Introduction
The neurological determination of death, or brain death (BD) must follow rigorous medical standards in order to not allow mistakes [
1,
2,
3]. Although in some countries the diagnosis of BD is given solely by clinical examination, in others, such as in Brazil, ancillary exams are necessary to complement the neurological assessment [
4,
5]. Therefore, examination methods which can aid on the diagnostic process have been widely studied, being the most used techniques the transcranial Doppler (TCD), computed tomography angiography and electroencephalogram [
6,
7,
8].
ICP monitoring is also considered a complementary examination in BD diagnosis, since if ICP overreach mean arterial pressure, cerebral perfusion pressure (CPP) is null [
6]. However, this technique is an invasive procedure, costly and restricted to some cases of acute brain injuries. Moreover, it is associated with complications and risks, mainly infection, hemorrhage, and obstruction [
9,
10,
11]. As BD is a condition of perfusion collapse because of extremely high ICP, one rationale on its monitoring could be the ICP waveforms (ICPW), considering that ICPW change according to reduction in intracranial compliance [
12].
Recently, the development of a non-invasive system to evaluate the ICPW (nICPW) allowed assessing the behavior of ICPW beyond the neurointensive care environment. This method has clinically [
9,
13,
14] and experimentally [
15,
16] demonstrated a high correlation with its invasive predicate, reproducing the same pulse shape profile and extracting numerical parameters from its different peaks amplitudes. Therefore, this pilot study aims to evaluate the behavior of nICPWin patients with a confirmed diagnosis of BD. Our specific goal was to analyze the quantitative differences between ICPW features comparing health individuals and patients undergoing brain death protocol.
Material and Methods
Study Design
The study is an exploratory and analytical pilot case-control study. It was carried out in Getulio Vargas University Hospital and João Lucio Hospital, in Manaus, Brazil. In compliance with the ethical aspects and the requirements, this study was approved by the Amazonas Federal University Research Ethics Committee, under the registration number: 82714517.2.0000.5020.
Neuromonitoring
Engineering and clinical applications of the nICPW system have been described in detail elsewhere [
17]. In summary, the system is able to capture micrometric skull deformations according to ICP variation and the influence of the cardiac cycle represents the pulse slopes of the ICP waveform. Moreover, thru an artificial intelligence processing, numerical parameters are derived from the waveforms, as pulse amplitude (AMP), P2/P1 ratio and time-to-peak (TTP), which also have been described elsewhere and change in accordance with ICC exhaustion [
12].
Inclusion Criteria
Adults (18 yo and older) of any gender and admission diagnostic, with a positive BD examination (a clear reason for coma docummented, absence of sedation, absence of brain stem reflexes and apnea test) were included. In the control group, healthy volunteers not submitted to sedation, without signs or symptoms of intracranial hypertension or diagnosis of previous neurological disease that could be responsible for altered ICP were included. These individuals were matched for gender and age with the participants in the case group.
Exclusion Criteria
Patients who underwent craniectomy and patients suffering from perforating or penetrating skull injuries were excluded.
Data Collection
Data collection was performed for 5 minutes with the individual in a supine position with the head elevated to 30 degrees. Physiological and epidemiological data were collected. Parameters derived from nICPW were collected and systematically analyzed offline.
Statistical Analysis
Continuous variables were presented as mean and standard deviation using a 95% confidence interval. The Wilcoxon test was used for comparisons between continuous variables. The waveforms were sorted in training (10 patients) and validation (5 patients). The models were adjusted by logistic regression, establishing the area under the curve (AUC). The data separation and adjustment were performed 500 times. P <0.05 was considered statistically significant, and all tests were two-tailed. Data were analyzed using the STATA 12.0 program.
Results
15 patients were included, being a control group of six healthy volunteers (female: male = 3:3); and 9 patients undergoing BD diagnostic protocol. The average age was 44,83 years ranging from 21 to 53. The case group comprised nine patients (F: M = 4:5); the average age was 48.5 years, ranging from 21 to 61. The underlying diseases of these patients were: hemorrhagic stroke (4), brain neoplasms (1), and closed head injury (1). The complimentary exam performed for brain death diagnosis was the TCD in all brain-dead patients concomitantly with the nICPW system.
