1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder characterized by degeneration of the nigrostriatal dopaminergic pathway; however, the causal factor is not clearly understood yet. PD is the second most common neurodegenerative disorder; It is clinically characterized by resting tremor, rigidity, bradykinesia, and postural instability. The main factors involved in neuronal death are an increase in iron content, mitochondrial dysfunction, free radicals' over-production, and diminished antioxidant response, among others [
1]. PD is a multifactorial disorder covering genetic and environmental factors [
2]. Accordingly, PD is a disorder that impacts the quality of life, without a curative treatment and has difficult symptoms to control, affecting predominantly men over women (1.5:1) in people over 65 years old [
3]. Currently, there are no pharmacological treatments that slow the progression of the disease or reduce the mortality rate of the remaining neurons.
Administration of MPP+ (1-methyl-4-phenyl pyridine), the active metabolite of neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), reproduces the main neurochemical characteristics of PD in laboratory animals. The MPP+ neurotoxic potential is based on its ability to inhibit complex I of the mitochondrial electron transport chain (METC), inducing energy depletion, free radicals’ over-production, oxidative stress, and metal dyshomeostasis [
4]. Moreover, previous data indicates a relation between MPP+-induced microglial activation and the degeneration of dopaminergic neurons. PC12 cellular line treated with MPP+ induced increases in the mRNA and protein levels of interleukin-6, IL-1β and tumor necrosis factor-alpha[
5]; likewise, MPP+ activates the NLRP3 inflammasome in microglia, and the NLRP3 inflammasome-activated microglia plays a pivotal role in the neurodegeneration associated with PD [
6].
Simvastatin is an inhibitor of hepatic 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase, the major rate-limiting enzyme in cholesterol synthesis. Like other members of the “statins”, simvastatin lowers total serum cholesterol and particularly low-density lipoprotein (LDL) cholesterol concentrations, thereby reducing the risk of atherosclerosis and its complications. Until now, there is enough accumulated evidence of the effect of simvastatin in some murine models of Parkinson's disease.
In this sense, the aim of this study has been to indicate the effect of simvastatin against MPP+-evoked oxidative and inflammatory damage. Since we consider that the protein expression map can clarify the differential expression profile induced by simvastatin and its effect on the neurotoxicity of MPP+ and repurposing its use as a potential drug as a coadjutant therapy for the dopaminergic neuron preservation in patients with Parkinson´s disease.
2. Results
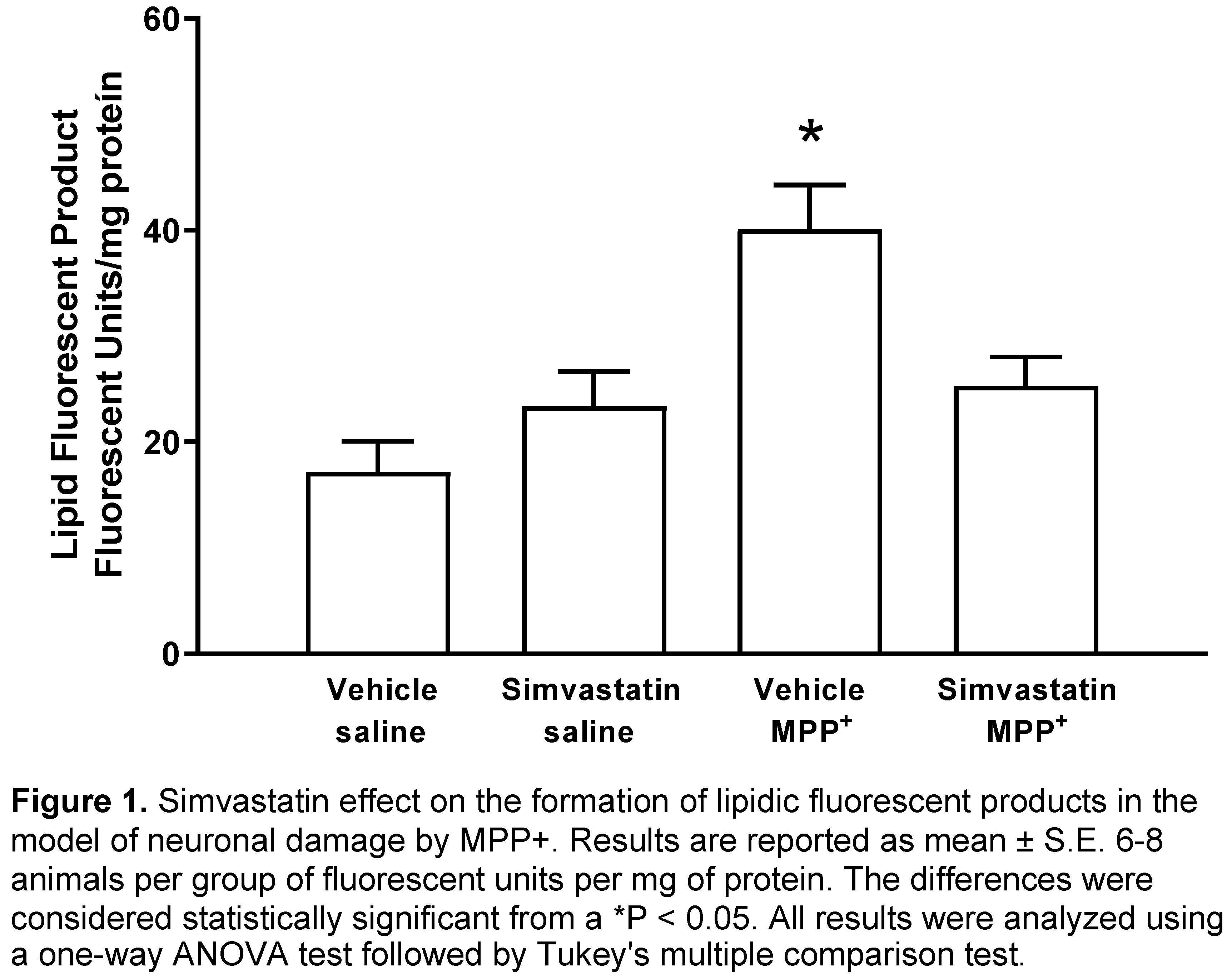
2.1. Fluorescent Lipidic Products
Figure 1 shows the results of the effect of simvastatin pre-administration schedule (40 mg/kg/day), which produced no statistic changes in striatal formation of fluorescent lipidic products (23.4 ± 3.25 Fluorescent Units (FU)/mg protein) versus control group (17.2 ± 2.88 FU/mg protein). Whereas MPP+-infusion statistically increased (p < 0.0001) the formation of lipid fluorescents products (40.1 ± 4.18 FU/mg protein); nonetheless, in the experimental group, the MPP+ damage was reversed (p < 0.007) by simvastatin treatment (25.3 ± 2.7 FU/mg protein).
