1. Introduction
According to the Diagnostic and Statistical Manual of Mental Disorders 5th edition (DSM 5), Borderline personality disorder (BPD) is characterized by a pervasive pattern of dysregulation on affect (anger outburst, depression episodes, anxiety), cognition (dissociative experiences, self-image disturbances) interpersonal relationships (unstable relationships, fear of abandonment), together with marked impulsivity [
1,
2]. The disorder has a 3% prevalence in general population [
3,
4] and is associated with significant impairment of patients’ psychological functioning [
4,
5,
6].
In the last twenty years, several neuroimaging studies, have tried to understand and delineate the neural marker of BPD. So far, neuroanatomical model of BPD indicate significantly reduction in grey matter volume and density in the bilateral medial prefrontal cortex (mPFC) [
7], medial orbital frontal cortex (OFC) [
8,
9], bilateral anterior cingulate cortex (ACC) [
10], bilateral amygdale and right parahippocampal gyrus [
11,
12]. On the other hand, they also showed an increase of grey matter volume and density in the bilateral praecuneus, right medium/paracingulate gyrus and posterior cingulate gyrus [
7].
Different studies have shown cortical volume reductions using specific regions of interest (ROIs), including in the anterior cingulate cortex, the orbitofrontal cortex and the right parietal cortex [
13,
14,
15]. Other studies have also examined brain structure in BPD using voxel-based morphometry (VBM) [
16] showing structural alterations in the pars opercularis and triangularis of the right inferior frontal gyrus, in the precentral gyrus and in the right superior frontal gyrus and bilaterally in the temporal lobe cortex [
17,
18,
19]. Also, with VBM Structural imaging studies meta-analysis have observed, a reduced volume of the amygdala and hippocampus but on the contrary in another study there was no evidence of volume reductions in the hippocampus or amygdala [
20]. Functional alterations consistent with the structural alterations have been found too [
19,
21,
22,
23]. Two distinct (but not necessarily incompatible) neurobiological models of BPD pathology. In the first model, the emotional instability/impulsivity/emotion regulation of BPD has been characterized as the core element of this disorder and explained as enhanced processing of emotionally arousing stimuli. In support of this model, functional imaging studies consistently describe a possible phenotype of emotional instability as hyperreactivity of the amygdala (limbic system) reacting to highly arousing and negative emotional stimuli and impaired recruitment of cognitive processes regulating emotions mainly through activations of the dorsolateral prefrontal cortex [
1]; see also [
19]. The second explanatory model refers to disorders of abnormal interpersonal communication that are characteristic of patients with BPD [
17]. A key concern of our review will be to formulate the relationship between deficits in empathy and impulsivity, based on our knowledge of social cognition. These are manifestations of PD pathology that may be both reflected in the brain, possibly in two separate abnormal circuits: one more related to impulsivity and another more related to interpersonal deficits. However, previous studies suffer from major limitations. First, the dependence between the various voxels was not considered, in fact they were analyzed independently with a univariate mass analysis [
24,
25]. Second, the majority of the above cited studies focused only on specific and a-priori decided Regions of Interests (ROI), thus limiting the explanatory capacity of the results and possibly hiding the relevance of other structures [
26]. Thirdly, only a few studies have assessed group differences in both gray matter and white matter.
To overcome the limitations of massive univariate approaches and ROIs analyses, a recent study [
27] used a data driven supervised machine learning approach to elucidate whole brain GM alterations in a group of BPD compared to HC, and a clinical control group (Bipolar patients). This study showed that a circuit, including basal ganglia, amygdala, and portions of the temporal lobes and of the orbitofrontal cortex, correctly classified BPD against HC (84,62%), and against bipolars (80%). For what concerns the WM contribution, Lapomarda and colleagues [
25] applied unsupervised machine learning methods and found that BPD were characterized by WM alterations when compared to healthy controls and a clinical control group (Bipolar patients) [
25]. In particular, BPD patients showed increased white matter concentration in frontal-parietal and temporal regions possibly associated with a dysfunctional top-down emotion regulation [
25].
However, in this study, GM and WM were analyzed separately. As such, this analysis does not allow to capture cross-information between modalities [
28], as it is not sensitive to find linked hidden structures in the data or any integration between different datatypes [
28]. Using both the GM and WM in a data fusion approach, to find possible covariances, can greatly improve the sensitivity of results as both sets of features are used to find a separation between patients and controls [
29].
1.1. The present study
To overcome the limitations described above, in the present study we propose an alternative approach recently developed inside the machine learning field, that seeks to quantitatively examine the relationship amongst various alterations that can be measured using different imaging modalities. This machine learning method is multivariate in nature, thus taking into account the relationship among voxels, and uses cross-information from multimodal neuroimaging data to explore the complex interplay of brain alterations in different modalities. The method is known as multimodal Canonical Correlation Analysis (mCCA) in conjunction with joint Independent Component Analysis (jICA) [
30]. The use of mCCA in conjunction with jICA (mCCA+jICA) [
28] fuses two modalities (for example GM and WM), finds the correlations between them (a multimodal canonical covariate matrix, mCCA), and then separates the covariance matrix into independent covarying GM-WM circuits (jICA). Loading coefficients of GM-WM circuits are then tested for their differences between normal and abnormal populations. As such mCCA, allows a multimodal fusion (MMF) that helps identifying the unique and shared variance associated with each imaging modality that underlies cognitive functioning in healthy controls and impaired mental illness [
30,
31,
32]. Previous studies have shown the benefit of MMF for understanding complex syndromes such as schizophrenia [
30,
31,
33] and bipolar disorder [
33]. Another advantage of this method is that the ICA part allows a data driven and more biological plausible decomposition of the brain into independent networks, compared to standard atlas-based parcellations [
34].
Thus, the first aim of the present study, was to apply a MMF approach (mCCA+jICA) to BPD patients, to find latent abnormal covariance patterns of the gray and white matter as compared to healthy controls. We predict to find a GM and WM circuit including temporal and frontal regions, as well as the insula, the amygdala and the hippocampus in line with previous observations [
17,
18,
19,
27]. These regions have been found to be compromised in previous studies [
17,
18,
19,
25,
27].
