’s phosphate-citrate buffers, pH 4.5 and 6.5 were prepared by mixing 5.46 ml or 2.9 ml of 1 M citric acid with 18.16 ml or 28.4 ml of 0.5 M Na2HPO4, respectively. Water was added to 100 ml, and the pH was adjusted, if necessary.
Artificial serum/plasma II (Cat. ASP-002) was obtained from Biopanda Diagnostics (Belfast, UK). Isotonic NaCl solution (0.9%) was from B. Braun (Melsungen, Germany) and AMC (7-Amino-4-methylcoumarin, Cat. A9891) was from Sigma-Aldrich (Taufkirchen, Germany). AMC was dissolved in DMSO at 10 mg/ml, and the AMC standards were prepared as 5 µM dilution in the artificial serum. AMC and Asp-AMC stocks and working solutions were stored at -20°C.
4.3. Validation of the Enzyme Activity Measurement
Experimental validation of the AGA activity measurement from human serum samples was performed using EMA guidelines [
21]. As a starting point, our published AGA activity protocol for cell culture lysates was used [
6,
7], which was here adapted and validated for human serum samples. The following parameters were validated:
4.3.1. Linearity and Lower Limit of Quantification (LLOQ)
Calibration samples were prepared in artificial serum matrix and analyzed in 5 separate runs. Each calibration sample contained a defined amount of AMC and Asp-AMC. The sum of AMC and Asp-AMC was kept constant at 12.5 µM (
Table 1). All samples were incubated for 24 h at 37°C after what 200 µl of McIlvain’s phosphate-citrate buffer, pH 4.5, was added. All tubes were vortexed, and 200 µl out of each tube were pipetted into a black 96-well plate. The plates were measured (excitation: 355 nm, emission: 460 nm) with a Tecan Infinite M200 plate reader (gain 120, 4x4 reads per well). Each calibration curve was fitted by linear regression. Slope, Y-intercept, and R-square were calculated using Microsoft Excel. Concentrations of the calibration standards were back-calculated and should be within ±15% of the nominal value, except for LLOQ, which should be within ±20%. The five individual calibration curves were averaged, and the average LLOQ was calculated.
4.3.2. Accuracy and Precision
Accuracy describes the closeness of the obtained value to the nominal value, reported as %, and is determined by spiking of samples with known amounts of the measured analyte (i.e., AMC). Accuracy was determined in artificial serum, in patient serum, and in healthy serum. 20 µl of artificial serum, or patient serum, or 10 µl of healthy donor serum were spiked with 0 – 100 pmol AMC. 20 µl of Asp-AMC was added, and the samples were incubated for 24 h at 37°C. Thereafter, the reaction was stopped by adding 200 µl of McIlvain’s phosphate-citrate buffer pH 4.5. Fluorescence measurements were performed as described for the calibration standards. Accuracy was calculated as determined value expressed as percentage of the true value. Between-run accuracy was evaluated by analyzing the variation coefficient of three independent experiments.
Precision is an indication for the degree of scatter of repeated measurements and is defined as (standard deviation/mean) x 100%. Within-run precision was determined for 6 healthy donors and 6 AGU patients, each measured in triplicates. Between-run precision of at least 3 different runs was assessed for 8 healthy donors and 20 AGU-patients. Both accuracy and precision should be within ±15%, except for the LLOQ (±20%).
4.3.3. Dilution Integrity, Carry-Over and Stability
Dilution integrity was demonstrated with 1-10 µl of serum from healthy donors. The volume difference was balanced with isotonic NaCl or artificial serum matrix. R-square and coefficient of variation were calculated with GraphPad Prism 5 (San Diego, CA, USA).
Carry-over of the analyte does not occur, because all samples are pipetted and measured independently of each other. AMC and Asp-AMC aliquots were stored at -20°C and used several times, without loss of potency due to degradation. Serum samples were stored at -80°C. Freezing and thawing for at least 3 times did not result in any obvious loss of AGA enzyme activity (See
Figure 4 and
Supplementary Figure S1).