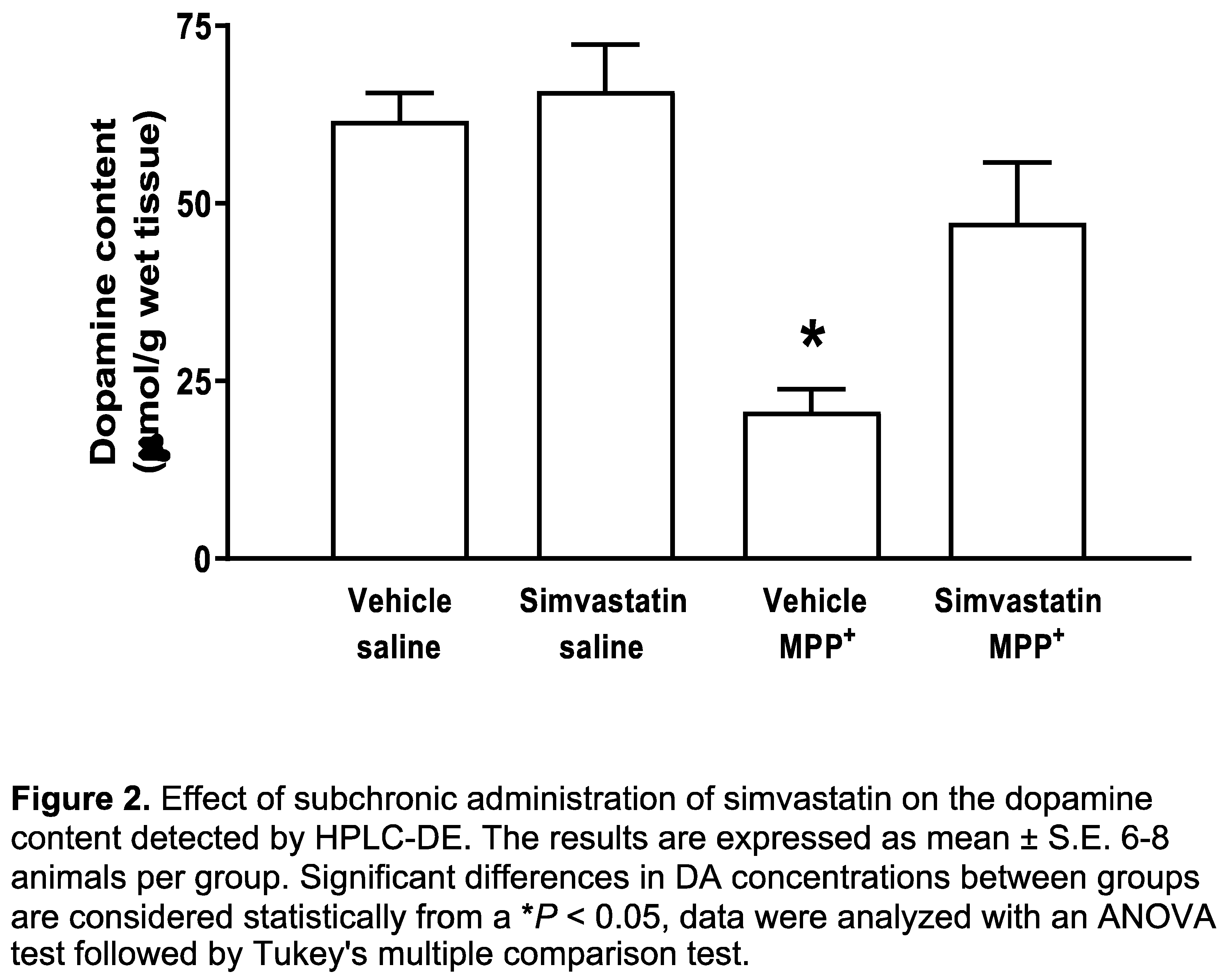
2.2. DOPAMINE (DA) Content in Striatal Tissue
The group of animals administered both oral pathway and intracerebral surgery with simvastatin and sterile saline, respectively, was considered as control group. Basal levels of DA were 61.6 ± 3.91 μmol/g wet tissue. The simvastatin pre-administration schedule with 40 mg/kg/day did not affect striatal DA levels (65.8 ± 6.5 μmol/g wet tissue). In contrast, after microinjection of MPP+ (10 μg/8 μL), a significant decrease (p < 0.001) was found in DA concentrations (20.6 ± 3.1 μmol/g wet tissue), whereas that the simvastatin pre-administration schedule to MPP+-treated rats produced a significant (p < 0.03) preservation of DA content (47.3 ± 8.4 μmol/g wet tissue) (Figure 2).
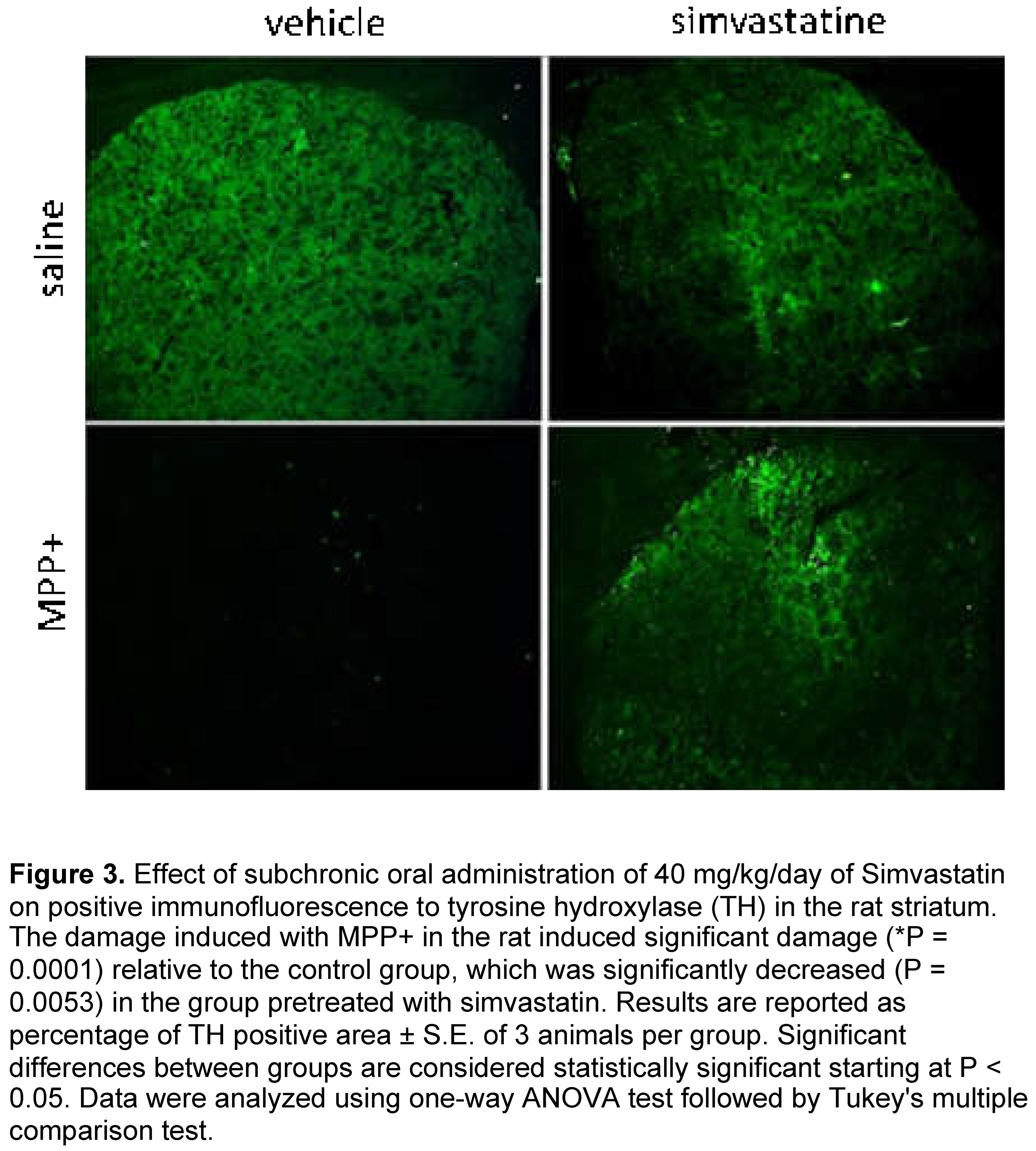
2.3. Neurodegenerative Damage
MPP+-induced significantly (p = 0.0001) reduced the positive signal for tyrosine hydroxylase (TH) compared to the control group (vehicle plus saline solution). This damage was significantly reduced (p= 0.0053) by sub chronic administration of 40/mg/kg when we compared the effect of simvastatin with the control group. Systemic administration of simvastatin plus intrastriatal administration of saline solution presented similar levels of positive signal for TH when compared to the control group (Figure 3).