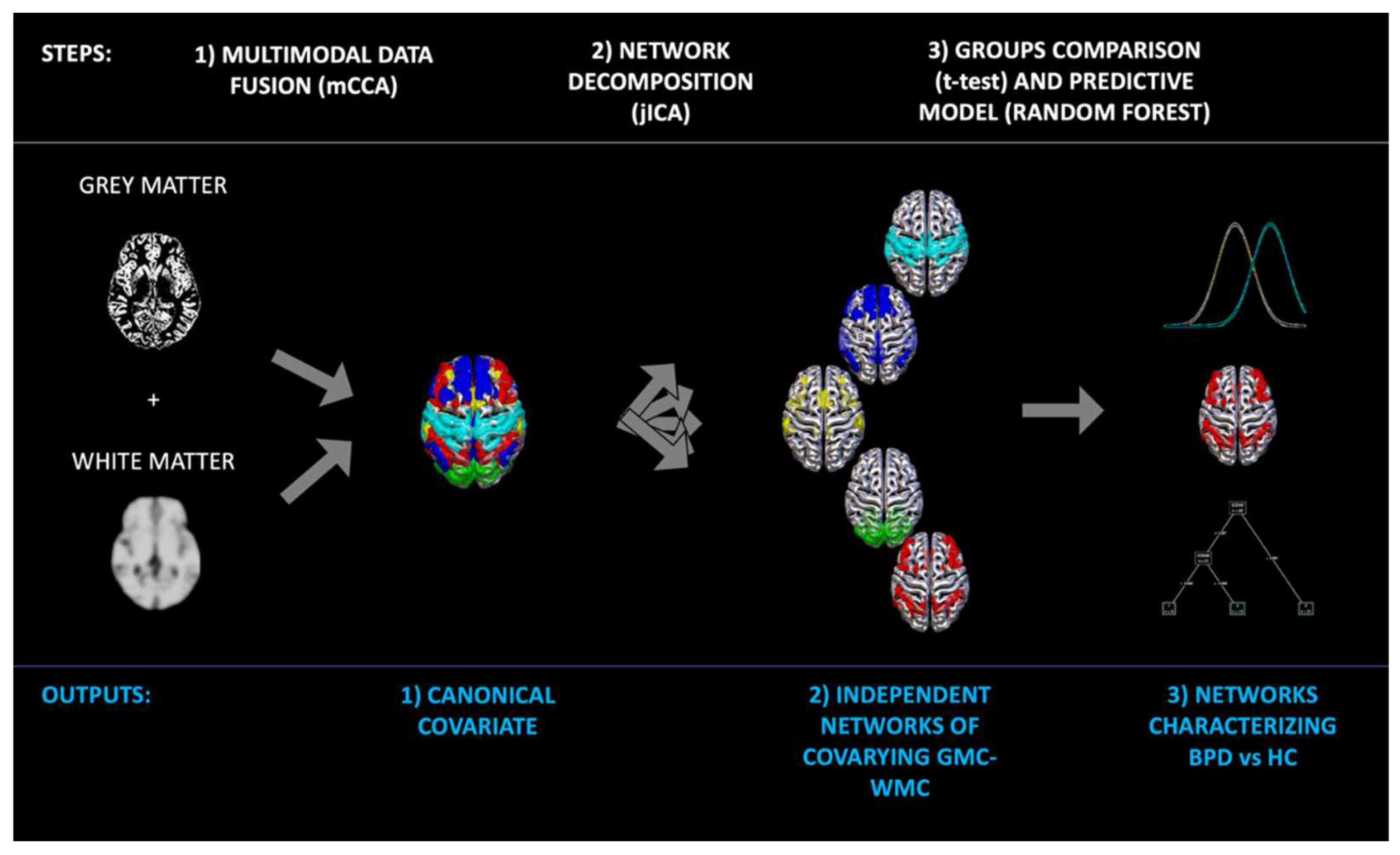
Once the covarying GM-WM circuits that differ between BPD and HC have been identified, supervised machine learning approach known as Random Forest will be used to test for the generalization of our results to new unobserved cases. Random forests are an ensemble learning method for classification based on forest of decision trees [
35,
36,
37]. The hold out method will be used to train the model on the 80% of participants, and to test it on the remaining (not used in the training phase) 20%. As such, this method can return a measure of generalizability. We predict that the same circuits found in aim 1, allows the generalization to new cases. Moreover, being Random Forest a hierarchical approach (all the networks are estimated and ordered from the best to the worst predictive), it may be that other networks will display a high level of predictability of BPD diagnosis.
Among relevant clinical data that may contribute to the identification of relevant neural mechanisms involved in BPD, in the present study we considered traumatic experiences in childhood. Meta-analyses of cross-sectional studies indicated that BPD patients are more likely to report childhood trauma history than nonclinical individuals, including experiences of sexual and physical abuse, neglect, maladaptive parenting, and parental conflict [
38], and more likely to report childhood trauma experiences than other psychiatric groups [
39]. Conversely, studies comparing maltreated and non-maltreated children on the prevalence of borderline features show that maltreated children were significantly more likely to present borderline features [
40,
41]. Prospective longitudinal research provided further evidence for the hypothesis that exposure to adverse events in childhood increased the risk of being diagnosed with BPD in adulthood [
42,
43,
44,
45]. In a previous attempt to assess the impact of traumatic experiences on the brain of BPD patients, Dadomo and colleagues [
46] reported evidence that a circuit including the amygdala, the Heschl area, the Caudate, the Putamen, and portions of the Cerebellum was predictive of sexual abuse. They also reported that another circuit involving temporal and cerebellar regions was predictive of interpersonal problems. However, this study considered only the contribution of GM, ignoring the fact that early traumatic experiences may affect WM too, and that WM may contribute to specific symptoms. Also, that study considered every factor in isolation (independent statistical models), without assessing the contribution of all factors in one unique model to assess for their relative contribution. In the present study, by contrast, we aim at building one unique model, to test a specific hypothesis that early life traumatic events (as measured by the Childhood Traumatic Questionnaire (CTQ) [
47], affects the abnormal brain networks found in aim 1, and how they in turn support specific symptoms as measured by the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) [
48]. In sum, the second aim of the present study, was to assess the impact of early traumatic experiences on specific brain circuits, and how such altered brain circuits give rise to specific BPD symptoms. Mediation analysis will be used to assess the mediating role of the brain networks found in aim 1, in explaining the link between specific child traumas and specific symptoms. We predict that the networks that differ between BPD and HC, are affected by specific traumatic experiences such as emotional and physical mistreatments, and that this abnormal neural circuits explain at least some of the psychological problems BPD suffer from such as for example, interpersonal and impulsivity problems. In sum, we predict that these analyses will shed light on an abnormal impulsivity network and a more interpersonal network.
2. Materials and Methods
2.1. Participants
Twenty patients with BPD (mean age = 35.75, SD = 8.61), and 45 healthy participants as controls (HC) without history of psychiatric and neurological disease (mean age 36.69, SD =8.46), matched for age and sex were considered. All the data were extracted from the shared OpenNeuro database [
49]. Demographic information about participants is displayed in
Table 1. The recruitment was in outpatient and support services from around Edinburgh. The exclusion criteria were the presence of neurological disease, or mental illness rather than BPD (SCID-II, SCID- IV), and the use of psychoactive substance, pregnancy, MRI contraindications. The BPD diagnosis was verified using Structured Clinical Interview for DSM-IV (SCID-II). See
Table 1.
2.2. Questionnaires
To reach our aims, the Child Trauma Questionnaire (CTQ) [
47] and the Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) [
48] scores were taken into consideration. The CTQ is a self-assessment questionnaire developed to evaluate traumatic experiences experienced during childhood that includes five sources of traumas: Physical neglect, Emotional neglect, Physical abuse, Emotional abuse and Sexual abuse. The Zan-BPD was designed to capture the severity of symptoms in four main sectors: the Affective sector indicating anger outbursts, feelings of emptiness and mood instability; the Cognitive sector relative to identity disturbance disassociation and paranoia; the Impulsivity sector relative to impulsivity such as self-mutilative/suicidal efforts; and the Interpersonal sector indicating intense, unstable relationships and frantic efforts to avoid abandonment.
2.3. Preprocessing
T1-weighted images were pre-processed through SPM12 (Statistical Parametric Mapping,
https://www.fil.ion.ucl.ac.uk/) [
50] and CAT12 toolbox (Computational Anatomy Toolbox for SPM,
http://www.neuro.uni-jena.de/cat/) [
51]. After having manually re-oriented all the images placing the anterior commissure as the origin, the segmentation into gray matter, white matter and cerebrospinal fluid was computed. The Diffeomorphic Anatomical Registration using Exponential Lie algebra tools for SPM12 (DARTEL) [
52] was used for registration. Finally, the normalization to the MNI space with a spatial Gaussian smoothing of 8 was performed.