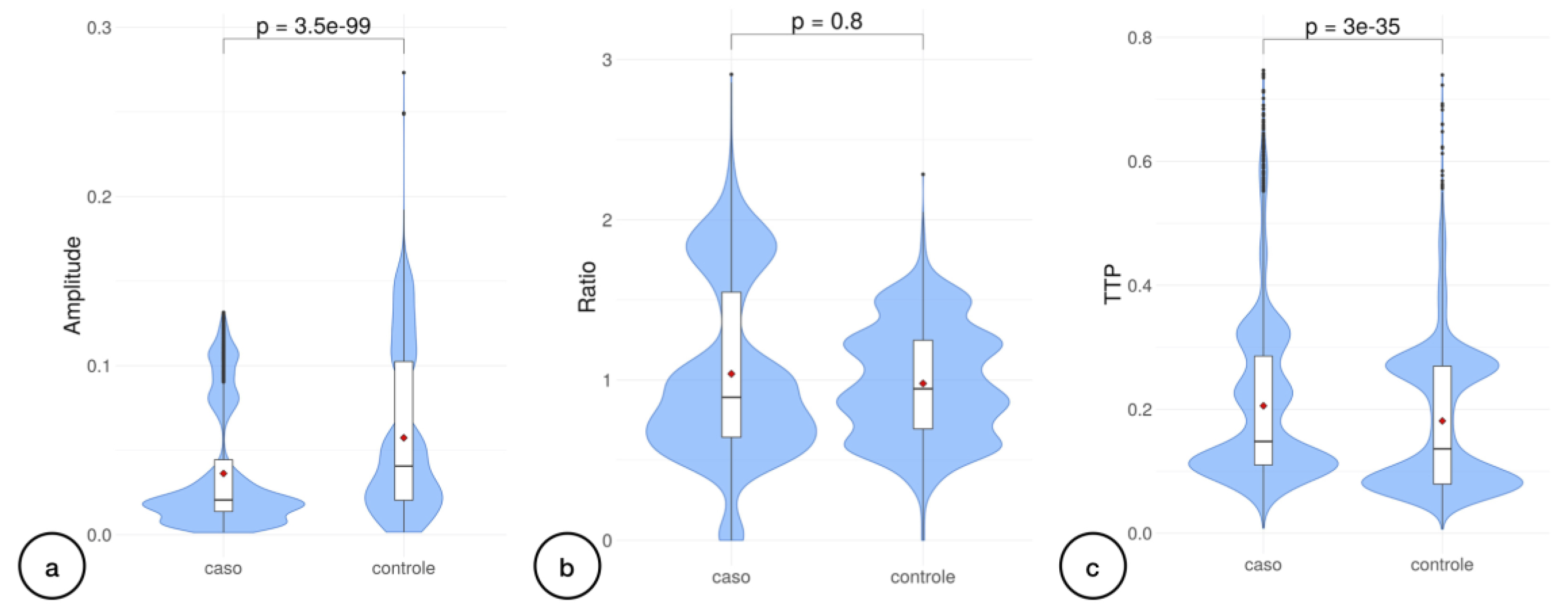
Regarding the nICPW analyses, we achieved 6,172 observations obtained pulse to pulse, including both control and case groups. Pulse amplitude had lower values in patients diagnosed with BD (p<0.0001) (
Figure 1.a). In contrast, the P2/P1 ratio median was not significant (p=0.80). TTP medians’ comparison revealed significant differences amidst case and control groups (p<0.00001) (
Figure 1.c).