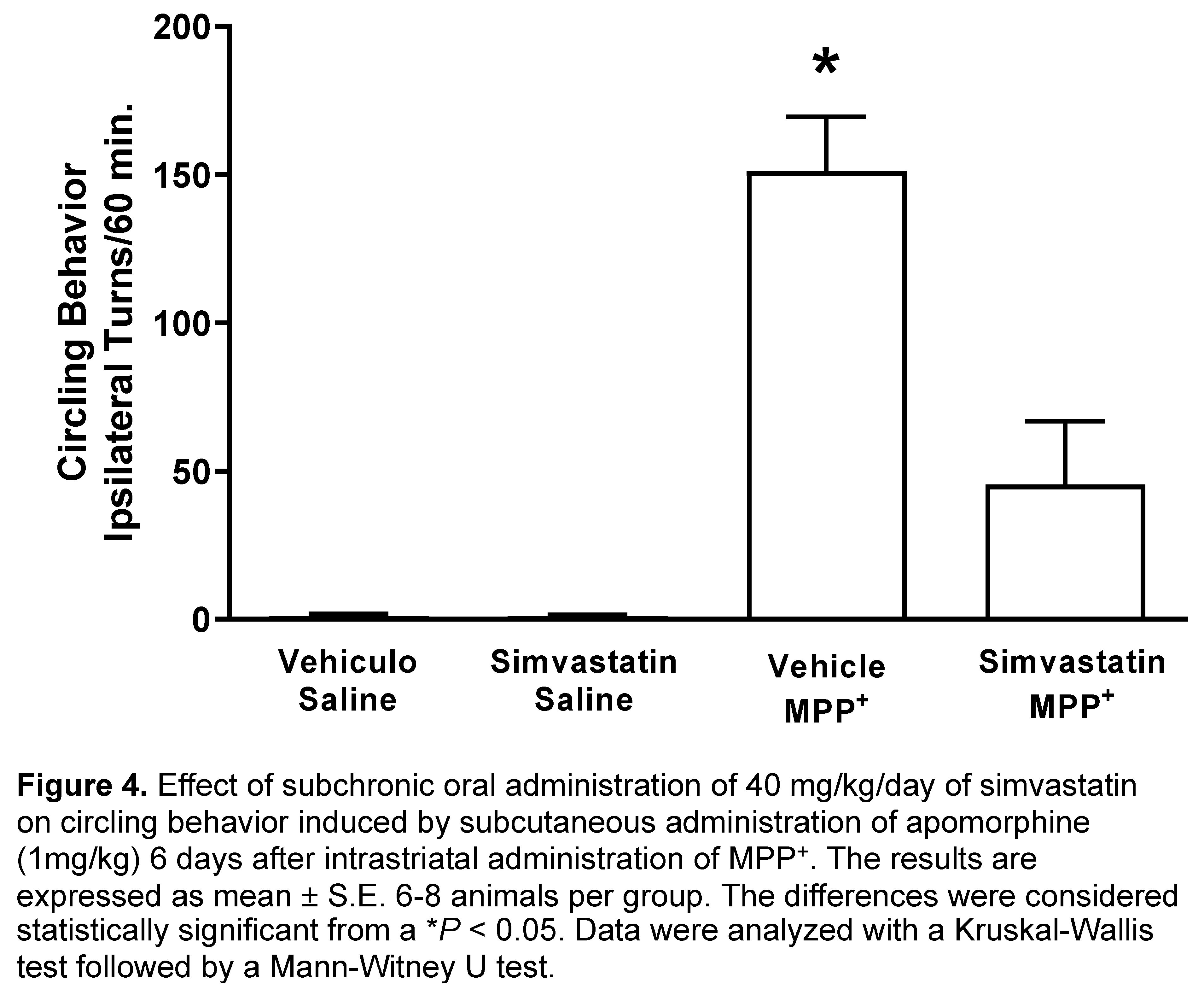
2.4. Circling Behavior
6 days after of MPP+ intrastriatal infusion, we observed a marked effect on circling behavior after apomorphine administration (151.1 ± 18.3 turns/60 min), which was significantly (p < 0.0007) in comparison with control groups (vehicle and saline; simvastatin and saline) as shown in Figure 3. Simvastatin pretreatment schedule reduced statistically (p < 0.008) the number of turns (45.5 ± 21.3 turns/60 min) MPP+-induced (Figure 4).
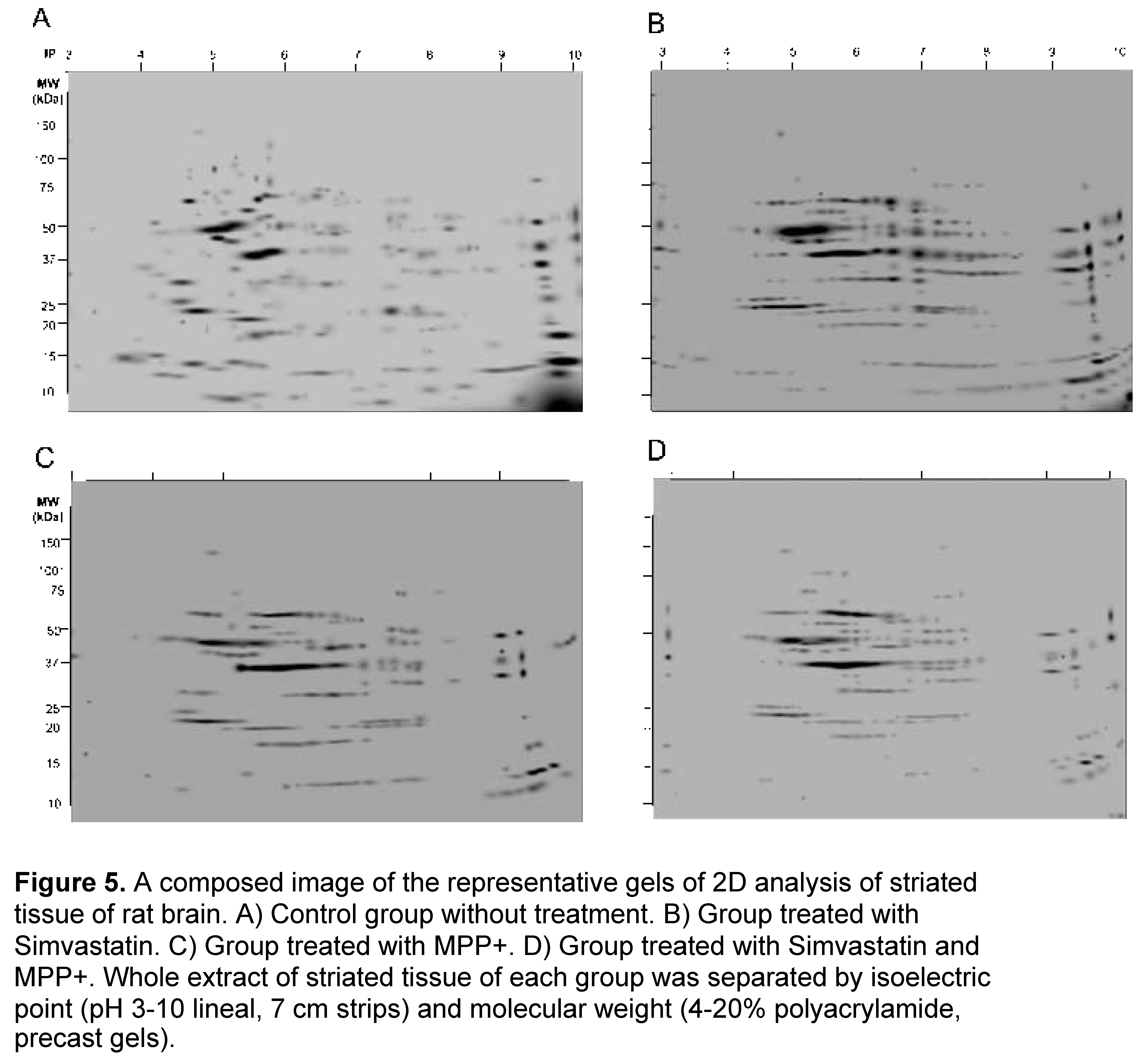
2.5. Protein Expression Patterns from Striatum from Control, Simvastatin, MPP+ and Simvastatin-MPP+-Treated Rats by 2D-SDS-Page
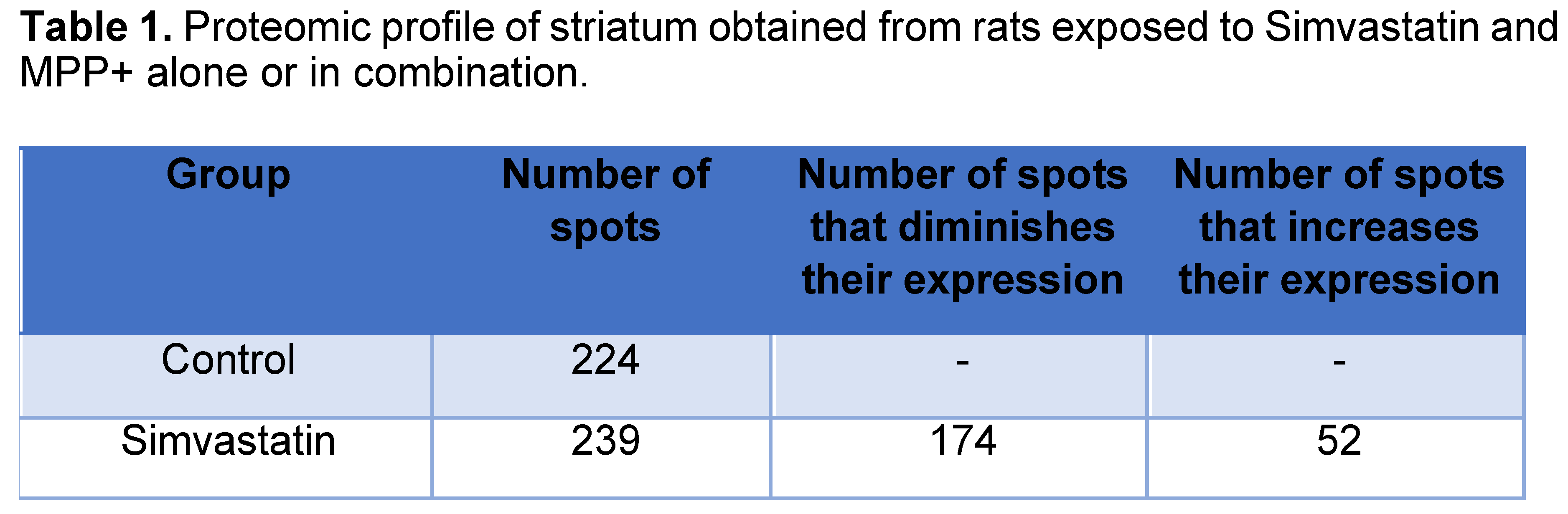
Representative gel images of the 4 experimental groups are shown in Figure 1, which depicts A) Control group without treatment, B) Group treated with Simvastatin, C) Group treated with MPP+ and D) Group treated with Simvastatin and MPP+. Since we confirm and extend in a previous report finding that SMV exerted a protective effect in the rat Parkinson model that seemed to be mediated by an alteration in the cytokine profile in the Nigro striatum. To dissect differences in protein expression, a 2-DE proteomic analysis was performed in the same section of the brain (Figure 5). As depicted in Figure and Table 1, a different protein profile was found for each experimental group. The initial analysis showed that the total number of spots was decreased in the Simvastatin / MPP+ treated samples as deduced by the number of total spots of 2D gels, while the group exposed to Simvastatin alone exhibited the greatest number of spots (Figure 5 and Table 1).
