Submitted:
08 February 2023
Posted:
08 February 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Algal Blooms as Eco-health Epitome
1.2. Complex Marine Ecosystems
1.3. Ecological Patterns as Chlorophyll-a Spreading Networks
2. Materials and Methods
2.1. Datasets
2.2. Ecosystem Organization and Connectome
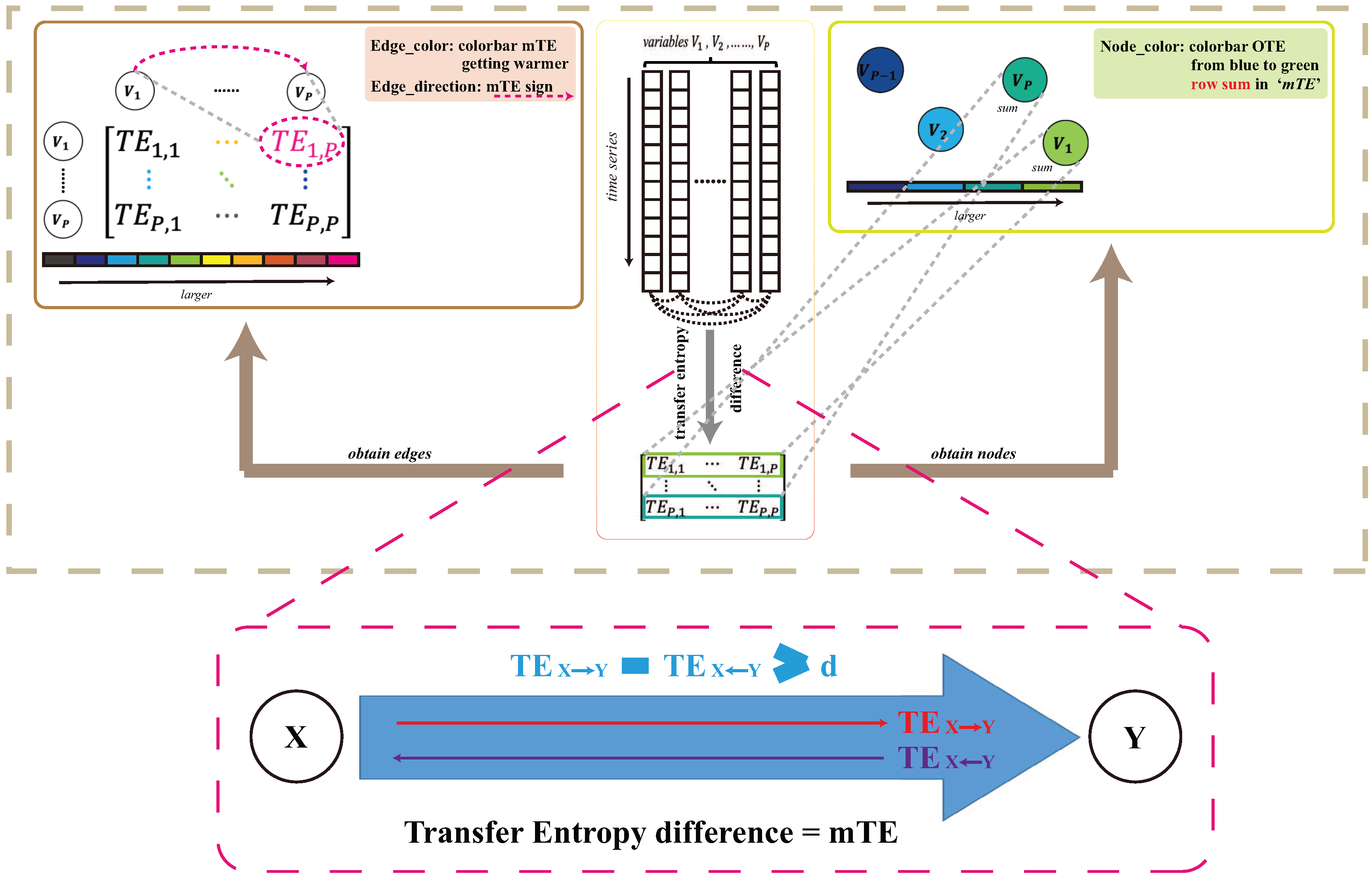