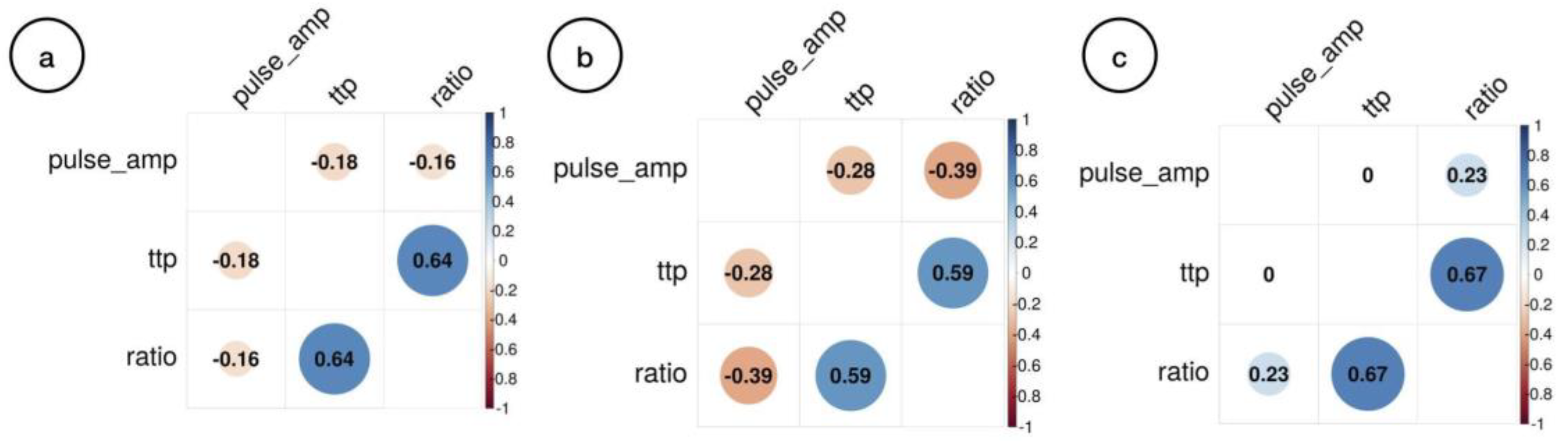
Joint correspondence analysis of all the observations obtained from the data collection, case group, and control group are discriminated in
Figure 2.
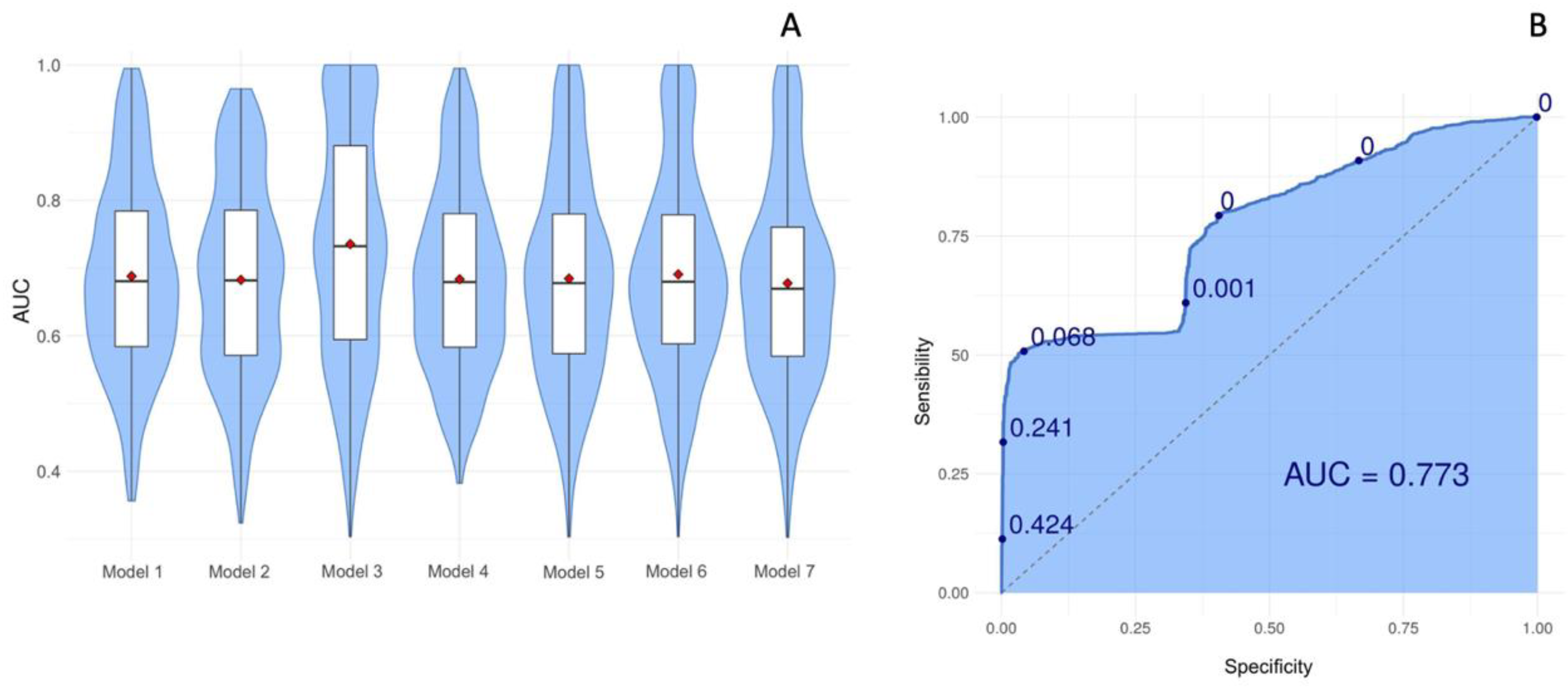
Seven models were tested for validation and model 3 obtained the best performance since it presented the greater average AUC, which value corresponds to 0.77 (Confidence interval [CI] = 2.076; 2.439) (
Figure 3).
Discussion
This exploratory study has shown the possibility of the nICPW to be statistically different among healthy and brain-dead subjects. However, our preliminary analysis led to a sole moderate accuracy, with consequences that may not be extrapolated to the clinical practice yet, as a complimentary exam to testify BD.
Scherzer et al. [
18] refers to ICP monitoring as an investigation method useful in the early timing, in order not to delay the diagnosis of BD and its medical consequences. Invasive ICP evaluation in BD was described as reaching a peak maximum in 5 to 12 hours, subsequently initiating a wave amplitude decrease [
19]. All of the 31 BD diagnosed patients who had ICP levels analyzed by Salih et al. [
20] presented severely elevated ICP at the moment of diagnosis (95.5 mmHg ± 9.8 mmHg), as well as reduced CPP. Roth et al. [
21] performed retrospective data of 18 patients with ICP monitoring during the development of BD due to primary brain lesions. All patients in this study experienced ICP values > 95 mmHg and CPP < 10mmHg. Interestingly, the ICPW obtained from invasive ICP analysis has been already described in BD. Domínguez et al. [