2.6. Bioinformatic Analysis
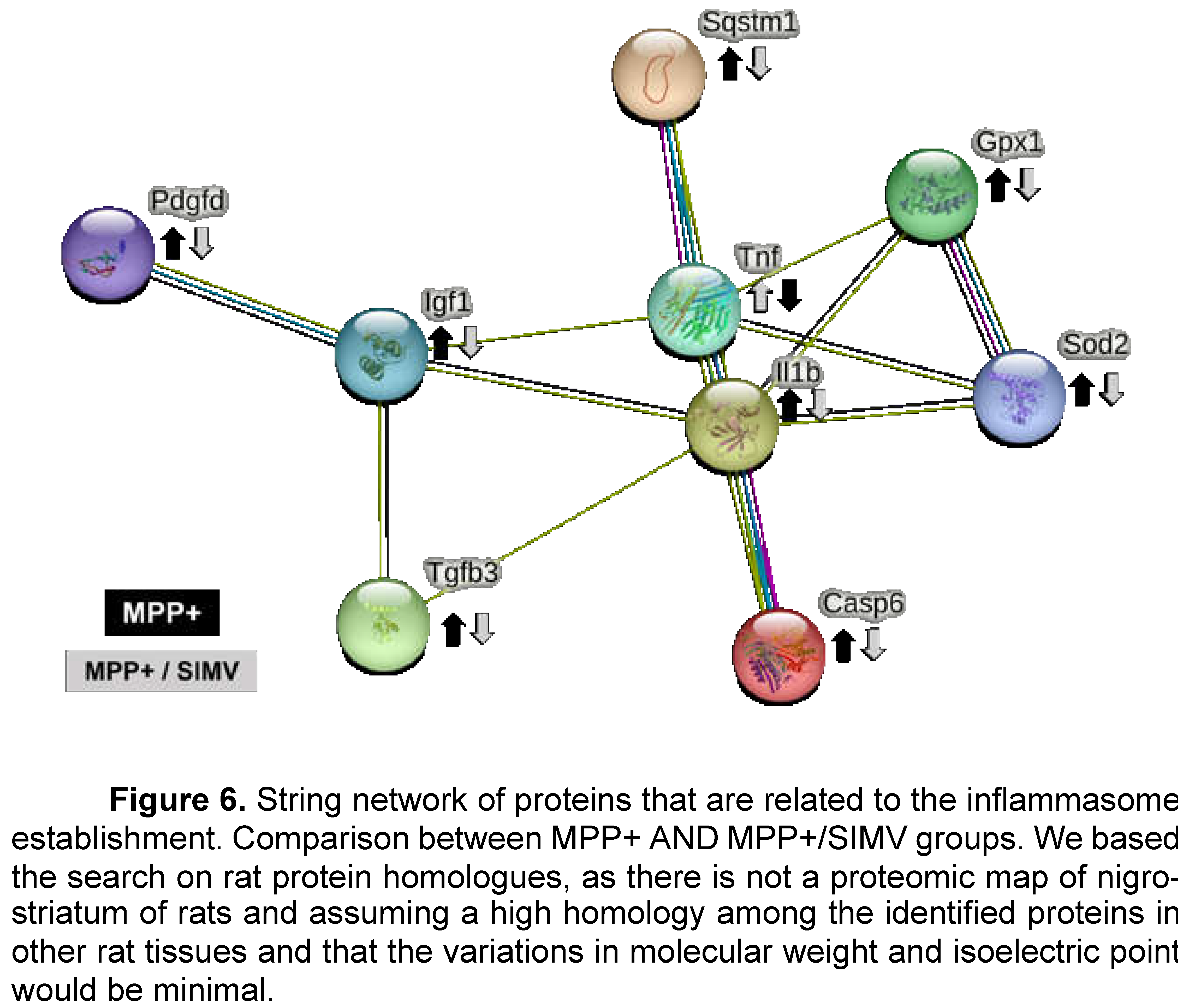
The neurotoxin MPP+ reproduces most of the biochemical and pathological hallmarks of Parkinson's disease, such as toxicity and therefore inflammation by oxidative stress inducers, and production of ROS by activation of the NADPH-oxidase complex. Some proteins that are involved in MPP+ inflammation response that were identified in the group injured by MPP+ were Platelet-derived growth factor D (Pdgfd), Insulin like growth factor 1 (Ilgf), IL-1β, GPX1 and Interferon alpha-1 (IFN-α1) (
Table 2 and Figure 6).
As apoptosis promotes neuroinflammation and neuronal degeneration in neurodegenerative pathologies such as Parkinson’s disease, we focused the search of proteins that are related to the establishment of inflammation response (Figure 6 and
Table 2). Platelet-derived growth factor D (Pdgfd) is a protein involved in leukocyte migration and more interestingly is involved in cell survival via ERK pathway and indirectly by the brain derived neurotrophic factor pathway. This protein reduces its expression in rats that were treated with simvastatin.
Insulin-like growth factor I (Igf1) is a protein involved in the positive regulation of T cell proliferation, it positively regulates IL-4, Jun, and Bcl-2 pathways, while negatively regulating TNF, IL-1β, Casp3, Bax, and NFκb1. This protein reduces its expression in rats that were exposed to MPP+ toxin. TGF-β3 and TGF- β1 exhibited a similar profile expression. TGF- β1 is involved in the positive regulation of glial cell differentiation, and both are involved in the regulation of inflammatory response. Even more, TGF- β1 has been reported to present a protective action against MPP+ injury in the rat model. MPP+ resulted in a reduction of TGF-β1 production in the substantia nigra and primary VM neurons and microglia [
7].
Allograft inflammatory factor 1 (Aif1) is involved in the positive regulation of leukocyte migration. S100A8 has a role in leukocyte aggregation. IL-1α involved in the inflammatory response. Tumor necrosis factor ligand superfamily member 6 (Faslg) is involved in the death of T cells. IL-6r, a part of the receptor for interleukin 6. Binds to IL6 with low affinity but does not transduce a signal. Signal activation depends on the association with IL6ST. Activation may lead to the regulation of the immune response, and acute-phase reactions. Carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) a protein involved in negative regulation of immune effector process that it’s over expressed when rats are treated with simvastatin.
IL-1β was detected in the Simvastatin and MPP+ groups; surprisingly the higher expression was exhibited by the simvastatin groups. Sequestosome-1(Sqstm1) is overexpressed in the simvastatin group. This protein is involved in the regulation of I-kappa B kinase/NF-kappa B signaling and is involved in the regulation of apoptosis triggered by inflammatory cytokines. Caspase-6 is involved in the activation cascade of caspases responsible for apoptosis execution. This protein reduces its expression in the MPP+/Simvastatin group. Finally, IFN-α1 expression is reduced in the MPP+ group.
In a previous report, we demonstrated that EB-pretreated rats injured with MPP
+ toxin, increase PON2 expression at a similar level to that shown by the control, while SOD2 expression was increased in EB, M, and EB/M groups, compared to the control. SOD2 expression was virtually absent in the control group without treatment, in the present study we found a similar pattern of SOD2 protein, which was highly expressed in the MPP+ group and present in the simvastatin group, but undetectable in the control group and the Simvastatin / MPP+ groups. Catalase expression was detected only in the simvastatin group, and GPX1 was overexpressed in the simvastatin group, a little higher in the MPP+ compared to the control but absent in the simvastatin / MPP+ group (
Table 3).