2.3. Eco-Environmental Network Inference
2.4. Eco-Environmental Factor Predictive Causality
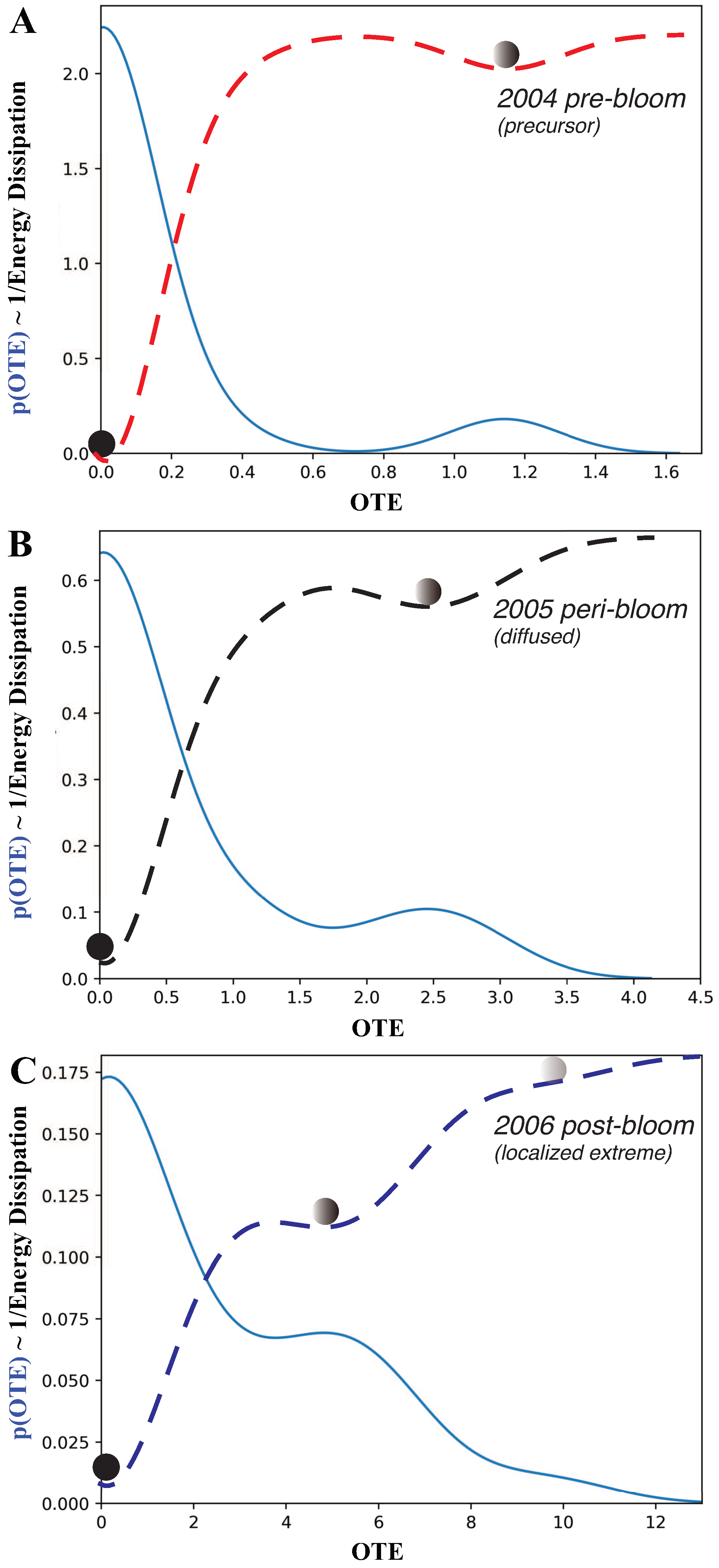
3. Results and Discussion
3.1. Spatio-temporal Spreading and Fluctuations
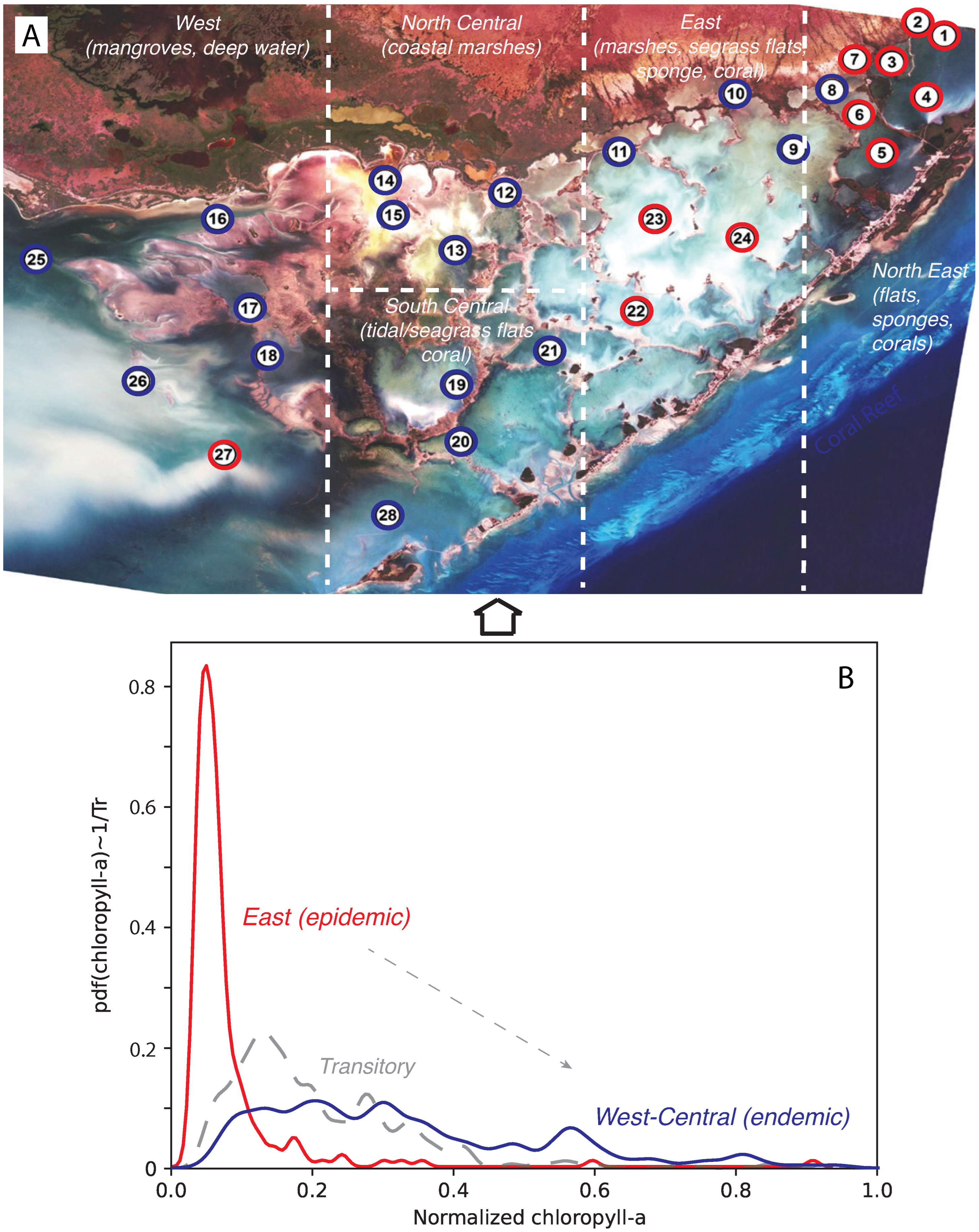
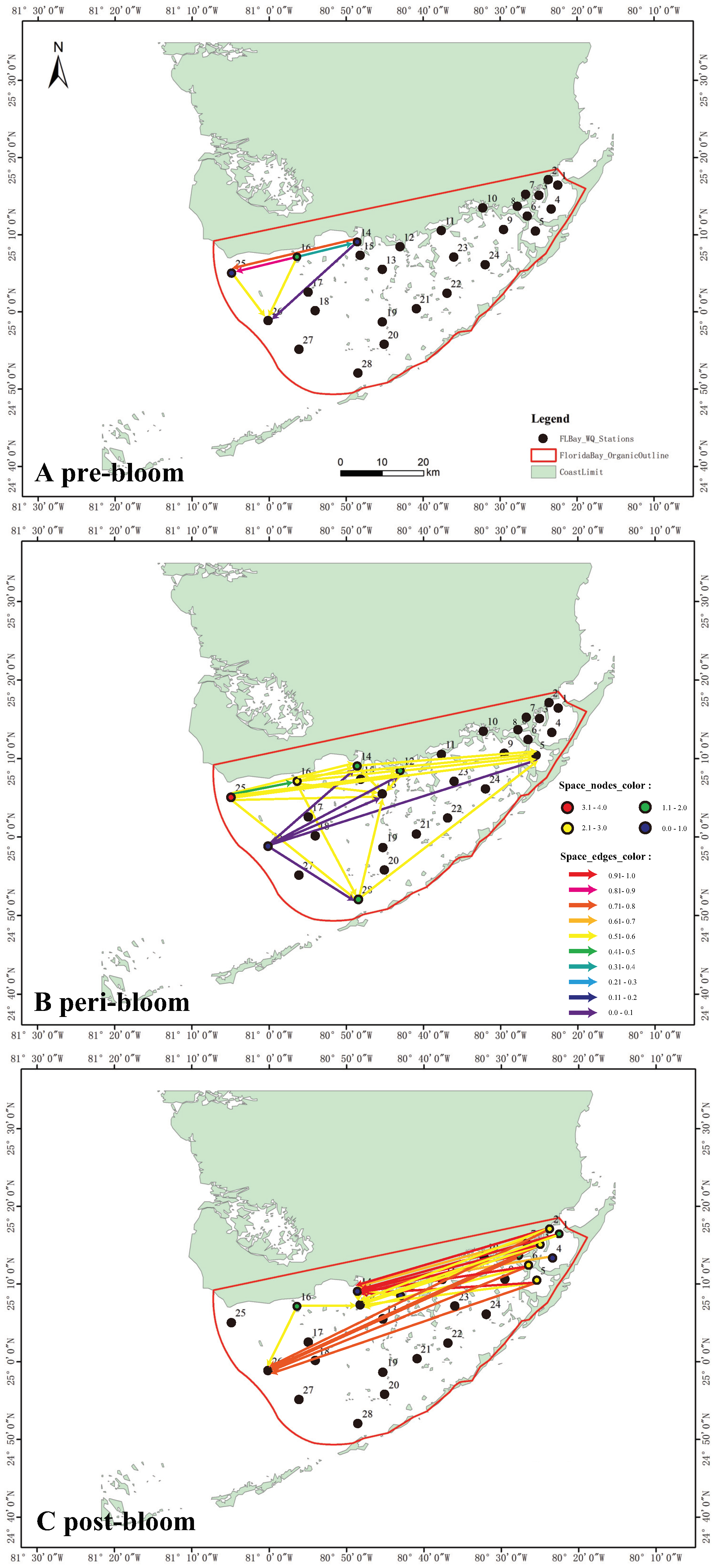
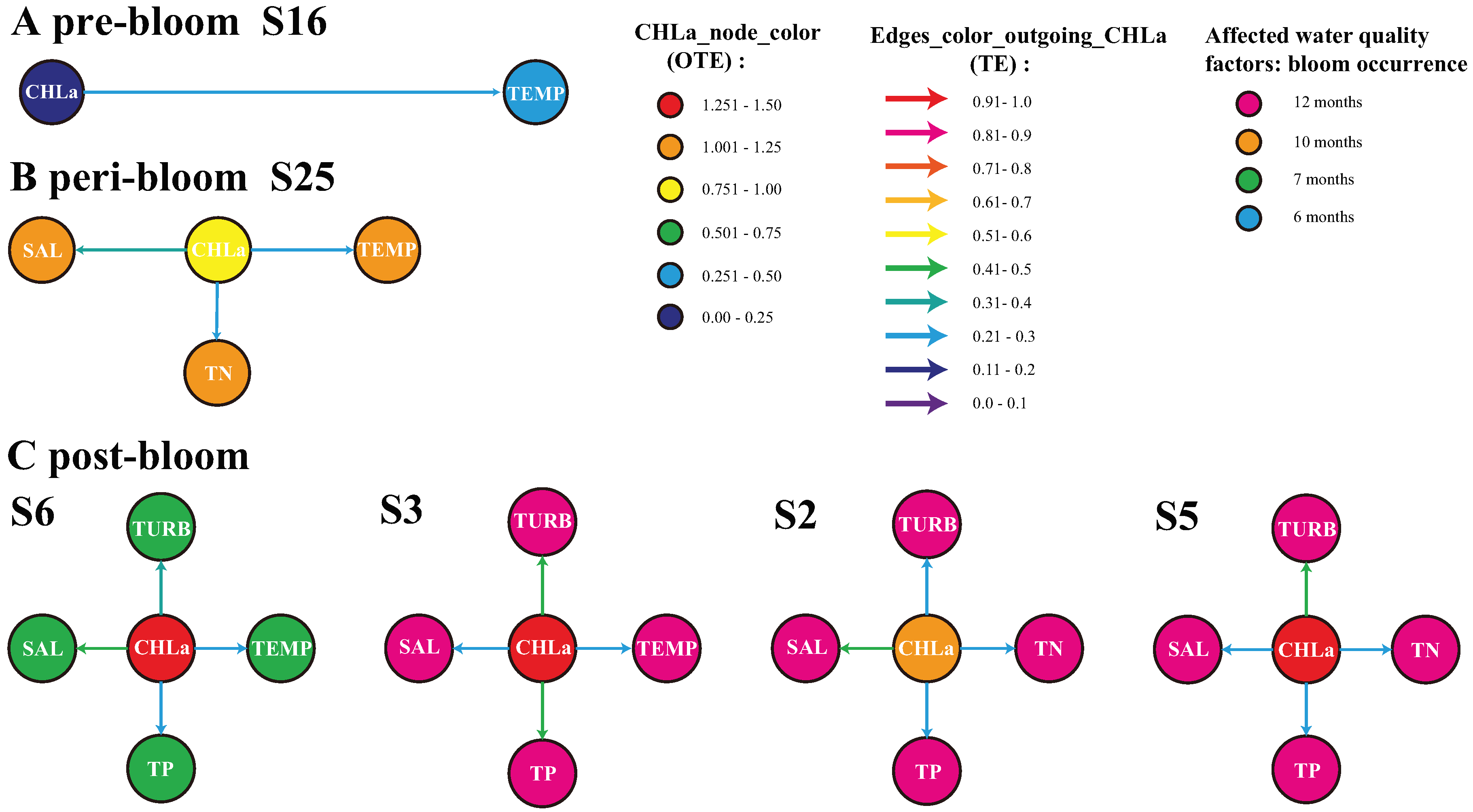
3.2. Water Quality Trends and Bloom Impacts
3.3. Bloom Intensity and Area Dependency
4. Conclusions
Author Contributions
Acknowledgments
References
- Albert, Réka and Albert-László Barabási. 2002. Statistical mechanics of complex networks. Reviews of modern physics 74(1), 47. [CrossRef]
- Andrade Jr, José S, Hans J Herrmann, Roberto FS Andrade, and Luciano R Da Silva. 2005. Apollonian networks: Simultaneously scale-free, small world, euclidean, space filling, and with matching graphs. Physical review letters 94(1), 018702.
- Boccaletti, Stefano, Vito Latora, Yamir Moreno, Martin Chavez, and D-U Hwang. 2006. Complex networks: Structure and dynamics. Physics reports 424(4-5), 175–308. [CrossRef]
- Boyer, Joseph N and Henry O Briceño. 2007. South florida coastal water quality monitoring network. FY2006 Cumulative Report South Florida Water Management District, Southeast Environmental Research Center, Florida International University (http://serc.fiu.edu/wqmnetwork/).