22] identified a P2-predominant pattern, although not specific to BD, once observed in both BD and survivors groups. The disappearance of P3 in most BD diagnosed patients was associated with the compromise of cerebral venous outflow, suggesting the presence of severe disturbance of blood flow in this pathology. There is agreement between these observations with the classic study from Nucci et al. that observed that P3 would become of higher amplitude than p1 in severe states of intracranial hypertension and the consequence is a pyramidal pulse shape [
23].
However, to our knowledge, no studies performed with invasive ICP monitoring focused on the ICPW quantitative data. Mainly because of the difficulties in extracting these parameters quantitatively. Rozsa et al. [
24] compared ultrasonic pulse waves assessment in eight BD patients and 34 neurologically healthy volunteers. Although no specific enough to use it as a criterion for BD, the patient group waveform was typical. Sub-wave values for P1, P2, and P3 were obtained, and intracranial pulses amplitude were significantly lower than controls, similar to what we have found in the present study. In BD, the P1 peak is low because there is no rapid increase in cerebral blood volume or consequent rapid increase in cerebrospinal fluid volume. In contrast, P2 and P3 peaks may stay high since there is no rapid venous outflow [
25]. However, in our data, we could not see an increase in the P2/P1 ratio, probably because ICP has reached an extremely high value and no more intracranial volume changes are seen during the cardiac cycle.
This study is the first to evaluate nICPW in BD patients. It is considered a new bedside strategy that provides real-time monitoring. ICP pulse morphology presented substantial differences amid BD patients when compared to the control group. The pulse amplitude and TTP variables values in the case group were statistically significant. Hence, this new nICPW monitoring device may be a useful screening tool to identify possible brain-dead patients.