2.4. Data fusion unsupervised machine learning to decompose the brain into independent covarying GM-WM networks
mCCA+jICA was applied to structural data using the FusionICA Toolbox (FIT,
http://mialab.mrn.org/software/fit) [
53] in the MATLAB 2021b environment (
https://it.mathworks.com/products/matlab.html) (
MATLAB (R2021b), 2021). The number of components was estimated for both modalities with Information theoretic criteria [
54]. To assess the consistency of each modality, ICASSO [
55,
56] was run ten times and Infomax algorithm was selected. The resulting output consists in a matrix with the number of subjects (rows) and the loading coefficients for each component (columns). Loading coefficients represent how each component is expressed for every subject. Eventual differences between groups is calculated with t-test on loading coefficients of GM-WM circuits. As a final step we converted the independent components into Talairach coordinates in order to specify the brain areas. Areas with both positive and negative values, if present, were considered and plotted in Surf Ice (
https://www.nitrc.org/projects/surfice/), using a different template for gray and white matter.
2.5. Predictive model
Beside testing for differences between groups by using frequentists approaches (e.g. t-tests) whose results are limited to the sample considered, we also aim to use supervised machine learning (SML) approach to extract a statistical model to predict new cases. In other words, we aim to test our results for their generalization. To do that a SML method known as Random Forest classification will be used. Random forest is an ensemble learning method for classification based on forest of decision trees [
35,
36,
37]. For classification tasks, the output of the random forest is the class selected by most trees. Random forests derive the name from Decision Trees, another SML for classification, but it uses multiple trees, and then averages their performance (bagging method). Of note, Random decision forests outperforms the decision trees (and other SML algorithms) for having less overfitting problems [
57]. One of the main reasons Random forests was used in this study is that it allows for ranking the importance of variables in a classification problem. In other words, the output is a hierarchical model that allows to estimate the most important feature to the least important. As such this method can help us understanding not only the brain circuits that differ between BPD and HC (frequentist approach), but also to assess which circuits predict new unobserved cases. The algorithm is indeed trained to correctly classify a part of the sample (including BPD and HC), and then tested for its predictive power on the unobserved subsample. The statistical results refer to the prediction of new unobserved cases and as such can be used as a measure of generalization. See
Figure 1.
3. Results
3.1. Groups comparison
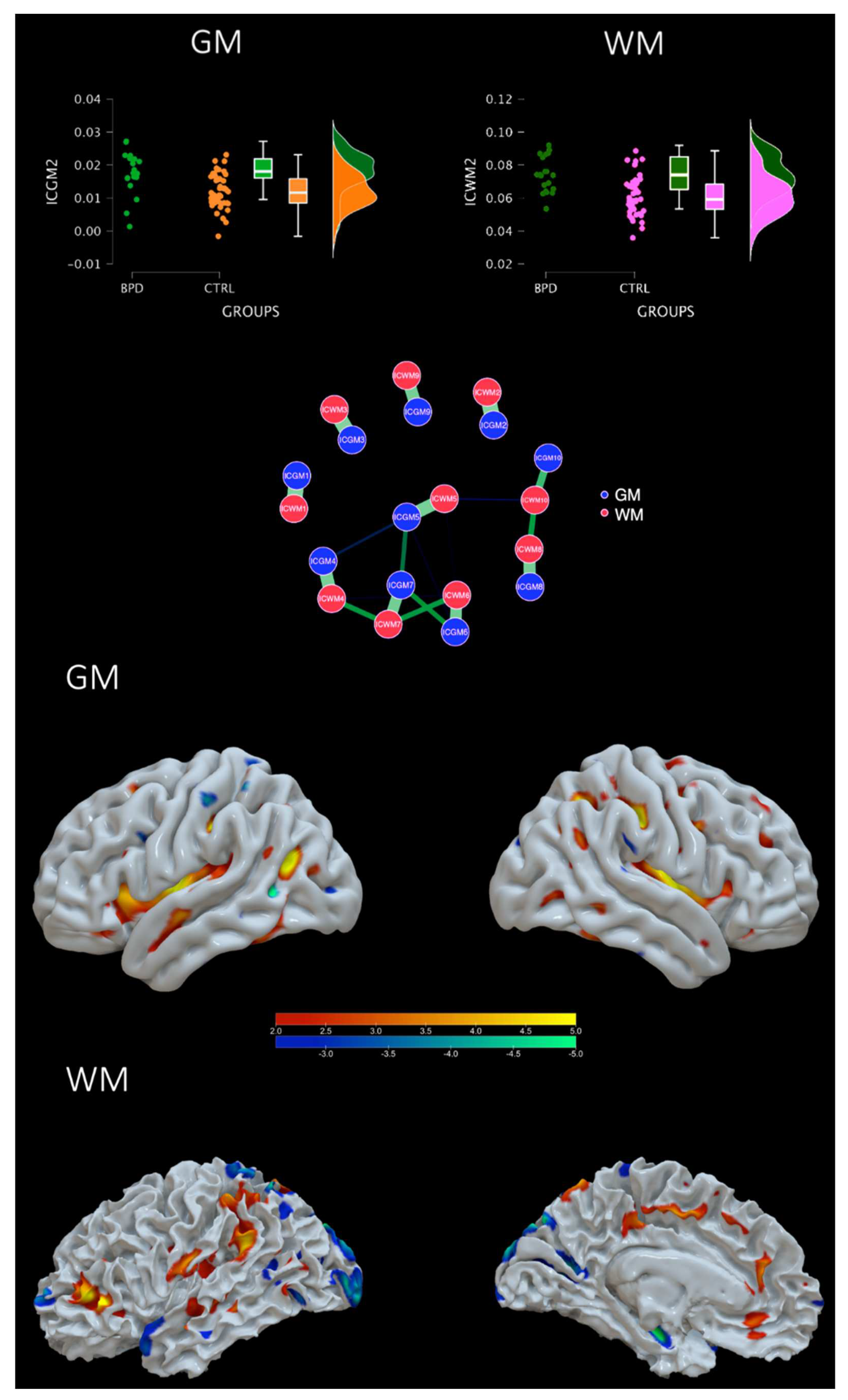
The Information theoretic criteria estimated 10 independent covarying gray (IC-GM) and white (IC-WM) matter networks. The positive values of these networks indicate increased gray/white matter concentration, whereas negative values indicate decreased concentration. The meaning of the covariation between a gray matter and a white matter component refers to a similar pattern of gray/white matter concentration. Results indicate that the following components ordered for importance, statistically differed between groups: ICGM2 (t= 3.715, p<0.001) and ICWM2 (t= 4.189, p<0.001), ICGM6 (t= 2.625, p=0.011) and ICWM6 (t= 2.501, p=0.015), ICGM8 (t=- 2.384, p=0.020), ICWM8 (t= -2.513, p=0.015), ICGM4 (-2.335, p=0.023) but not ICWM4 (t= -1.23,1p=0.223). Whereas the others did not (ICGM1 p= 0.881, ICWM1 p=0.701; ICGM3 p=0.843, ICWM3 p=0.382; IGGM5 p=0.719, ICWM5 p=0.716; ICGM7 p=0.273, ICWM7 p=0.514, ICGM9 p= 0.308, ICWM9 p=0.975; ICM10 p=0.038 ICWM10 p=0.447). However, after applying a Bonferroni corrected threshold (p=0.00125) only the ICGM2-ICWM2 survived. Regions included in the ICGM2 were the post central and precentral gyri, the superior middle temporal gyrus, the insula, the superior, middle and inferior frontal gyrus, the parietal lobule, the uncus (including the amygdala), cerebellar portions and the hippocampus among others. See
Figure 2 and
Table 2,
Table 3,
Table 4 and
Table 5. GM and WM of IC2 were also highly correlated each other (r=0.857) indicating a strong common profile of alterations in a network that differs between BPD and HC. See
Figure 2, network plot, for a representation of each GM-WM component and the strength of correlation.