- Boyer, Joseph N, Christopher R Kelble, Peter B Ortner, and David T Rudnick. 2009. Phytoplankton bloom status: Chlorophyll a biomass as an indicator of water quality condition in the southern estuaries of florida, usa. Ecological indicators 9(6), S56–S67. [CrossRef]
- Briceño, Henry O and Joseph N Boyer. 2010. Climatic controls on phytoplankton biomass in a sub-tropical estuary, florida bay, usa. Estuaries and Coasts 33(2), 541–553. [CrossRef]
- Burd, Adrian B and George A Jackson. 2002. An analysis of water column distributions in florida bay. Estuaries 25(4), 570–585. [CrossRef]
- Butler IV, Mark J, John H Hunt, William F Herrnkind, Michael J Childress, Rodney Bertelsen, William Sharp, Thomas Matthews, Jennifer M Field, and Harold G Marshall. 1995. Cascading disturbances in florida bay, usa: cyanobacteria blooms, sponge mortality, and implications for juvenile spiny lobsters panulirus argus. Marine Ecology Progress Series 129, 119–125. [CrossRef]
- Cloern, James E and Alan D Jassby. 2010. Patterns and scales of phytoplankton variability in estuarine–coastal ecosystems. Estuaries and coasts 33(2), 230–241. [CrossRef]
- Convertino, M, A Reddy, Y Liu, and C Munoz-Zanzi. 2021. Eco-epidemiological scaling of leptospirosis: Vulnerability mapping and early warning forecasts. Science of The Total Environment 799, 149102. [CrossRef]
- Convertino, Matteo and L James Valverde Jr. 2019. Toward a pluralistic conception of resilience. Ecological Indicators 107, 105510. [CrossRef]
- Convertino, Matteo and Haojiong Wang. 2022. Envirome disorganization and ecological riskscapes: The algal bloom epitome. In Risk Assessment for Environmental Health, pp. 327–346. CRC Press.
- Coscia, Michele, Fosca Giannotti, and Dino Pedreschi. 2011. A classification for community discovery methods in complex networks. Statistical Analysis and Data Mining: The ASA Data Science Journal 4(5), 512–546.
- Cosper, Elizabeth M, William C Dennison, Edward J Carpenter, V Monica Bricelj, James G Mitchell, Susan H Kuenstner, David Colflesh, and Maynard Dewey. 1987. Recurrent and persistent brown tide blooms perturb coastal marine ecosystem. Estuaries 10(4), 284–290. [CrossRef]
- Costa, L da F, Francisco A Rodrigues, Gonzalo Travieso, and Paulino Ribeiro Villas Boas. 2007. Characterization of complex networks: A survey of measurements. Advances in physics 56(1), 167–242. [CrossRef]
- Donges, Jonathan F, Yong Zou, Norbert Marwan, and Jürgen Kurths. 2009. Complex networks in climate dynamics. The European Physical Journal Special Topics 174(1), 157–179.
- Dorogovtsev, Sergey N, Alexander V Goltsev, and José Ferreira F Mendes. 2002. Pseudofractal scale-free web. Physical review E 65(6), 066122. [CrossRef]
- Duan, Ziheng, Haoyan Xu, Yida Huang, Jie Feng, and Yueyang Wang. 2022. Multivariate time series forecasting with transfer entropy graph. Tsinghua Science and Technology 28(1), 141–149. [CrossRef]
- Feldhoff, Jan H, Stefan Lange, Jan Volkholz, Jonathan F Donges, Jürgen Kurths, and Friedrich-Wilhelm Gerstengarbe. 2015. Complex networks for climate model evaluation with application to statistical versus dynamical modeling of south american climate. Climate dynamics 44(5), 1567–1581. [CrossRef]
- Galbraith, Elroy and Matteo Convertino. 2021. The eco-evo mandala: Simplifying bacterioplankton complexity into ecohealth signatures. Entropy 23(11), 1471. [CrossRef]
- Galbraith, Elroy, PR Frade, and Matteo Convertino. 2022. Metabolic shifts of oceans: Summoning bacterial interactions. Ecological Indicators 138, 108871. [CrossRef]
- Gfeller, D, P De Los Rios, A Caflisch, and F Rao. 2007. Complex network analysis of free-energy landscapes. Proceedings of the National Academy of Sciences 104(6), 1817–1822.
- Hidalgo, Jorge, Jacopo Grilli, Samir Suweis, Miguel A Munoz, Jayanth R Banavar, and Amos Maritan. 2014. Information-based fitness and the emergence of criticality in living systems. Proceedings of the National Academy of Sciences 111(28), 10095–10100. [CrossRef]
- Ilany, Amiyaal, Andrew S Booms, and Kay E Holekamp. 2015. Topological effects of network structure on long-term social network dynamics in a wild mammal. Ecology letters 18(7), 687–695. [CrossRef]
- Kumar, Ravi, Prabhakar Raghavan, Sridhar Rajagopalan, D Sivakumar, Andrew Tomkins, and Eli Upfal. 2000. Stochastic models for the web graph. In Proceedings 41st Annual Symposium on Foundations of Computer Science, pp. 57–65. IEEE.