Comparing the expression of proteins that participates in the inflammatory response of MPP+ and MPP+/Simvastatin groups (Figure 6), we proposed that MPP+ injury establishes an inflammatory response in the substantia nigra that induces the secretion of IL1β, a proinflammatory cytokine that stimulates, among others, the secretion of TNF, another proinflammatory cytokine. The activation of both cytokines may be partially regulated by the Sequestosome-1 protein; which is also involved in the autophagy of peroxisomes in response to reactive oxygen species (ROS); these ROS could be regulated by SOD2 and Gpx1. The inflammatory environment could lead to the activation of caspase 6, which promotes the apoptosis regulated by caspases. Probably as a response, IGF1 increases its expression to down-regulate apoptosis, while TGFβ3 could be pro- or anti-inflammatory depending on the environment. Platelet-derived growth factor D recruits macrophages. All these proteins reduce their expression in the group that received simvastatin after MPP+, in comparison with the group that only was injured with MPP+ (Figure 6).
3. Discussion
Parkinson's disease is a neurodegenerative disorder characterized by an imbalance in transition metals (iron, copper, manganese, and zinc), decreased activity of complex I of the mitochondrial electron transport chain, reduced content of GSH, overproduction of free radicals and oxidative stress [
1] [
8]; moreover, the stress of the aging process promotes chronic low-level inflammation in the brain [
9]. Currently, diverse treatments are used as a therapy to control the clinical symptoms of Parkinson's disease; however, to date, no pharmacology treatment cures the illness, and not exist treatments that can preserve the remnant dopaminergic neurons or other drugs that can increase neuron half-life in the nigro-striatal pathway; that is, we don’t have drugs that can stop the progression of the disease.
Simvastatin is a long-established hydroxy-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor. At the maximal recommended dose, it produces an average reduction in low-density lipoprotein cholesterol (LDL-C), accompanied by reductions in very LDL-C, triglycerides, and apolipoprotein B, and a modest increase in high-density lipoprotein cholesterol [
10]. The present study evaluated the neuroprotective effect of simvastatin against MPP+-induced neurotoxicity. MPP+ (1-methyl-4 -phenyl pyridine) the active metabolite of neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), is a molecule that reproduces the main neurochemical alterations observed in Parkinson's disease [
11]. The effect of simvastatin on MPP+-induced damage has been proven for a long time [
12].
According to the findings of this study, a simvastatin dose (40 mg/kg/day), showed a significate antioxidant effect from the order of lovastatin administrated in a dose of 5 mg/kg [
13]. Another aspect of simvastatin in the central nervous system showed its effect against the LPS-induced inflammatory damage and not in the oxidative damage produced by MPP+ [
14]. However, in this work the effect of simvastatin was effective upon three damage markers: lipid peroxidation, dopamine content, and circling behavior; the first two, were evaluated as short term and the last, was considered as long-term endpoint damage markers; in consequence, the neuroprotective effect of simvastatin to make evident a broad temporal spectrum of neuroprotection against MPP+-induced neurotoxicity, since it was able of counteract the oxidative damage exerted by MPP+ during the first 24 hours characterized by oxidative damage preferably.
In a previous report, we demonstrated that estradiol benzoate (EB) pretreated rats injured with MPP
+ toxin, increase PON2 expression to a similar level to that shown by the control [
15]; while SOD2 expression increased in EB, MPP+ and EB/MPP+ groups, respect to the control, while SOD2 expression was virtually absent in control group without treatment; in the present study, we found a similar pattern of SOD2 protein, which was highly expressed in the MPP+ group and present in the simvastatin group, but undetectable in the control group and the simvastatin/MPP+ groups.
Likewise, catalase expression was detected only in the simvastatin group; and GPX1 was overexpressed in the simvastatin group, a little higher in the MPP+ compared to the control but absent in the simvastatin/MPP+ group (
Table 3), which is the cause of that simvastatin possess a clear effect against MPP+-induced oxidative stress. MPP+-evoked nitrosative damage is manifested mainly from the first 48 to 72 hours, this effect is characterized by the overexpression of inducible nitric oxide synthase (iNOS) Ca
2+-independent [
16], [
8]; also becomes clear that simvastatin protected against MPP+-evoked neuroinflammation given long term, all the which was indirectly observed through circling behavior.
In the same way, it has been shown that simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intra-nigral injection of LPS through the decrease of the induction of interleukin-1beta (IL- 1β), tumor necrosis factor-alpha (TNF-α), and nitric oxide synthase (iNOS) [
17]; decreasing astrocytes activation as well [
18]. This effect of simvastatin is associated with its ability to modulate the N-methyl-D-aspartic acid (NMDA) receptor and reduce 6-hydroxydopamine (6-OHDA) damage [
19]; also, simvastatin reduces the mRNA and protein levels of the N-methyl-D-aspartic acid receptor 1 (NMDAR1) and IL-6 in PC12 cells stimulated with 6-OHDA [
20].
Also, has been indicated that simvastatin mediates a protective effect on dopaminergic neurons in the substantia nigra compacta in the LPS-PD model, possibly by promoting neuronal repair and regeneration through induction of brain-derived neurotrophic factor (BDNF) expression, and by inhibiting oxidative stress, thus improving substantia nigra function. However, currently exists strong evidence that ERK1/2-mediated modulation of the antioxidant system after simvastatin treatment may partially explain the antioxidant activity in experimental parkinsonian models [
19].
On the other hand, is well to known that the apoptotic processes promote neuroinflammation and neuronal degeneration in neurodegenerative pathologies such as Parkinson’s disease [
21]. In the present study, we focused the search of proteins that are related to the establishment of inflammation response (Figure 5 and
Table 2). Platelet-derived growth factor-D (PDGF-D) is a protein involved in leukocyte migration and more interestingly is involved in cell survival via ERK pathway and indirectly by the brain derived neurotrophic factor (BDNF) pathway [
22]. In addition to the overexpression of proteins involved in the response to oxidative stress by simvastatin administration induces at least a couple of other proteins: carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1), a protein involved in negative regulation of immune effector process [
21], and sequestosome-1(Sqstm1), which is overexpressed in the simvastatin group. This protein is involved in the regulation of I-kappaB kinase/NF-kappaB signaling, is involved in the regulation of apoptosis triggered by inflammatory cytokines [
23].
Insulin-like growth factor I (Igf1) is a protein involved in the positive regulation of T cell proliferation, it positive regulates IL-4, Jun, and Bcl-2 pathways, while negative regulates TNF, IL-1β, Casp3, Bax and NFκ-B1. This protein reduces its expression in rats that were exposed to MPP+ toxin [
24].
TGF-β3 and TGF- β1 exhibited a similar profile expression. TGF- β1 is involved in positive regulation of glial cell differentiation, and both are involved in the regulation of inflammatory response. Even more, TGF- β1 has been reported to present a protective action against MPP+ injury in rat model [
25],[
15]. MPP+ resulted in a reduction of TGF-β1 production in the substantia nigra and in primary VM neurons and microglia [
25]. Allograft inflammatory factor 1 (Aif1) is involved in the positive regulation of leukocyte migration. S100A8 has a role in leukocyte aggregation. IL-1α involved in the inflammatory response. Tumor necrosis factor ligand superfamily member 6 (Faslg) that is involved in the death of T cells. IL-6r, a part of the receptor for interleukin 6. Binds to IL6 with low affinity but does not transduce a signal. Signal activation depends on the association with IL6ST. Activation may lead to the regulation of the immune response, acute-phase reactions. IL-1β was detected in the Simvastatin and MPP+ groups; surprisingly the higher expression was exhibited by the simvastatin groups. Caspase-6 is involved in the activation cascade of caspases responsible for apoptosis execution. This protein reduces it expression in the MPP+/Simvastatin group. Finally, IFN-α1 expression is reduced in the MPP+ group.
4. Materials and Methods
4.1. Ethics Statement
The protocol used in this study was approved by The Committee on Ethics and Use in Animal Experimentation of the Institute of Neurology and Neurosurgery and the standards of the National Institutes of Health of Mexico (Permit number INN-2017-2023). The study was done following the guidelines of Mexican regulations (NOM-062-ZOO-1999) and the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, 8th Edition to ensure compliance with the established international regulations and guidelines.
4.2. Animals and Treatments
Male Wistar rats (250-280 g) were housed in acrylic box cages and placed under constant conditions of temperature, humidity, and lighting (12 h light/dark cycles). Animals were provided with a standard commercial rat chow diet and water
ad libitum. Experimental rats were administered in a sub-chronic schedule with a single dose by oral pathway (op) of 40 mg/kg/day of simvastatin (Kendrick Pharmaceutical, Reg. No. 470M2002 SSA-IV) in tween 80 to 1%, as vehicle or vehicle solution alone for seven days. Two hours after the last administration of simvastatin, the MPP
+ was administered by means of an intracerebral surgery under ketamine/xylazine anesthesia (70/10 mg/kg). Animals were infused with MPP
+ (10 μg/8 μl) or saline solution for the control group, into the right striatum with stereotaxic coordinates: 0.5 mm posterior, -3.0 mm lateral to the bregma and -4.5 mm ventral [
26].