3.2. Predictive model results
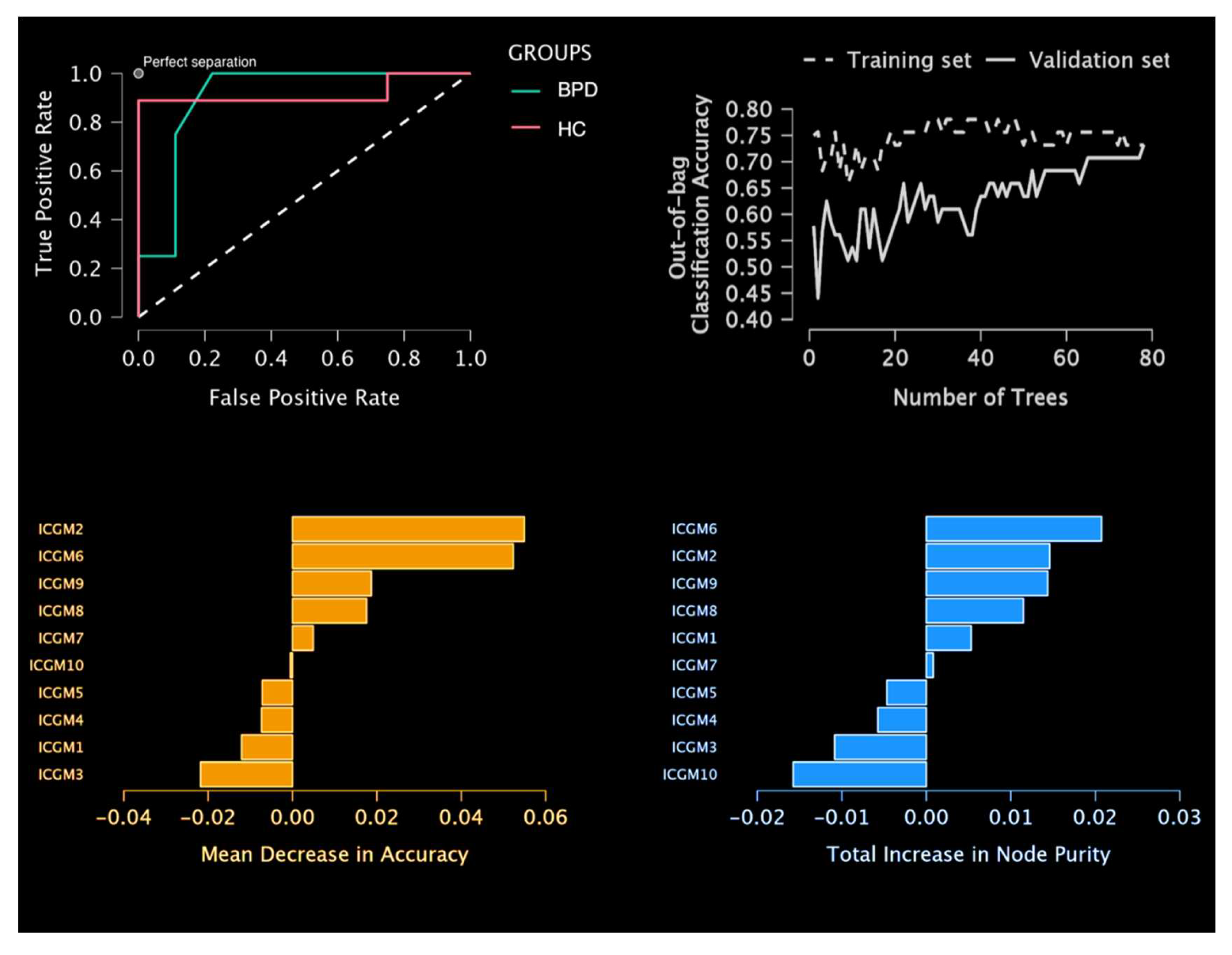
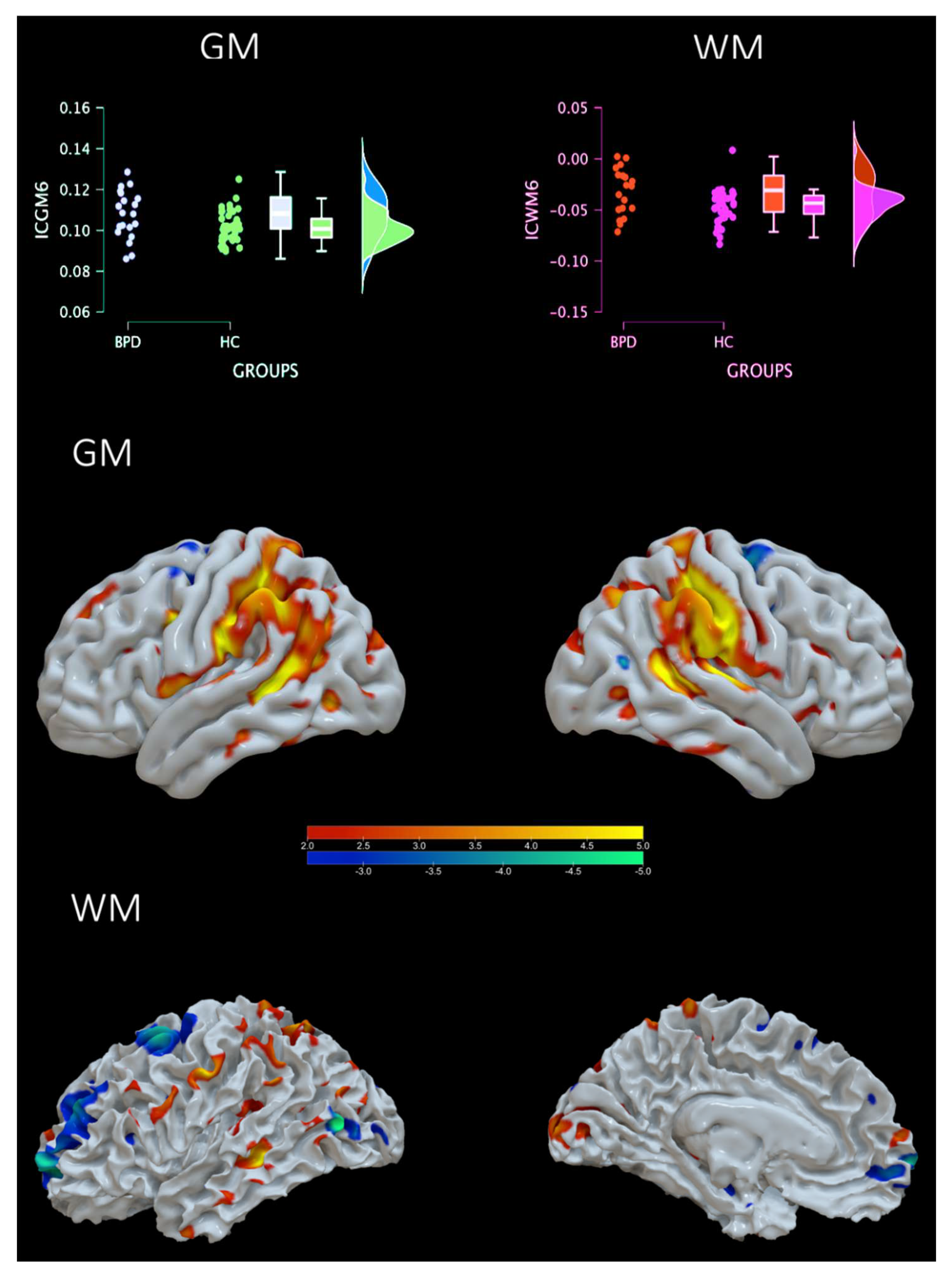
For the Random Forest classification the hold out method of 20% for validation and 20% for testing was selected. In other words, the model was trained on 39 subjects, the model was validated during learning on 13 subjects, and finally tested on 13 unobserved before subjects. The proportion of BPD and HC was kept among the three partitions with a 30% BPD and 70% HC. The number of trees to reach optimal performance was of 78 with 3 features er split. Random forest classification returned a test accuracy of 84.6% for both BPD and HC, a precision (positive predictive value) of 75% for BPD, and 88.9% for HC, a recall (true positive rate) of 75% for BPD, and 88.9% for HC, a false positive rate of 11.1% for BD and 25% for HC, a false discovery rate of for 25% BD and 11.1% for HC. The Area under the curve was 0.861 for BPD and of 0.889 for HC (average= 0.875). The mean decrease in purity confirmed the importance of ICGM2 as main predictor and main split in trees. Then followed in order of decreased importance: ICGM6, ICGM9, ICGM8, ICGM7. See
Figure 3. Of note, ICGM2 and ICGM6 were very similar in the power of prediction (respectively, mean decrease of accuracy 0.055, 0.052; and the total increase in node purity 0.015, 0.021, even better of ICGM6). Building on this result, we decided to comment further also the ICGM-WM6 component. This component included regions such as temporal-parietal regions, the parahippocampus, the cingulate, the fusiform gyrus, the cuneus and insula. GM and WM of IC6 were also highly correlated each other (r=0.777) indicating a strong common profile of alterations in a network that differs between BPD and HC. See
Table 6,
Table 7,
Table 8 and
Table 9 and
Figure 4.