- Li, Jie and Matteo Convertino. 2019. Optimal microbiome networks: macroecology and criticality. Entropy 21(5), 506. [CrossRef]
- Li, Jie and Matteo Convertino. 2021a. Inferring ecosystem networks as information flows. Scientific reports 11(1), 1–22. [CrossRef]
- Li, Jie and Matteo Convertino. 2021b. Temperature increase drives critical slowing down of fish ecosystems. PLoS One 16(10), e0246222. [CrossRef]
- Lin, Jingyi and Yifang Ban. 2013. Complex network topology of transportation systems. Transport reviews 33(6), 658–685. [CrossRef]
- Lotze, Heike K, Boris Worm, and Ulrich Sommer. 2000. Propagule banks, herbivory and nutrient supply control population development and dominance patterns in macroalgal blooms. Oikos 89(1), 46–58. [CrossRef]
- Martinello, Matteo, Jorge Hidalgo, Amos Maritan, Serena Di Santo, Dietmar Plenz, and Miguel A Muñoz. 2017. Neutral theory and scale-free neural dynamics. Physical Review X 7(4), 041071. [CrossRef]
- Nelson, Natalie G, Rafael Munoz-Carpena, and Edward J Phlips. 2017. A novel quantile method reveals spatiotemporal shifts in phytoplankton biomass descriptors between bloom and non-bloom conditions in a subtropical estuary. Marine Ecology Progress Series 567, 57–78. [CrossRef]
- Newman, Mark EJ. 2003. The structure and function of complex networks. SIAM review 45(2), 167–256. [CrossRef]
- Paerl, Hans W, Rolland S Fulton, Pia H Moisander, and Julianne Dyble. 2001. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. TheScientificWorldJournal 1, 76–113. [CrossRef]
- Phlips, Edward J, Susan Badylak, and Tammy C Lynch. 1999. Blooms of the picoplanktonic cyanobacterium synechococcus in florida bay, a subtropical inner-shelf lagoon. Limnology and Oceanography 44(4), 1166–1175. [CrossRef]
- Reijneveld, Jaap C, Sophie C Ponten, Henk W Berendse, and Cornelis J Stam. 2007. The application of graph theoretical analysis to complex networks in the brain. Clinical neurophysiology 118(11), 2317–2331. [CrossRef]
- Sanders, Robert. 1987. The pareto principle: its use and abuse. Journal of Services Marketing 1(2), 37–40. [CrossRef]
- Schreiber, Thomas. 2000. Measuring information transfer. Physical review letters 85(2), 461. [CrossRef]
- Shannon, Claude Elwood. 1948. A mathematical theory of communication. The Bell system technical journal 27(3), 379–423.
- Smayda, Theodore J. 1997. What is a bloom? a commentary. Limnology and Oceanography 42(5part2), 1132–1136.
- Wang, H and M Convertino. 2023. Algal bloom ties: Systemic biogeochemical stress and chlorophyll-a shift forecasting. submitted –(–), –. [CrossRef]
- Wang, Xiao Fan. 2002. Complex networks: topology, dynamics and synchronization. International journal of bifurcation and chaos 12(05), 885–916.
- Xu, Li, Denis Patterson, Simon Asher Levin, and Jin Wang. 2023. Non-equilibrium early-warning signals for critical transitions in ecological systems. Proceedings of the National Academy of Sciences 120(5), e2218663120. [CrossRef]
- Zhang, Jie and Michael Small. 2006. Complex network from pseudoperiodic time series: Topology versus dynamics. Physical review letters 96(23), 238701. [CrossRef]
- Zhou, Changsong, Lucia Zemanová, Gorka Zamora, Claus C Hilgetag, and Jürgen Kurths. 2006. Hierarchical organization unveiled by functional connectivity in complex brain networks. Physical review letters 97(23), 238103. [CrossRef]
- Zhou, Changsong, Lucia Zemanová, Gorka Zamora-Lopez, Claus C Hilgetag, and Jürgen Kurths. 2007. Structure–function relationship in complex brain networks expressed by hierarchical synchronization. New Journal of Physics 9(6), 178. [CrossRef]
- Zou, Yong, Reik V Donner, Norbert Marwan, Jonathan F Donges, and Jürgen Kurths. 2019. Complex network approaches to nonlinear time series analysis. Physics Reports 787, 1–97. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