4.4. Validated Method for the Measurement of AGA Activity in Human Serum
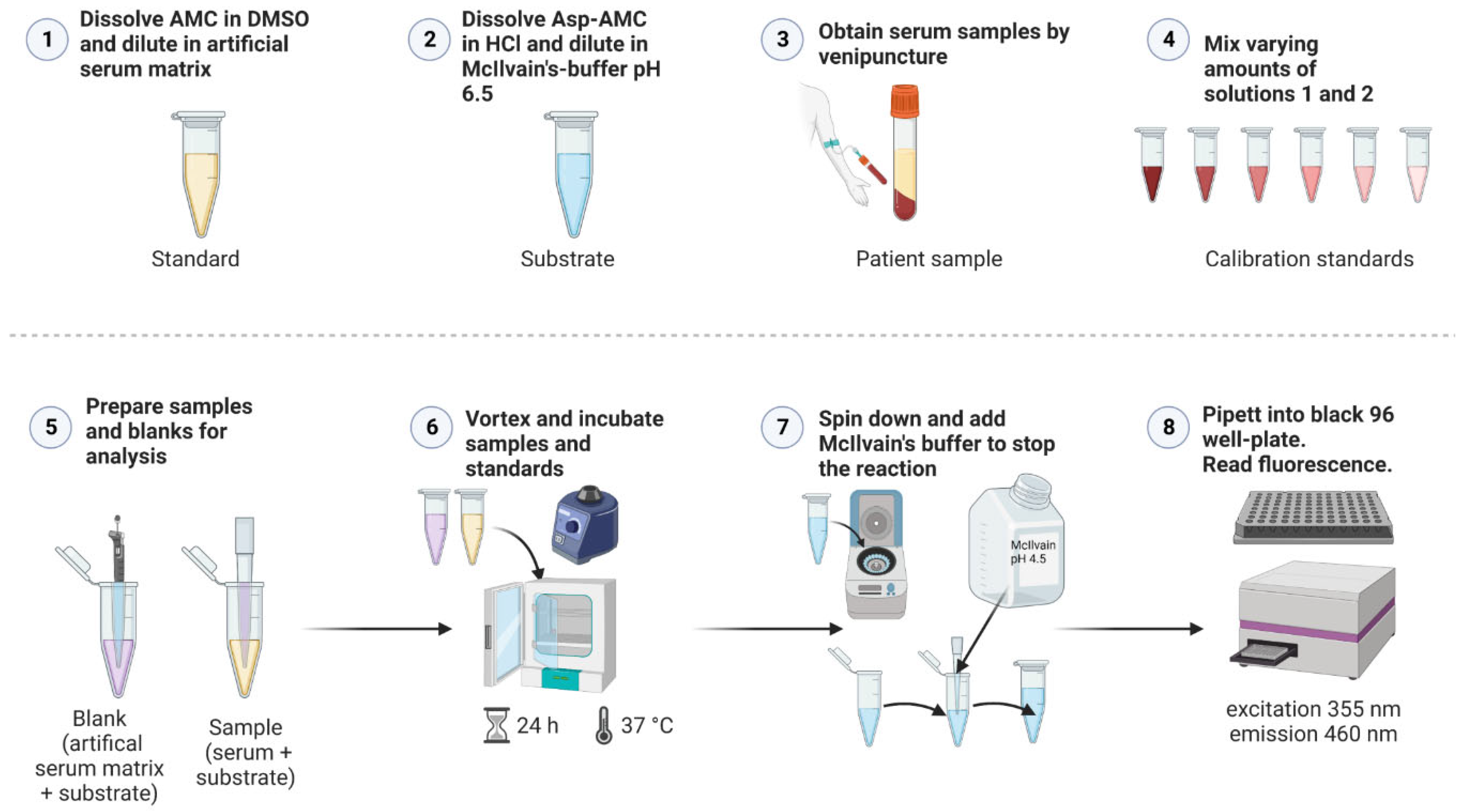
Figure 6 shows a general scheme for the AGA activity assay. For all samples, three 0.5 ml tubes were prepared with 20 µl of 25 µM Asp-AMC substrate solution. In these tubes, 20 µl patient serum was added, whereas for healthy controls, 10 µl serum and 10 µl isotonic NaCl were added. Triplicate blank samples were prepared with 20 µl artificial serum and 20 µl Asp-AMC substrate solution. Note that the blanks are identical to the calibration sample S1 of the standard curve (
Table 1). For the AMC standard curve, 16 tubes were prepared, containing increasing amounts of AMC in artificial serum, ranging from 0- 100 pmol AMC. Because of the background fluorescence of Asp-AMC, defined amounts of Asp-AMC substrate solution were added, so that the total amount of AMC and Asp-AMC was identical in all standards. Artificial serum was added to 40 µl (see
Table 1 for detailed pipetting scheme). All tubes were tightly closed, vortexed and incubated at 37°C for 24 h. After 24 h, all tubes were centrifuged for 10 s to collect any condensed fluid. Reactions were stopped by addition of 200 µl of McIlvain’s phosphate-citrate buffer, pH 4.5. All tubes were vortexed, 200 µl from each tube were transferred into a black 96-well plate. The plates were measured immediately (excitation: 355 nm, emission: 460 nm) with a Tecan Infinite M200 plate reader (gain 120, 4x4 reads per well). For calculation of AGA enzyme activity, the mean blank fluorescence was subtracted from all samples. According to the slope of the standard curve, the AGA activity values are expressed as mU/L (1 U = 1 µmol/min, 24 h corresponding to 1440 min).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org. Figure S1: Linearity and lower limit of quantification (LLOQ); Table S1: Coefficients of variation (CV) of the individual measurements of within-run and between-run precision.