3.3. Mediation Analysis
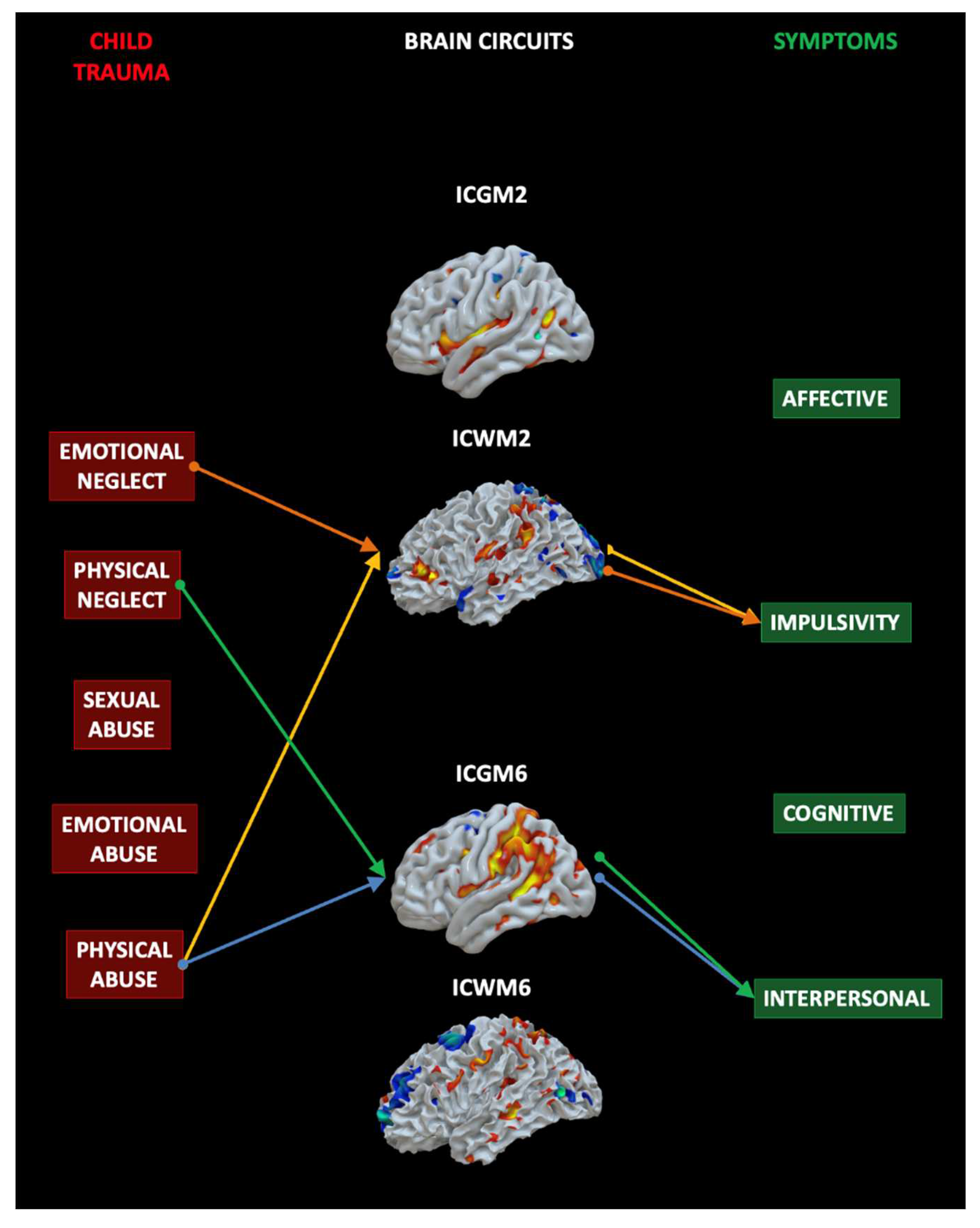
To test the hypothesis that early traumatic experiences as measured by the CTQ (Independent variables, IVs) may support specific symptoms as measured by the Zanarini BPD symptoms questionnaire (Dependent variab[es, DVs), via the contribution of specific neural circuits that differ between BPD and CTRL (Mediating variables, MAs), we ran a Mediation Analysis (MA). The MA included the 5 CTQ subscales (the IVs), the 4 neural circuits (ICGM2, ICWM2, ICGM6, ICWM6) (the MAs), and the 4 affective sectors of Zanarini (the DVs). See
Figure 5. For what concerns the indirect effect, or the impact of the IVs on the DV mediated by the MAs, of interest for the present study, results showed that Emotional Neglect (b=-0.300, 0.018) and Physical Abuse (b=0.519, p=0.016) predicted IC2 (WM) network and that this in turn predicted symptoms in the impulsivity domain. Physical Neglect (b=0.630, p=0.048) and Abuse (b=0.579, p=0.036) predicted IC6 (GM) and this in turn predicted interpersonal symptoms. In sum, at least one of the two modalities of each brain circuits are related with specific traumatic sources and support specific symptoms. See Supplementary material for all the details and all the MA results.