4.3. Striatal Dopamine Levels Measurement
Twenty-four hours after the simvastatin administration schedule and MPP+ infusion, HPLC with electrochemical detection was used to measure striatal levels of dopamine (DA) as described previously [
27]. Samples obtained 24 h after MPP
+ injection were sonicated into 10 volumes of perchloric acid-sodium metabisulfite solution (1 M 0.1% w/v), centrifuged at 10,000 x g for 10 min, and the supernatant was analyzed. Data were collected and processed by interpolation in a standard curve. Results are expressed as μmol of DA per gram of wet tissue.
4.4. Fluorescent Lipidic Products Assay
The simvastatin effect was evaluated on striatal lipid fluorescent products (LPF) formation 24 h after administration of MPP
+, as described previously [
28]. The striatal tissue was homogenized in 2.2 ml of sterile saline, and 1 ml of the homogenate was then added to 4 mL of a chloroform-methanol mixture (2:1, v/v). The tubes were capped and vortexed for 10 s, and the mixture was then ice-cooled for 30 min to allow phase separation. The aqueous phase was discarded, 1 ml of chloroform layer was transferred into a quartz cuvette, and 150 μl of methanol was added. Fluorescence was measured in a PerkinElmer LS50B luminescence spectrometer at 370 nm of excitation and 430 nm of emission. The protein content was measured as previously reported [
29]. Results are expressed as arbitrary fluorescence units/μg protein.
4.5. Immunofluorescence for Tyrosine Hydroxylase in the Striatum of the Rat
The effect of simvastatin on neurodegeneration induced-MPP+ in the rat striatum was measured by the presence of tyrosine hydroxylase (TH) – antibody dilution (1:100). 24 h after the behavioral evaluation, the animals (n=3-4) were anesthetized with an overdose of sodium pentobarbital (80 mg/kg) and subjected to intracardiac perfusion with 200 ml of saline solution, the perfusion was followed by 200 ml of paraformaldehyde at 4%. The brains were removed, stored in 30% sucrose solution, and kept refrigerated until processing. Subsequently, 22 µm coronal cuts were made from the injured area (AP +1.6 mm to AP -0.48 mm related to Bregma) in a Leica brand cryostat, model CM 1520. The cuts were made in the injured area with to carry out an optical percentage evaluation for the positive signal to TH.
Tissues were washed with PBS 3% (10 min) and antigen recovery was performed in citrate solution for 1 h. Three more washes were made with PBS and blocking was carried out with BSA (6 mg) plus horse serum (4 µl) dissolved in 200 µl of PBS 3% for 1 h. Subsequently, the samples were incubated overnight with a mouse anti-TH antibody (1:100; sc-25269, Santa Cruz Biotechnology). Subsequently, three more washes were carried out with PBS-tween 3% and immediately the samples were incubated for 2 h with a secondary antibody (AffiniPure Goat Anti-Mouse IgG 1:100 Fluorescein (FITC)- AB_2338589 JacksonimmunoResearch). Finally, the tissues were mounted on previously silanized slides plus 3 µl of antioxidant solution. Nine slices of each brain were taken, at the coordinates already mentioned, from which photographs of 6 fields of the striatum of the injured hemisphere were taken for the determination of the TH signal. The photographs were processed as binary figures using Image J software, evaluating the percentage areas of both signals in each slide. Results were reported as the average area percent signal for TH.
4.6. Circling Behavior
Apomorphine-Induced circling behavior was assessed in rats as previously described [
28], which was considered as end-point brain toxicity. Six days after MPP
+ intrastriatal injection, animals were subcutaneously treated with apomorphine plus ascorbic acid (1 mg/kg and 1mg/kg, respectively) and then, were placed into individual box cages. Five minutes later, the number of ipsilateral rotations to the lesioned striatum was recorded for 60 min. Rotations were considered as 360º turns, and results were expressed as the total number of ipsilateral turns in one h period (turns/h).
4.7. Samples Protein Extraction
Striatal tissue (St) was washed three times with 1 ml of PBS, then homogenized for 1 min in the ice bath and the proteins were precipitated with acetone for 48 h; samples were centrifuged 6000 rpm/5 min/4° (3X); pellets were solubilized with 500 ml of 2D sample buffer (4% urea, 2% thiourea, 2% CHAPS, 160 mM DTT); then were precipitated with methanol-chloroform; pellets were solubilized with 200 ml of 2D sample buffer, were centrifuged at maximum speed in microfuge 5 min at 4°, and supernatants were used for two-dimensional analysis.
4.8. 2D-SDS-Page
300 μg of Mc and St of all groups were individually loaded in IPG-strips pH 3-10 of 7 cm. After 16 h of passive rehydration, IEF was performed as described below: step 1, 50 V/20 min /rapid; step 2, 70 V/30 min/rapid; step 3, 250 V/20 min/lineal; step 4, 4000 V/2 h/lineal; step 5, 4000 V/20000 Vh/rapid. Strips were treated with equilibration buffer with 2% (w/v) DTT (6 M urea, 2% SDS, 0.375 M TRIS-HCl pH 8.8, 20% glycerol and 1% bromophenol blue) for 15 min, and then with equilibration buffer with 2.5% (w/v) IAA for 20 min. Strips were loaded in precast 4-20% polyacrylamide gels and electrophoresis was run for approximately 35 minutes at 200 V. Gels were stained with Coomassie blue.
4.9. Bioinformatic Analysis
After Coomassie blue staining of the gels, they were scanned with ChemidocTM XRS Device (Bio-Rad Laboratories, Segrate, Milan, Italy) at 252 dpi resolution and analyzed using the PDQuest program. Spots that exhibited differential expression compared to the control were analyzed in the SWISS 2DPAGE public repository (
https://world-2dpage.expasy.org/swiss-2dpage?pi_mw), to determine the identity of the proteins by comparison of MW and Ip with available 2D proteomic maps.
4.10. String Analysis
To determine the main signaling pathways involved in the simvastatin effect, we chose the proteins that exhibited differential expression between MPP+ and MPP+ / Simvastatin groups for their analysis in the STRING database (
https://version-11-5.string-db.org). The analysis showed that selected proteins (Figure 6) are biologically connected, exhibiting a PPI enrichment p-value of 7.64e-09.
4.11. Statistical Analysis
Results from dopamine content and lipid fluorescent products were analyzed by one-way ANOVA followed by Tukey test. Data obtained from evaluating the circling behavior were analyzed by Kruskal-Wallis’ followed by Mann-Whitney´s test. Statistical significance was set at p < 0.05.
5. Conclusions
Simvastatin showed a significate antioxidant and more importantly neuroprotection effect against MPP+-induced neurotoxicity since it was able to counteract the oxidative damage exerted by MPP+, improving three damage markers: lipid peroxidation, dopamine content, and circling behavior; the first two, were evaluated as short term and the last, was considered as long-term endpoint damage markers. We also identified proteins responsible for inflammation response that should be further studied.
Author Contributions
Conceptualization, JMM, CR and MRO; methodology, CTGL, SM, CRu, and AM; software, CTGL; validation, JMM, MRO. and AM; formal analysis, JMM, MRO, CTGL; investigation, MRO, JMM, CTGL; CR, Cru, AM, resources, JMM and MRO; data curation, MRO and CTGL; writing—original draft preparation, MRO and JMM; writing—review and editing, MRO, JMM; supervision, MRO, CTGL and JMM; project administration, JMM, MRO and CTGL; funding acquisition, JMM and MRO. All authors have read and agreed to the published version of the manuscript.”
Funding
This project has been supported partially both Grant SEP/CONACYT 287959 and 257092. Also, by Grant IN-202723 from Programa de Apoyo a Proyectos de Innovación Tecnológica (PAPIIT), Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México (UNAM).
Institutional Review Board Statement
The animal study protocol was approved by The Committee on Ethics and Use in Animal Experimentation of the Institute of Neurology and Neurosurgery and the standards of the National Institutes of Health of Mexico (Permit number INN-2017-2023). The study was done following the guidelines of Mexican regulations (NOM-062-ZOO-1999) and the Guide for the Care and Use of Laboratory Animals of the National Institute of Health, 8th Edition to ensure compliance with the established international regulations and guidelines.
Data Availability Statement
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Acknowledgments
Carmen T. Gómez de León was a recipient of a Post-Doctoral fellowship from Grant FC 2016-2125 from Fronteras en la Ciencia, Consejo Nacional de Ciencia y Tecnología (CONACYT). We thank the technical support given to the realization of the present study to Biologist Gilberto Hevyn Chávez Cortés.