Author Contributions
Clinical samples from AGU patients were obtained by M.L. A.B. performed the activity assay validation, and A.B. and R.T. drafted the figures and wrote the manuscript draft. R.T. and M.L. obtained funding for the study. All authors reviewed and edited the manuscript. All authors agreed with the final manuscript version.
Funding
This study was funded by the Jane and Aatos Erkko Foundation (to R.T. and M.L.), and Suomen AGU r.y. (to R.T.). The funders played no role in the design of the study, the interpretation of the data, or decision to publish.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and was approved by the Institutional Ethics Committee of Helsinki and Uusimaa Hospital District, Board for women, children and psychiatry (number HUS/2604/2017, approved on 19.10.2017).
Informed Consent Statement
Written informed consent was obtained from all subjects involved in the study, or from their legal custodians.
Data Availability Statement
Primary data are available from the corresponding author upon a justified request.
Acknowledgments
The authors wish to thank the patients and their families, as well as the volunteers for participating in this study. Eeva Rantanen and Niina Brunner from Suomen AGU r.y. are acknowledged for their great support in recruiting the patients. We thank Petra Janson and Simone Kegel for their skilled technical assistance.
Conflicts of Interest
A.B. declares no conflict of interest. M.L. declares consulting activities for Neurogene Inc. R.T. has received research funding from Neurogene Inc. for studies related on AGU, but not directly concerning the present study, and funding from Argenx BV, GC Pharma, and Topas Therapeutics for unrelated studies.