4. Discussion
In the present study, we contributed to the clarification of the neural basis of BPD by taking advantage of a novel combination of two methodologies see [
59] for a similar method. First, we used an unsupervised data fusion machine learning approach known as mCC+jICA to find latent abnormal covariance patterns of the gray and white matter that separate BPD patients from healthy controls. T-test clarified that one of these networks significantly differ between BPD and controls. Then, we tested the possibility to predict the diagnoses of BPD cases (Random Forest, a supervised machine learning approach) from these networks. Two networks were found to predict BPD new cases (hold-out method). Last but not least, we tested the role of these circuits as possible mediators between etiological factors of BPD (traumatic experiences in childhood) and different BPD symptoms. We found that the two networks that separate BPD from controls mediate traumas and symptoms. In what follows, we describe such patterns in term of “Impulsivity Network” and “Interpersonal Network”.
Impulsivity Network
The first network found as expected was specifically predictive of BPD symptoms in the impulsivity domain. Such network included alterations of white matter in several prefrontal regions, including the inferior frontal gyrus (IFG) and extended alterations of gray matter in the insula, postcentral and precentral gyri. Consistently with our results, all these areas have been previously mentioned as implicated in a variety of impulsivity-related functions [
6,
60,
61] and their structural alterations have been previously reported as associated with individual differences in impulsivity [
25,
62,
63,
64,
65]. As widely described in ‘top-down cognitive control’ models of impulsivity [
66,
67], PFC areas may act as a brake on impulsive tendencies by exerting inhibitory control, while subcortical structures propel the occurrence of impulsive behaviours. Among other components of executive control, the role of IFG in impulsivity has been largely investigated due to its role in contrasting impulsivity through the suppression of inappropriate behavioural responses [
49]. The insula, instead, appears to be involved in a specific component of impulsivity which concerns the preference of smaller immediate rewards instead of to wait for larger delayed rewards (delay discounting). Due to the relevance of impulsivity-related symptoms in BPD, the alterations such brain structures were expected from the present study and are in line with previous studies on BPD patients [
13,
14,
15]. Extending such previous studies, the present study suggests that it may be possible to derive an objective biomarker for BPD, generated from impulsivity-related brain structure abnormalities.
Interpersonal Network
The second network emerged from the present study was specifically predictive of BPS symptoms in the interpersonal domain. This network included an extended area of increased grey matter concentration centred in the temporal-parietal junction (TPJ), and other smaller areas located in anterior and posterior midline structures and in the insula. These brain structures correspond to a well-described Theory of Mind (ToM) network associated to the ability to think about mental states in oneself and especially in others [
68]. As such this network is a good candidate to explain the interpersonal and mentalizing problems these patients suffer from. As part of this network, the TPJ has a core role in the inference of mental states [
69], with relevant clinical implications due to the possible existence of individual differences in the ability to combine cognitive and affective information – including emotional/and somatic information from the anterior cingulate cortex and insula – in more or less complex representations of mental states [
69]. Even if most popular neurobiological models of psychopathology are mainly focused on deficits in executive/inhibitory functions, recent contributions have emphasized the additional relevance of semantic functions implicated in mental representation of self and others for emotion regulation [
70]. Such kind of semantic function alteration is also implicit in clinical models of BPD, which attribute interpersonal difficulties of BPD to impoverished representations of self and others reflected in the shift between positive and negative poles (or “black-and-white thinking”) [
71]. In the present study, beyond observing the relevance of ToM functions in BPD, we showed that even if between-group comparison showed only a relative significant difference in BPD patients compared to healthy controls (the significance did not survive to Bonferroni corrections), the pattern of brain alterations in the ToM network can be considered a significant marker of BPD able to distinguish BPD brains from healthy participants brains.
The impact of specific childhood traumas on the brain and symptoms
To complete the picture, from the Mediation Analysis, the candidate neural markers of BPD identified in the present study, significantly mediated the association between self-reported physical abuse in childhood and symptoms in impulsivity domain (Impulsivity Network) and interpersonal domain (ToM Network). Thus, the emergent hypothesis is that having had physical abuse in childhood may negatively influence the development brain structures devoted to cognitive inhibition (as brake of impulsive behaviour) and to interpersonal - ToM functions, predisposing to BPD in adulthood. Such negative influence of traumatic experiences in childhood have been largely theorized in clinical theories of BPD [
21,
25,
27], and the results on such association have been previously described in structural neuroimaging [
71,
72]. As reported in previous study on BPD, we also expected an effect of sexual abuse, mediated by limbic areas alterations [
21,
25], however we did not find such result. A previous study [
21] reported a link between sex trauma and a specific brain circuit. However, this circuit included areas different from the ones that we found in our study. Futures studies may want to better explore this issue.
5. Conclusions
Our study successfully found two covarying grey and white matter networks that significantly differ between BPD and controls by using an innovative combination of supervised and unsupervised machine learning approaches. A relationship between specific child traumas and symptoms was found to be mediated by such neural circuits. Our study does not come without limitations.
First, we must acknowledge that the sample size of patients with BPD was quite small. This limitation is something common in the scientific literature on BPD as there are limited number of studies and a few available open datasets. Future studies may want to use larger samples to replicate these findings. Second, we focused our analyses only on structural brain features. Future studies may want to explore a fusion between structural and functional data. Beside the limitations, this data fusion unsupervised combined with supervised machine learning approach has not been applied previously to understand the BPD brain.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: title; Table S1: title; Video S1: title.
Author Contributions
AG, IM, GS, HD conceptualized the study. GL and SS preprocessed the data and revised the manuscript. AG analyzed the data and wrote the methods and results sections. HD wrote the manuscript. IM and GS wrote and revised the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethics committee at the medical faculty of the University of Leipzig (154/13-ff).