Limitations
This study has several limitations. Firstly, the reduced sample size may underpower this study. Then, a better, accurate model was prevented. However, as this is an exploratory study, it gave some insights to be used in a larger study. Our second main limitation is the absence of patients with a different spectrum of consciousness disorders or sedated patients.
Conclusions
In this exploratory study, parameters extracted from noninvasive ICP waveform as pulse amplitude and the time interval for highest peak amplitude were significantly different between brain-dead patients and healthy subjects. Further studies with larger samples may determine the role of this technique on the brain death assessment complementation.
Author Contributions
MMLI and RLOA conceived and designed the analysis, selected the data, wrote final draft; LMO and MVDC: collected the data, wrote original draft; DBG, NACS and MCOP: Wrote final draft; WP and SB: manuscript revision.
Funding
No funding was received for this research.
Ethics approval
The protocol was approved by the Research Ethical Committee at Amazonas State University, Brazil.
Informed consent
Informed consent was obtained from all individual participants or next to kin included in the study.
Data availability statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Gustavo Frigieri for support with the Brain4care system.
Competing interests
Sérgio Brasil is consultant for Brain4care. The other authors declare no competing interests.
Consent for publication
The authors revised this final version and give consent for its publication.
References
- Brasil, S.; de Carvalho Nogueira, R.; de-Lima-Oliveira, M. Determination of Brain Death. JAMA 2021, 325, 493. [Google Scholar] [CrossRef]
- Brasil, S. Brain Death Diagnostic Security is Over Organ Donation. Transplant Proc 2021, 53, 2415. [Google Scholar] [CrossRef] [PubMed]
- Wijdicks, E.F.; Varelas, P.N.; Gronseth, G.S.; Greer, D.M.; American Academy of, N. Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2010, 74, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Brasil, S. Mistaken concepts on the use of ancillary testing in brain death diagnosis. Canadian Journal of Anesthesia/Journal canadien d’anesthésie 2022, 69, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Brasil, S.; Bor-Seng-Shu, E.; de-Lima-Oliveira, M.; M, K.A.; M, J.T.; Bernardo, L.; W, M.B. Role of computed tomography angiography and perfusion tomography in diagnosing brain death: A systematic review. J Neuroradiol 2016, 43, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Brasil, S.; Bor-Seng-Shu, E.; de-Lima-Oliveira, M.; Taccone, F.S.; Gattas, G.; Nunes, D.M.; Gomes de Oliveira, R.A.; Martins Tomazini, B.; Tierno, P.F.; Becker, R.A.; et al. Computed tomography angiography accuracy in brain death diagnosis. J Neurosurg 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ronconi, K.A.L.; Amorim, R.L.O.; Paschoal, F.M., Jr.; Oliveira, M.L.; Nogueira, R.C.; Paiva, W.S.; Gonçalves, D.B.; Farias, S.R.; Brasil, S.P.; Teixeira, M.J.; et al. Transcranial Doppler: A Useful Tool to Predict Brain Death Still Not Confirmed by Clinical Assessment. Transplant Proc 2021, 53, 1803–1807. [Google Scholar] [CrossRef] [PubMed]
- Powner, D.J.; Darby, J.M. Current considerations in the issue of brain death. Neurosurgery 1999, 45, 1222–1226. [Google Scholar] [CrossRef]
- Brasil, S.; Frigieri, G.; Taccone, F.S.; Robba, C.; Solla, D.J.F.; de Carvalho Nogueira, R.; Yoshikawa, M.H.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.S. Noninvasive intracranial pressure waveforms for estimation of intracranial hypertension and outcome prediction in acute brain-injured patients. J Clin Monit Comput 2022. [Google Scholar] [CrossRef]