References
- Arvio, M.; Mononen, I. Aspartylglycosaminuria: a review. Orphanet J Rare Dis 2016, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Goodspeed, K.; Feng, C.; Laine, M.; Lund, T.C. Aspartylglucosaminuria: Clinical Presentation and Potential Therapies. J Child Neurol 2021, 36, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E.; Baumann, M.; Gron, K.; Syvanen, A.C.; Enomaa, N.; Halila, R.; Aula, P.; Peltonen, L. Aspartylglucosaminuria: cDNA encoding human aspartylglucosaminidase and the missense mutation causing the disease. EMBO J 1991, 10, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Mononen, I.; Heisterkamp, N.; Kaartinen, V.; Williams, J.C.; Yates, J.R., 3rd; Griffin, P.R.; Hood, L.E.; Groffen, J. Aspartylglycosaminuria in the Finnish population: identification of two point mutations in the heavy chain of glycoasparaginase. Proc Natl Acad Sci U S A 1991, 88, 2941–2945. [Google Scholar] [CrossRef]
- Ikonen, E.; Enomaa, N.; Ulmanen, I.; Peltonen, L. In vitro mutagenesis helps to unravel the biological consequences of aspartylglucosaminuria mutation. Genomics 1991, 11, 206–211. [Google Scholar] [CrossRef]
- Banning, A.; Gulec, C.; Rouvinen, J.; Gray, S.J.; Tikkanen, R. Identification of Small Molecule Compounds for Pharmacological Chaperone Therapy of Aspartylglucosaminuria. Sci Rep 2016, 6, 37583. [Google Scholar] [CrossRef]
- Banning, A.; Schiff, M.; Tikkanen, R. Amlexanox provides a potential therapy for nonsense mutations in the lysosomal storage disorder Aspartylglucosaminuria. Biochim Biophys Acta Mol Basis Dis 2018, 1864, 668–675. [Google Scholar] [CrossRef]
- Banning, A.; Tikkanen, R. Towards Splicing Therapy for Lysosomal Storage Disorders: Methylxanthines and Luteolin Ameliorate Splicing Defects in Aspartylglucosaminuria and Classic Late Infantile Neuronal Ceroid Lipofuscinosis. Cells 2021, 10. [Google Scholar] [CrossRef]
- Chen, X.; Snanoudj-Verber, S.; Pollard, L.; Hu, Y.; Cathey, S.S.; Tikkanen, R.; Gray, S.J. Pre-clinical Gene Therapy with AAV9/AGA in Aspartylglucosaminuria Mice Provides Evidence for Clinical Translation. Mol Ther 2021, 29, 989–1000. [Google Scholar] [CrossRef]
- Uusimaa, J.; Kettunen, J.; Varilo, T.; Jarvela, I.; Kallijarvi, J.; Kaariainen, H.; Laine, M.; Lapatto, R.; Myllynen, P.; Niinikoski, H.; Rahikkala, E.; Suomalainen, A.; Tikkanen, R.; Tyynismaa, H.; Vieira, P.; Zarybnicky, T.; Sipila, P.; Kuure, S.; Hinttala, R. The Finnish genetic heritage in 2022 - from diagnosis to translational research. Dis Model Mech 2022, 15. [Google Scholar] [CrossRef] [PubMed]
- Hagemeijer, M.C.; van den Bosch, J.C.; Bongaerts, M.; Jacobs, E.H.; van den Hout, H.; Oussoren, E.; Ruijter, G.J.G. Analysis of urinary oligosaccharide excretion patterns by UHPLC/HRAM mass spectrometry for screening of lysosomal storage disorders. J Inherit Metab Dis 2023. [Google Scholar] [CrossRef]
- Mononen, I.; Kaartinen, V.; Mononen, T. Laboratory detection of aspartylglycosaminuria. Scand J Clin Lab Invest Suppl 1988, 191, 7–11. [Google Scholar] [CrossRef]
- Mononen, I.; Mononen, T.; Ylikangas, P.; Kaartinen, V.; Savolainen, K. Enzymatic diagnosis of aspartylglycosaminuria by fluorometric assay of glycosylasparaginase in serum, plasma, or lymphocytes. Clin Chem 1994, 40, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Mononen, I.T.; Kaartinen, V.M.; Williams, J.C. A fluorometric assay for glycosylasparaginase activity and detection of aspartylglycosaminuria. Anal Biochem 1993, 208, 372–374. [Google Scholar] [CrossRef]