Informed Consent Statement
The studies involving human participants were reviewed and approved by reuse of open access data, see details inside manuscript. The patients/participants provided their written informed consent to participate in this study.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found at: UCLA Consortium for Neuropsychiatric Phenomics - OpenNeuro database, accession number ds000030.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koenigsberg, H.W.; Harvey, P.D.; Mitropoulou, V.; Schmeidler, J.; New, A.S.; Goodman, M.; Silverman, J.M.; Serby, M.; Schopick, F.; Siever, L.J. Characterizing affective instability in borderline personality disorder. The American journal of psychiatry 2002, 159, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Zanarini, MC. Psychotherapy of borderline personality disorder. Acta Psychiatr Scand. 2009, 120, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Lenzenweger, M.F.; Lane, M.C.; Loranger, A.W.; Kessler, R.C. DSM-IV personality disorders in the National Comorbidity Survey Replication. Biological psychiatry 2007, 62, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Trull, T.J.; Jahng, S.; Tomko, R.L.; Wood, P.K.; Sher, K.J. Revised NESARC personality disorder diagnoses: gender, prevalence, and comorbidity with substance dependence disorders. J. Personal. Disord. 2010, 24, 412–426. [Google Scholar] [CrossRef]
- Tomko, R.L.; Trull, T.J.; Wood, P.K.; Sher, K.J. Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. Journal of personality disorders 2014, 28, 734. [Google Scholar] [CrossRef]
- De Panfilis, C.; Schito, G.; Generali, I.; Gozzi, L.; Ossola, P.; Marchesi, C.; Grecucci, A. Emotions at the border: Increased punishment behavior during fair interpersonal exchanges in Borderline Personality Disorder. Journal of Abnormal Psychology 2019, 128, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Meng, Y.J.; Li, X.J.; Zhang, C.; Liang, S.; Li, M.L.; ... Li, T. Common and distinct patterns of grey matter alterations in borderline personality disorder and bipolar disorder: voxel-based meta-analysis. The British Journal of Psychiatry 2019, 215, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Wolf, R.C.; Thomann, P.A.; Sambataro, F.; Vasic, N.; Schmid, M.; Wolf, N.D. Orbitofrontal cortex and impulsivity in borderline personality disorder: an MRI study of baseline brain perfusion. European archives of psychiatry and clinical neuroscience 2012, 262, 677–685. [Google Scholar] [CrossRef]
- Ducasse, D.; Courtet, P.; Olie, E. Physical and social pains in borderline disorder and neuroanatomical correlates: a systematic review. Current Psychiatry Reports 2014, 16, 1–12. [Google Scholar] [CrossRef]
- Wrege, J.S.; Ruocco, A.C.; Euler, S.; Preller, K.H.; Busmann, M.; Meya, L. ; .. & Walter, M. Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cognitive, Affective, & Behavioral Neuroscience 2019, 19, 1273–1285. [Google Scholar] [CrossRef]
- Lou, J.; Sun, Y.; Cui, Z.; Gong, L. Common and distinct patterns of gray matter alterations in borderline personality disorder and posttraumatic stress disorder: a dual meta-analysis. Neuroscience Letters 2021, 741, 135–376. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wang, S.; Qin, K.; Li, L.; Chen, Y.; Zhang, X.; ... Gong, Q. Common and Distinct Neural Patterns of Attention-Deficit/Hyperactivity Disorder and Borderline Personality Disorder: A Multimodal Functional and Structural Meta-analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging 2022. [Google Scholar] [CrossRef] [PubMed]
- Irle, E.; Lange, C.; Sachsse, U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biological psychiatry 2005, 57, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Lyoo, I.K.; Han, M.H.; Cho, D.Y. A brain MRI study in subjects with borderline personality disorder. Journal of affective disorders 1998, 50, 235–243. [Google Scholar] [CrossRef]
- Hazlett, E.A.; New, A.S.; Newmark, R.; Haznedar, M.M.; Lo, J.N.; Speiser, L.J. ... & Buchsbaum, M.S. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological psychiatry 2005, 58, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—the methods. Neuroimage 2000, 11, 805–812. [Google Scholar] [CrossRef] [PubMed]
- Soloff, P.H.; Pruitt, P.; Sharma, M.; Radwan, J.; White, R.; Diwadkar, V.A. Structural brain abnormalities and suicidal behavior in borderline personality disorder. Journal of psychiatric research 2012, 46, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Niedtfeld, I., I.; Schulze, L.; Krause-Utz, A.; Demirakca, T.; Bohus, M.; Schmahl, C. Voxel-based morphometry in women with borderline personality disorder with and without comorbid posttraumatic stress disorder. PloS one 2013, 8, e65824. [Google Scholar] [CrossRef]
- Schulze, L.; Schmahl, C.; Niedtfeld, I. Neural correlates of disturbed emotion processing in borderline personality disorder: a multimodal meta-analysis. Biological psychiatry 2016, 79, 97–106. [Google Scholar] [CrossRef]
- Aguilar-Ortiz S, Salgado-Pineda P, Marco-Pallarés J, Pascual JC, Vega D, Soler J, et al. Abnormalities in gray matter volume in patients with borderline personality disorder and their relation to lifetime depression: A VBM study. PLoS ONE 2018, 13, e0191946. [CrossRef]
- Dadomo, H.; Grecucci, A.; Giardini, I.; Ugolini, E.; Carmelita, A.; Panzeri, M. Schema Therapy for Emotional Dysregulation: Theoretical Implication and Clinical Applications. Frontiers in psychology 2016, 7, 1987. [Google Scholar] [CrossRef]
- Frederickson, J.J.; Messina, I.; Grecucci, A. Dysregulated Anxiety and Dysregulating Defenses: Toward an Emotion Regulation Informed Dynamic Psychotherapy. Frontiers in psychology 2018, 9, 2054. [Google Scholar] [CrossRef]
- Ruocco, A.C.; Amirthavasagam, S.; Zakzanis, K.K. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a meta-analysis of magnetic resonance imaging studies. Psychiatry Research: Neuroimaging 2012, 201, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sorella, S.; Lapomarda, G.; Messina, I.; Frederickson, J.J.; Siugzdaite, R.; Job, R.; Grecucci, A. Testing the expanded continuum hypothesis of schizophrenia and bipolar disorder. Neural and psychological evidence for shared and distinct mechanisms. NeuroImage. Clinical 2019, 23, 101854. [Google Scholar] [CrossRef] [PubMed]
- Lapomarda, G.; Grecucci, A.; Messina, I.; Pappaianni, E.; Dadomo, H. Common and different gray and white matter alterations in Bipolar and Borderline Personality Disorder. Brain Research 2021. [Google Scholar] [CrossRef] [PubMed]
- Pappaianni, E.; De Pisapia, N.; Siugzdaite, R.; Crescentini, C.; Calcagnì, A.; Job, R.; Grecucci, A. Less is more: Psychological and morphometric differences between low vs high reappraisers. Cogn. Affect. Behav. Neurosci 2019, 20, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Grecucci, A.; Lapomarda, G.; Messina, I.; Monachesi, B.; Sorella, S.; Siugzdaite, R. Structural features related to affective instability correctly classify patients with Borderline Personality Disorder. A Supervised Machine Learning approach. Frontiers in Psychiatry 2022, 13. [Google Scholar] [CrossRef]
- Sui, J.; Pearlson, G.; Caprihan, A.; Adali, T.; Kiehl, K.A.; Liu, J. ... & Calhoun, V.D. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage 2011, 57, 839–855. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A.; Viera, S. (Eds.) Machine learning: methods and applications to brain disorders; Academic Press.
- Calhoun, V.D.; Adali, T.; Giuliani, N.R.; Pekar, J.J.; Kiehl, K.A.; Pearlson, G.D. Method for multimodal analysis of independent source differences in schizophrenia: combining gray matter structural and auditory oddball functional data. Hum Brain Mapp 2006, 27, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Correa, N.M.; Li, Y.O.; Adali, T.; Calhoun, V.D. Canonical Correlation Analysis for Feature-Based Fusion of Biomedical Imaging Modalities and Its Application to Detection of Associative Networks in Schizophrenia. IEEE J Sel Top Sign Proces 2008, 2, 998–1007. [Google Scholar] [CrossRef]
- Plis, S.M.; Weisend, M.P.; Damaraju, E.; Eichele, T.; Mayer, A.; Clark, V.P. ... & Calhoun, V.D. Effective connectivity analysis of fMRI and MEG data collected under identical paradigms. Computers in biology and medicine 2011, 41, 1156–1165. [Google Scholar] [CrossRef]
- Sui, J.; He, H.; Yu, Q.; Chen, J.; Rogers, J.; Pearlson, G.D. ... & Calhoun, V.D. Combination of resting state fMRI, DTI, and sMRI data to discriminate schizophrenia by N-way MCCA+ jICA. Frontiers in human neuroscience 2013, 7, 235. [Google Scholar] [CrossRef] [PubMed]
- Grecucci, A.; Sorella, S.; Consolini, J. Decoding Individual Differences in Expressing and Suppressing Anger from Structural Brain Networks: a Supervised Machine Learning Approach. Behavioral Brain Research 2022, 439, 114245. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.K. The Random Subspace Method for Constructing Decision Forests. IEEE Transactions on Pattern Analysis and Machine Intelligence 1998, 20, 832–844. [Google Scholar] [CrossRef]
- Ho, B.C.; Andreasen, N.C. (2002). Do structural brain deficits worsen following onset of first-episode schizophrenia? In SCHIZOPHRENIA RESEARCH (Vol. 53, No. 3, pp. 98–98). PO BOX 211, 1000 AE AMSTERDAM, NETHERLANDS: ELSEVIER SCIENCE BV.