Conflicts of Interest
Authors declare there is no conflict of interest.
References
- Tolleson, C.M.; Fang, J.Y. Advances in the mechanisms of Parkinson's disease. Discov Med 2013, 15, 61–66. [Google Scholar]
- Beitz, J.M. Parkinson s disease: A review. Front. Biosci. 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef]
- Hare, D.J.; Adlard, P.A.; Doble, P.A.; Finkelstein, D.I. Metallobiology of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Metallomics 2013, 5, 91–109. [Google Scholar] [CrossRef] [PubMed]
- Bournival, J.; Plouffe, M.; Renaud, J.; Provencher, C.; Martinoli, M.-G. Quercetin and Sesamin Protect Dopaminergic Cells from MPP+-Induced Neuroinflammation in a Microglial (N9)-Neuronal (PC12) Coculture System. Oxidative Med. Cell. Longev. 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Lee, E.; Hwang, I.; Park, S.; Hong, S.; Hwang, B.; Cho, Y.; Son, J.; Yu, J.-W. MPTP-driven NLRP3 inflammasome activation in microglia plays a central role in dopaminergic neurodegeneration. Cell Death Differ. 2019, 26, 213–228. [Google Scholar] [CrossRef]
- Chen, X.; Liu, Z.; Cao, B.-B.; Qiu, Y.-H.; Peng, Y.-P. TGF-β1 Neuroprotection via Inhibition of Microglial Activation in a Rat Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 433–446. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson's Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Vitlic, A.; Lord, J.M.; Phillips, A.C. Stress, ageing and their influence on functional, cellular and molecular aspects of the immune system. AGE 2014, 36, 9631. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, T.R.; A Tobert, J. Simvastatin: a review. Expert Opin. Pharmacother. 2004, 5, 2583–2596. [Google Scholar] [CrossRef]
- Prasad, E.M.; Hung, S.-Y. Behavioral Tests in Neurotoxin-Induced Animal Models of Parkinson’s Disease. Antioxidants 2020, 9, 1007. [Google Scholar] [CrossRef] [PubMed]
- Selley, M.L. Simvastatin prevents 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced striatal dopamine depletion and protein tyrosine nitration in mice. Brain Res. 2005, 1037, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Vidal, Y.; Montes, S.; Tristan-López, L.; Anaya-Ramos, L.; Teiber, J.; Ríos, C.; Baron-Flores, V.; Monroy-Noyola, A. The neuroprotective effect of lovastatin on MPP + -induced neurotoxicity is not mediated by PON2. NeuroToxicology 2015, 48, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Santiago, M.; Hernández-Romero, M.C.; Machado, A.; Cano, J. Zocor Forte® (simvastatin) has a neuroprotective effect against LPS striatal dopaminergic terminals injury, whereas against MPP+ does not. Eur. J. Pharmacol. 2009, 609, 58–64. [Google Scholar] [CrossRef]
- Aguirre-Vidal, Y.; Morales-Montor, J.; de Leon, C.T.G.; Ostoa-Saloma, P.; Diaz-Zaragoza, M.; Montes, S.; Arteaga-Silva, M.; Monroy-Noyola, A. Protection induced by estradiol benzoate in the MPP+ rat model of Parkinson's disease is associated with the regulation of the inflammatory cytokine profile in the nigro striatum. J. Neuroimmunol. 2020, 349, 577426. [Google Scholar] [CrossRef] [PubMed]
- Brzozowski, M.J.; Jenner, P.; Rose, S. Inhibition of i-NOS but not n-NOS protects rat primary cell cultures against MPP+-induced neuronal toxicity. J. Neural Transm. 2014, 122, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Romero, M.d.C.; Argüelles, S.; Villarán, R.F.; De Pablos, R.M.; Delgado-Cortés, M.J.; Santiago, M.; Herrera, A.J.; Cano, J.; Machado, A. Simvastatin prevents the inflammatory process and the dopaminergic degeneration induced by the intranigral injection of lipopolysaccharide. J. Neurochem. 2007, 105, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiao, L.; Wu, J.; Fan, H.; Sun, J.; Zhang, Y. Simvastatin Protects Dopaminergic Neurons Against MPP+-Induced Oxidative Stress and Regulates the Endogenous Anti-Oxidant System Through ERK. Cell. Physiol. Biochem. 2018, 51, 1957–1968. [Google Scholar] [CrossRef]
- Yan, J.; Sun, J.; Huang, L.; Fu, Q.; Du, G. Simvastatin prevents neuroinflammation by inhibiting N-methyl-D-aspartic acid receptor 1 in 6-hydroxydopamine-treated PC12 cells. J. Neurosci. Res. 2014, 92, 634–640. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Yuan, L.; Li, W.; Li, J.-Y. Ferroptosis in Parkinson’s disease: glia–neuron crosstalk. Trends Mol. Med. 2022, 28, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Vasefi, M.; Beazely, M.A. Neuroprotective effects of direct activation and transactivation of PDGFβ receptors. Vessel. Plus 2020, 2020. [Google Scholar] [CrossRef]
- Zou, B.; Liu, J.; Klionsky, D.J.; Tang, D.; Kang, R. Extracellular SQSTM1 as an inflammatory mediator. Autophagy 2020, 16, 2313–2315. [Google Scholar] [CrossRef]
- Cui, X.; Tang, J.; Hartanto, Y.; Zhang, J.; Bi, J.; Dai, S.; Qiao, S.Z.; Cheng, K.; Zhang, H. NIPAM-based Microgel Microenvironment Regulates the Therapeutic Function of Cardiac Stromal Cells. ACS Appl. Mater. Interfaces 2018, 10, 37783–37796. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, Z.; Cao, B.-B.; Qiu, Y.-H.; Peng, Y.-P. TGF-β1 Neuroprotection via Inhibition of Microglial Activation in a Rat Model of Parkinson’s Disease. J. Neuroimmune Pharmacol. 2017, 12, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Osornio, M.; Orozco-Ibarra, M.; Díaz-Ruiz, A.; Brambila, E.; Boll, M.-C.; Monroy-Noyola, A.; Guevara, J.; Montes, S.; Ríos, C. Copper sulfate pretreatment prevents mitochondrial electron transport chain damage and apoptosis against MPP + -induced neurotoxicity. Chem. Interactions 2017, 271, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Osornio, M.; Montes, S.; Pérez-Severiano, F.; Aguilera, P.; Floriano-Sánchez, E.; Monroy-Noyola, A.; Rubio, C.; Ríos, C. Copper reduces striatal protein nitration and tyrosine hydroxylase inactivation induced by MPP+ in rats. Neurochem. Int. 2009, 54, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Osornio, M.; Montes, S.; Heras-Romero, Y.; Guevara, J.; Rubio, C.; Aguilera, P.; Rivera-Mancia, S.; Floriano-Sánchez, E.; Monroy-Noyola, A.; Ríos, C. Induction of ferroxidase enzymatic activity by copper reduces MPP+-evoked neurotoxicity in rats. Neurosci. Res. 2013, 75, 250–255. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
Table 2.
Proteins related to immune response that changed their expression respect to the control.
Table 2.
Proteins related to immune response that changed their expression respect to the control.