- Voznyi Ya, V.; Keulemans, J.L.; Kleijer, W.J.; Aula, P.; Gray, G.R.; van Diggelen, O.P. Applications of a new fluorimetric enzyme assay for the diagnosis of aspartylglucosaminuria. J Inherit Metab Dis 1993, 16, 929–934. [Google Scholar] [CrossRef] [PubMed]
- Enns, G.M.; Shashi, V.; Bainbridge, M.; Gambello, M.J.; Zahir, F.R.; Bast, T.; Crimian, R.; Schoch, K.; Platt, J.; Cox, R.; Bernstein, J.A.; Scavina, M.; Walter, R.S.; Bibb, A.; Jones, M.; Hegde, M.; Graham, B.H.; Need, A.C.; Oviedo, A.; Schaaf, C.P.; Boyle, S.; Butte, A.J.; Chen, R.; Chen, R.; Clark, M.J.; Haraksingh, R.; Consortium, F.C.; Cowan, T.M.; He, P.; Langlois, S.; Zoghbi, H.Y.; Snyder, M.; Gibbs, R.A.; Freeze, H.H.; Goldstein, D.B. Mutations in NGLY1 cause an inherited disorder of the endoplasmic reticulum-associated degradation pathway. Genet Med 2014, 16, 751–758. [Google Scholar] [CrossRef]
- Haijes, H.A.; de Sain-van der Velden, M.G.M.; Prinsen, H.; Willems, A.P.; van der Ham, M.; Gerrits, J.; Couse, M.H.; Friedman, J.M.; van Karnebeek, C.D.M.; Selby, K.A.; van Hasselt, P.M.; Verhoeven-Duif, N.M.; Jans, J.J.M. Aspartylglycosamine is a biomarker for NGLY1-CDDG, a congenital disorder of deglycosylation. Mol Genet Metab 2019, 127, 368–372. [Google Scholar] [CrossRef]
- Mueller, W.F.; Zhu, L.; Tan, B.; Dwight, S.; Beahm, B.; Wilsey, M.; Wechsler, T.; Mak, J.; Cowan, T.; Pritchett, J.; Taylor, E.; Crawford, B.E. GlcNAc-Asn is a biomarker for NGLY1 deficiency. J Biochem 2022, 171, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Ikonen, E.; Syvanen, A.C.; Peltonen, L. Dissection of the molecular pathology of aspartylglucosaminuria provides the basis for DNA diagnostics and future therapeutic interventions. Scand J Clin Lab Invest Suppl 1993, 213, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.; Konig, J.F.; Gray, S.J.; Tikkanen, R. Functional Analysis of the Ser149/Thr149 Variants of Human Aspartylglucosaminidase and Optimization of the Coding Sequence for Protein Production. Int J Mol Sci 2017, 18. [Google Scholar] [CrossRef]
- European Medicines Agency. Guideline on Bioanalytical Method Validation. EMEA/CHMP/EWP/192217/2009 Rev. 1 Corr. 2. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 31.01.2023).
- Aula, P.; Raivio, K.; Autio, S. Enzymatic diagnosis and carrier detection of aspartylglucosaminuria using blood samples. Pediatr Res 1976, 10, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Kaartinen, V.; Mononen, I. Assay of aspartylglycosylaminase by high-performance liquid chromatography. Anal Biochem 1990, 190, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Banning, A.; Zakrzewicz, A.; Chen, X.; Gray, S.J.; Tikkanen, R. Knockout of the CMP-Sialic Acid Transporter SLC35A1 in Human Cell Lines Increases Transduction Efficiency of Adeno-Associated Virus 9: Implications for Gene Therapy Potency Assays. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- EU Clinical Trials Register. Available online: https://www.clinicaltrialsregister.eu/ctr-search/search?query=2017-000645-48 (accessed on 10.02.2023).
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).