- Breiman, L. Random Forests. Machine Learning 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Fossati, A.; Madeddu, F.; Maffei, C. Borderline personality disorder and childhood sexual abuse: a meta-analytic study. Journal of personality disorders 1999, 13, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Palmier-Claus, J.; Branitsky, A.; Mansell, W.; Warwick, H.; Varese, F. Childhood adversity and borderline personality disorder: A meta-analysis. Acta Psychiatrica Scandinavica 2020, 141, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Cicchetti, D.; Rogosch, F.A.; Hecht, K.F.; Crick, N.R.; Hetzel, S. Moderation of maltreatment effects on childhood borderline personality symptoms by gender and oxytocin receptor and FK506 binding protein 5 genes. Development and psychopathology 2014, 26, 831–849. [Google Scholar] [CrossRef]
- Hecht, K.F.; Cicchetti, D.; Rogosch, F.A.; Crick, N.R. Borderline personality features in childhood: The role of subtype, developmental timing, and chronicity of child maltreatment. Development and psychopathology 2014, 26, 805–815. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Arseneault, L.; Bleidorn, W.; Fonagy, P.; Goodman, M.; Houts, R.; Moffitt, T.E. Etiological features of borderline personality related characteristics in a birth cohort of 12-year-old children. Development and psychopathology 2012, 24, 251–265. [Google Scholar] [CrossRef]
- Gratz, K.L.; Latzman, R.D.; Tull, M.T.; Reynolds, E.K.; Lejuez, C.W. Exploring the association between emotional abuse and childhood borderline personality features: The moderating role of personality traits. Behavior therapy 2011, 42, 493–508. [Google Scholar] [CrossRef]
- Winsper, C.; Zanarini, M.; Wolke, D. Prospective study of family adversity and maladaptive parenting in childhood and borderline personality disorder symptoms in a non-clinical population at 11 years. Psychological medicine 2012, 42, 2405–2420. [Google Scholar] [CrossRef] [PubMed]
- Widom, C.S.; Czaja, S.J.; Paris, J. A prospective investigation of borderline personality disorder in abused and neglected children followed up into adulthood. Journal of personality disorders 2009, 23, 433–446. [Google Scholar] [CrossRef]
- Dadomo, H.; Salvato, G.; Lapomarda, G.; Ciftci, Z.; Messina, I.; Grecucci, A. Structural features predict sexual trauma and interpersonal problems in borderline personality disorder but not in controls: A multi-voxel pattern analysis. Frontiers in Human Neuroscience 2022. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, D.P.; Fink, L.; Handelsman, L.; Foote, J. Childhood trauma questionnaire. Assessment of family violence: A handbook for researchers and practitioners; 1998. [Google Scholar]
- Zanarini, M.C. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD): a continuous measure of DSM-IV borderline psychopathology. Journal of personality disorders 2003, 17, 233. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the right inferior frontal cortex. Trends in cognitive sciences 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Penny, W.D.; Mattout, J.; Trujillo-Barreto, N. Bayesian model selection and averaging. In Statistical Parametric Mapping: The analysis of functional brain images. London: Elsevier; 2006. [Google Scholar]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E. CAT-a computational anatomy toolbox for the analysis of structural MRI data. BioRxiv. 2022. [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Acar, E.; Levin-Schwartz, Y.; Calhoun, V.D.; Adali, T. (2017, May). Tensor-based fusion of EEG and FMRI to understand neurological changes in schizophrenia. In 2017 IEEE International Symposium on Circuits and Systems (ISCAS) (pp. 1-4). IEEE. [CrossRef]
- Wax, M.; Kailath, T. Detection of signals by information theoretic criteria. IEEE Transactions on acoustics, speech, and signal processing 1985, 33, 387–392. [Google Scholar] [CrossRef]
- Himberg, J.; Hyvärinen, A.; Esposito, F. Validating the independent components of neuroimaging time series via clustering and visualization. Neuroimage 2004, 22, 1214–1222. [Google Scholar] [CrossRef]
- Himberg, J.; Hyvarinen, A. (2003, September). Icasso: software for investigating the reliability of ICA estimates by clustering and visualization. In 2003 IEEE XIII Workshop on Neural Networks for Signal Processing (IEEE Cat. No. 03TH8718) (pp. 259-268). IEEE. [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The elements of statistical learning; Springer series in statistics: New York, NY, USA, 2001. [Google Scholar]
- Kozak, M.J.; Cuthbert, B.N. The NIMH research domain criteria initiative: background, issues, and pragmatics. Psychophysiology 2016, 53, 286–297. [Google Scholar] [CrossRef]
- Baggio, T.; Grecucci, A.; Meconi, F.; Messina, I. Anxious brains: A combined data fusion machine learning approach to predict trait anxiety from morphometric features. Sensors 2023, 23. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.R.; Potenza, M.N. Recent insights into the neurobiology of impulsivity. Current addiction reports 2014, 1, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: behavioral and neural basis of response control. Progress in neurobiology 2013, 108, 44–79. [Google Scholar]
- Lim, J.E.; Kim, S.; Seo, S.; Kang, W.; Kim, A.; Kang, Y. ... & Han, K.M. Association of prefrontal cortex thinning with high impulsivity in healthy adults. Psychiatry Investigation 2021, 18, 570. [Google Scholar]
- Lee, T.M.; Chan, C.C.; Han, S.H.; Leung, A.W.; Fox, P.T.; Gao, J.H. An event-related fMRI study on risk taking by healthy individuals of high or low impulsiveness. Neuroscience Letters 2008, 438, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Pan, N.; Wang, S.; Zhao, Y.; Lai, H.; Qin, K.; Li, J. ... & Gong, Q. Brain gray matter structures associated with trait impulsivity: A systematic review and voxel-based meta-analysis. Human Brain Mapping 2021, 42, 2214–2235. [Google Scholar]
- Hirjak, D.; Thomann, A.K.; Kubera, K.M.; Wolf, R.C.; Jeung, H.; Maier-Hein, K.H.; Thomann, P.A. Cortical folding patterns are associated with impulsivity in healthy young adults. Brain imaging and behavior 2017, 11, 1592–1603. [Google Scholar] [CrossRef]
- Fineberg, N.A.; Chamberlain, S.R.; Goudriaan, A.E.; Stein, D.J.; Vanderschuren, L.J.; Gillan, C.M. ... & Potenza, M.N. New developments in human neurocognition: clinical, genetic, and brain imaging correlates of impulsivity and compulsivity. CNS spectrums 2014, 19, 69–89. [Google Scholar]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, compulsivity, and top-down cognitive control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef]
- Carrington, S.J.; Bailey, A.J. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human brain mapping 2009, 30, 2313–2335. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B. ... & Kanske, P. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychological Bulletin 2021, 147, 293. [Google Scholar] [PubMed]
- Messina, I.; Sambin, M.; Beschoner, P.; Viviani, R. Changing views of emotion regulation and neurobiological models of the mechanism of action of psychotherapy. Cognitive, Affective, & Behavioral Neuroscience 2016, 16, 571–587. [Google Scholar]
- Kernberg, O. Borderline personality organization. Journal of the American psychoanalytic Association 1967, 15, 641–685. [Google Scholar] [CrossRef] [PubMed]
- Roy, A. Childhood trauma and impulsivity. Possible relevance to suicidal behavior. Archives of suicide Research 2005, 9, 147–151. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).