- Dattilo, M. Noninvasive methods to monitor intracranial pressure. Curr Opin Neurol 2023, 36, 1–9. [Google Scholar] [CrossRef]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): an international, prospective observational cohort study. Lancet Neurol 2021, 20, 548–558. [Google Scholar] [CrossRef]
- Brasil, S. Intracranial pressure pulse morphology: the missing link? Intensive Care Med 2022. [Google Scholar] [CrossRef]
- Brasil, S.; Solla, D.J.F.; Nogueira, R.d.C.; Teixeira, M.J.; Malbouisson, L.M.S.; Paiva, W.d.S. A Novel Noninvasive Technique for Intracranial Pressure Waveform Monitoring in Critical Care. Journal of Personalized Medicine 2021, 11, 1302. [Google Scholar] [CrossRef]
- de Moraes, F.M.; Rocha, E.; Barros, F.C.D.; Freitas, F.G.R.; Miranda, M.; Valiente, R.A.; de Andrade, J.B.C.; Neto, F.; Silva, G.S. Waveform Morphology as a Surrogate for ICP Monitoring: A Comparison Between an Invasive and a Noninvasive Method. Neurocrit Care 2022. [Google Scholar] [CrossRef] [PubMed]
- Cabella, B.; Vilela, G.H.; Mascarenhas, S.; Czosnyka, M.; Smielewski, P.; Dias, C.; Cardim, D.A.; Wang, C.C.; Mascarenhas, P.; Andrade, R.; et al. Validation of a New Noninvasive Intracranial Pressure Monitoring Method by Direct Comparison with an Invasive Technique. Acta Neurochir Suppl 2016, 122, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Frigieri, G.; Andrade, R.A.P.; Wang, C.C.; Spavieri, D., Jr.; Lopes, L.; Brunelli, R.; Cardim, D.A.; Verzola, R.M.M.; Mascarenhas, S. Analysis of a Minimally Invasive Intracranial Pressure Signals During Infusion at the Subarachnoid Spinal Space of Pigs. Acta Neurochir Suppl 2018, 126, 75–77. [Google Scholar] [CrossRef] [PubMed]
- Andrade, R.d.A.P.; Oshiro, H.E.; Miyazaki, C.K.; Hayashi, C.Y.; Morais, M.A.; Brunelli, R.; Carmo, J.P. A nanometer resolution wearable wireless medical device for non invasive intracranial pressure monitoring. IEEE Sensors Journal 2021, 1. [Google Scholar] [CrossRef]
- Scherzer, E. [Intracranial pressure and brain death]. Wien Med Wochenschr 1990, 140, 562–564. [Google Scholar] [PubMed]
- Agapejev, S.; Da Silva, P.P.; Zanini, M.A.; Piza, E.T. [Intracranial pressure monitoring as a complementary tests for diagnosing brain death. Preliminary observation through the report of 2 cases]. Arq Neuropsiquiatr 1997, 55, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Salih, F.; Holtkamp, M.; Brandt, S.A.; Hoffmann, O.; Masuhr, F.; Schreiber, S.; Weissinger, F.; Vajkoczy, P.; Wolf, S. Intracranial pressure and cerebral perfusion pressure in patients developing brain death. J Crit Care 2016, 34, 1–6. [Google Scholar] [CrossRef]
- Roth, C.; Ferbert, A.; Matthaei, J.; Kaestner, S.; Engel, H.; Gehling, M. Progress of intracranial pressure and cerebral perfusion pressure in patients during the development of brain death. J Neurol Sci 2019, 398, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Roldan, J.M.; Barrera-Chacon, J.M.; Martin-Bermudez, R.; Murillo-Cabezas, F.; Garcia-Alfaro, C.; Rincon-Ferrari, M.D. Changes in the intracranial pulse pressure waveform associated with brain death. Transplant Proc 1999, 31, 2597–2598. [Google Scholar] [CrossRef] [PubMed]
- Nucci, C.G.; De Bonis, P.; Mangiola, A.; Santini, P.; Sciandrone, M.; Risi, A.; Anile, C. Intracranial pressure wave morphological classification: automated analysis and clinical validation. Acta Neurochir (Wien) 2016, 158, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Rozsa, L.; Szabo, S.; Gombi, R.; Miko, L.; Balazs, E. [Intracranial pressure increase and changes in cerebrovascular circulation, associated with brain death, studied by transcranial Doppler sonography]. Orv Hetil 1991, 132, 2785–2788. [Google Scholar]
- Karaali, K.; Cevikol, C.; Senol, U.; Arici, G.; Kabaalioglu, A.; Ramazanoglu, A.; Bircan, O. [Orbital Doppler sonography findings in cases of brain death. AJNR Am J Neuroradiol 2000, 21, 945–947. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).