| SPOT |
Protein name |
UniProtK B<break/>Accession number |
Theoretical Mr/pI |
Experimental <break/>Mr/pI |
Possible function |
Relative expression |
| ST1 |
ST2 |
ST3 |
ST4 |
| 3501 |
Carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1) |
P16573 |
50.76 / 5.31 |
50.27 / 5.31 |
Cell adhesion,<break/>Positive regulation of activation-induced cell death of T cells |
+ |
++++ |
++ |
++++ |
| 5103 |
TGF-β2 |
Q07257 |
12.69 / 6.88 |
12.09 / 6.91 |
Cytokine |
+ |
- |
- |
- |
| 7403 |
TGF-β1 (Tgfb1) |
P17246 |
44.33 / 8.59 |
40.94 / 8.52 |
Inflammatory response,<break/>Positive regulation of activation-induced cell death of T cells,<break/>Negative regulation of macrophage cytokine production |
++ |
+ |
- |
- |
| 4603 |
TGF-β3 (Tgfb3) |
Q07258 |
47.12 / 6.10 |
48.11 / 6.06 |
Activation of MAPK activity |
++ |
++ |
+ |
- |
| 6401 |
Proteinase-activated receptor 1 |
P26824 |
43.31 / 7.39 |
41.24 / 7.41 |
Inflammatory response |
+ |
- |
- |
- |
| 4402 |
Heme oxygenase 1 |
P06762 |
33.00 / 6.08 |
33.57 / 6.09 |
Oxidoreductase, Apoptosis |
+ |
- |
- |
- |
| 3302 |
IL-1α |
P16598 |
30.86 / 5.59 |
30.32 / 5.53 |
Inflammatory response |
+ |
++ |
- |
- |
| 8301 |
IL-1β |
Q63264 |
30.64 / 8.37 |
31.80 / 8.34 |
Inflammatory response |
- |
++ |
+ |
- |
| 3104 |
Interleukin-23 subunit alpha (IL-23α) |
Q91Z84 |
19.60 / 5.63 |
17.92 / 5.62 |
Immunity, Inflammatory response |
+ |
- |
- |
- |
| 3004 |
Protein S100-A8 |
P50115 |
10.10 / 5.69 |
9.46 / 5.64 |
Inflammatory response |
++ |
+ |
- |
- |
| 1302 |
Amyloid-beta A4 protein |
P08592 |
30.03 / 4.15 |
26.92 / 4.13 |
Apoptosis, Cell adhesion, Endocytosis, Notch signaling pathway |
+ |
- |
- |
- |
| 6206 |
Allograft inflammatory factor 1 (Aif1) |
P55009 |
16.69 / 7.83 |
18.75 / 7.87 |
Cytoskeleton |
++ |
+ |
- |
- |
| 5302 |
B- and T-lymphocyte attenuator |
Q6PNM1 |
31.11 / 6.77 |
30.35 / 6.77 |
Adaptive immunity |
+ |
- |
- |
- |
| 3003 |
TYRO protein tyrosine kinase-binding protein |
Q6X9T7 |
9.54 / 5.69 |
8.29 / 5.63 |
Immunity |
+ |
- |
- |
- |
| 6203 |
IL-6r |
P22273 |
24.36 / 7.77 |
22.37 / 7.66 |
Response to lipopolysaccharide |
+ |
++ |
- |
- |
| 5504 |
Corticotropin-releasing factor receptor 2 |
P47866 |
47.69 / 6.85 |
47.18 / 6.86 |
G-protein coupled receptor |
+ |
+ |
++++ |
++ |
| 2203 |
Leucine repeat adapter protein 25 |
Q566R4 |
18.71 / 4.95 |
18.97 / 4.95 |
Negative regulation of transforming growth factor beta receptor signaling pathway |
+ |
++ |
- |
- |
| 6503 |
Serine protease HTRA1 |
Q9QZK5 |
48.97 / 7.55 |
49.83 / 7.56 |
Negative regulation of transforming growth factor beta receptor signaling pathway |
+ |
- |
- |
- |
| 9103 |
Insulin-like growth factor I (Igf1) |
P08025 |
17.83 / 9.77 |
17.91 / 9.75 |
Growth factor |
++++ |
+++ |
++ |
- |
| 2404 |
cAMP-dependent protein kinase type I-alpha regulatory subunit |
P09456 |
43.09 / 5.28 |
43.18 / 5.27 |
cAMP, cAMP-binding, Nucleotide-binding |
+ |
++ |
++ |
- |
| 8003 |
Caspase-8 |
Q9JHX4 |
10.83 / 9.13 |
11.21 / 9.13 |
Apoptosis |
+ |
- |
- |
- |
| 3204 |
Myc box-dependent-interacting protein 1 |
O08839 |
25.23 / 5.72 |
24.85 / 5.73 |
Positive regulation of astrocyte |
+ |
- |
- |
- |
| 2604 |
Myelin-associated glycoprotein |
P07722 |
67.17 / 4.94 |
68.81 / 4.93 |
Axon regeneration |
+ |
- |
- |
- |
| 4207 |
Matrilysin |
P50280 |
18.93 / 6.22 |
19.35 / 6.21 |
Metalloprotease |
+ |
- |
- |
- |
| 8102 |
WAP four-disulfide core domain protein 2 |
Q8CHN3 |
12.39 / 8.89 |
12.50 / 8.88 |
Serine protease inhibitor |
+ |
- |
- |
- |
| 7002 |
Protein WFDC9 |
Q6IE41 |
6.39 / 8.19 |
6.78 / 8.20 |
Unknown |
+ |
- |
- |
- |
| 6201 |
Vascular endothelial growth factor B |
O35485 |
19.56 / 7.36 |
18.92 / 7.41 |
Angiogenesis |
+ |
- |
- |
- |
| 2501 |
Calreticulin |
P18418 |
46.34 / 4.34 |
46.96 / 4.34 |
Chaperone, Metal-binding, Zinc |
+ |
- |
- |
- |
| 6101 |
Macrophage migration inhibitory factor |
P30904 |
12.34 / 7.28 |
12.62 / 7.38 |
Inflammatory response |
+ |
- |
- |
- |
| 2702 |
Cadherin-17 |
P55281 |
89.56 / 4.72 |
87.22 / 4.72 |
Cell adhesion |
+ |
- |
- |
- |
| 3704 |
Protein artemis |
Q5XIX3 |
78.18 / 5.63 |
79.45 / 5.62 |
DNA repair, Immunity |
+ |
- |
- |
- |
| 6205 |
Interferon alpha-1 (Ifna1) |
P05011 |
19.42 / 7.83 |
22.54 / 7.84 |
Cytokine pro inflamamtory |
+++ |
++ |
+ |
++ |
| 8104 |
Platelet-derived growth factor D (Pdgf-d) |
Q9EQT1 |
13.96 / 9.39 |
13.22 / 9.35 |
Developmental protein, Growth factor, Mitogen |
++ |
- |
+ |
- |
| 8005 |
Tumor necrosis factor ligand superfamily member 6 (Faslg) |
P36940 |
8.52 / 9.24 |
8.88 / 9.24 |
Proinflammatory |
++ |
+ |
- |
- |
| 2001 |
Protein S100-B |
P04631 |
10.61 / 4.53 |
11.74 / 4.56 |
Positive regulation of myelination |
+ |
- |
- |
- |
| 2705 |
Ubiquitin carboxyl-terminal hydrolase 10 |
Q3KR59 |
87.18 / 5.05 |
87.64 / 5.05 |
DNA repair |
+ |
- |
- |
- |
| 2503 |
Sequestosome-1 (Sqstm1) |
O08623 |
47.55 / 5.05 |
48.01 / 5.07 |
Apoptosis, Autophagy, Differentiation, Immunity |
+++ |
+++++ |
++ |
+ |
| 3201 |
Cdc42 effector protein 2 |
Q5PQP4 |
22.91 / 5.31 |
23.14 / 5.30 |
Rho protein signal transduction |
+ |
- |
- |
- |
| 5204 |
Metalloproteinase inhibitor 4 |
P81556 |
22.55 / 6.88 |
22.76 / 6.87 |
Notch signaling pathway, central nervous system development |
+ |
- |
- |
- |
| 5201 |
Caspase-6 |
O35397 |
18.06 / 6.48 |
18.10 / 6.49 |
Apoptosis |
++ |
+ |
+ |
- |
| 5606 |
Synaptic functional regulator FMR1 |
Q80WE1 |
66.78 / 6.77 |
68.63 / 6.78 |
Translation regulation |
+ |
- |
- |
- |
| 2607 |
Interleukin-2 receptor subunit beta |
P26896 |
57.71 / 5.20 |
56.75 / 5.18 |
Cytokine pro inflamamtory |
+ |
- |
- |
- |
| 6601 |
Ectonucleoside triphosphate diphosphohydrolase 1 |
P97687 |
57.40 / 7.49 |
63.42 / 7.45 |
Regulate purinergic neurotransmission |
++ |
+ |
- |
- |
| 2410 |
Tumor necrosis factor (TNF-α) |
P16599 |
25.81 / 5.14 |
24.27 / 5.19 |
Cytokine pro inflammatory |
+ |
+++ |
++ |
++++ |
Table 3.
Differential expression of antioxidant enzymes in striated tissue of rat brain.
Table 3.
Differential expression of antioxidant enzymes in striated tissue of rat brain.
| SPOT |
Protein name |
UniProtKB<break/>Accession number |
Theoretical Mr/pI |
Experimental<break/> Mr/pI |
Possible function |
Relative expression |
| ST1 |
ST2 |
ST3 |
ST4 |
| 3306 |
Superoxide Dismutase 2 (SOD2) |
P07895 |
15.91 / 5.89 |
19.55 / 5.88 |
Vasodilation |
- |
++ |
+++ |
- |
| 5705 |
Catalase (CAT) |
P04762 |
59.63 / 7.15 |
58.07 / 7.20 |
Positive regulation of NF-kappaB transcription factor activity |
- |
+ |
- |
- |
| 7203 |
Glutathione Peroxidase 1 (GPX1) |
P04041 |
22.35 / 7.70 |
21.51 / 7.70 |
Angiogenesis |
++ |
+++ |
+ |
- |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).