1. Introduction
Plant viruses and their respective virus-like particles (VLP) offer an exciting nanotechnological platform for a variety of applications. Their propensity to self-assemble into monodisperse and highly symmetric nanometer-range particles makes them obvious candidates for the entrapment and delivery of biomedically active cargos
in vivo [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10]. In addition, the rigid protein capsids may be genetically or chemically modified to introduce (bio)chemical moieties to be displayed on the viruses' surface [
11,
12,
13,
14,
15,
16]. The resulting conjugates exhibit strong polyvalency while preserving the bioavailability of these nanomolecular building blocks and are used as carriers for the presentation of antigens or other biochemically relevant units [
2,
17].
Cowpea chlorotic mottle virus (CCMV) is a plant virus from the family
Bromoviridae, and its particles have an icosahedral symmetry with a diameter of 28 nm [
18,
19]. The capsid consists of 180 identical copies of the coat protein [
20]. A 3D model is shown in
Figure 1 [
20,
21]. CCMV can be produced with high yields of 0.1-1 mg per gram of infected leaf tissue [
22]. Hence, CCMV is an attractive building block in many scientific fields. For example, Lam
et al. used CCMV as a carrier for gene therapy to deliver siRNAs [
23]. Apart from heterologous RNAs, a wide range of other non-viral cargo has also been packaged by self-assembled viral capsid proteins of CCMV, for example, anionic polymers [
24], mineralized salts [
25], gold nanoparticles [
26], and fluorescent proteins [
27]. In addition, CCMV VLPs were recently studied as potential candidates for
in situ cancer vaccines [
28] and scaffolds to display SARS-CoV-2 antigens [
29].
However, the success of future applications of this nanotechnological platform strongly depends on the availability and purity of the starting material. So far, native CCMV is propagated in the host plant
Vigna unguiculata (cowpea) and isolated by repeated precipitation with polyethylene glycol (MW 8000) and NaCl [
30,
31]. After resuspension of the second pellet containing the enriched but still impure CCMV, a final polishing step is necessary – usually density gradient ultracentrifugation [
22,
32] and sometimes ultrafiltration [
33]. It should be noted that ultracentrifuges are quite expensive equipment and unsuitable for upscaling and relate to severe safety issues [
34]. The availability of a suitable ultracentrifuge often limits access to purified virus preparations. In addition, the purity of the isolated CCMV and the characterization of the purification steps have not been the subject of in-depth studies. Obviously, applying plant viruses
in vivo demands the highest standards to avoid any unwanted contamination. Virus-like particles (VLP) of CCMV are commonly produced recombinantly [
35,
36]. A Coomassie-stained SDS-PAGE gel is usually considered to be sufficient for characterizing the eluate of the coat proteins after Ni-NTA purification (IMAC).
To improve the availability and purity of CCMV, we developed a new two-step method for the purification of CCMV from infected leaves (see
Figure 2). After propagation of CCMV in its natural host,
Vigna unguiculata, symptomatic leaves are harvested and homogenized to a crude extract. Then, CCMV is precipitated once using PEG 8000. Next, the resuspended CCMV is purified by affinity extraction and subsequent elution on a novel CCMV-binding peptide aptamer immobilized on a monolithic glass column. This powerful and fast purification step eliminates the need for multiple PEG precipitations or any density gradient ultracentrifugation. In our protocol, we qualified the impurities at each step using, among other methods, silver-stained SDS-PAGE, MALDI-TOF-MS, and size exclusion chromatography combined with UV detection. Furthermore, the purity of the virus isolates was determined by reversed-phase HPLC/UV, and the total yield of CCMV in each step was calculated using an enzyme-linked immunosorbent assay (ELISA) based on a new monoclonal antibody.
2. Materials and Methods
2.0. Biochemicals and other reagents
Buffers: PBS (10x powder, from AppliChem, A0965,9010); Acetate binding buffer: sodium acetate (30 mM), acetic acid (20 mM), Na2-EDTA (1 mM), pH 4.8; Acetate elution buffer: acetic acid (6 mM), Na2-EDTA (1 mM), pH 3.6; Acetate neutralization buffer: sodium acetate (584 mM), acetic acid (292 mM); SEC running buffer: Sodium acetate (30 mM), acetic acid (20 mM), sodium chloride (150 mM), Na2-EDTA (1 mM), pH 4.8; phosphate binding buffer: Na2HPO4 · 2 H2O (8.7 mM), NaH2PO4 · 2 H2O (3.3 mM), pH 7.4; phosphate elution buffer: NaH2PO4 · 2 H2O (6.0 mM), H3PO4 (6.0 mM), pH 2.3; 2x loading buffer for SDS-PAGE (reducing): Glycerol (24%), tris base (900 mM, pH 8.45) (72%), sodium dodecyl sulfate SDS (4%), TCEP (100 mM), bromophenol blue (0.02%); 1x SDS running buffer: tris base (100 mM), tricine (100 mM), SDS (1%); Solvents: Ethanol (absolute for HPLC, Labsolute, 2222).
Other reagents: Acetic acid (Chemoslute,2289-1L); EDTA disodium salt 2-hydrate (AppliChem, 131669.1209); formaldehyde (Sigma, 252549-25ML); (3-glycidyloxypropyl)trimethoxysilane (Sigma-Aldrich, 440167, CAS 2530-83-8); hydrochloric acid (ChemSolute, 857,1011); Mucasol ® (Brand, 230091); polyethylene glycol MW 8000 (Sigma-Aldrich P2139); potassium dihydrogen phosphate (ChemSolute 1648.0250); silver nitrate (Roth, 9370.4); sodium acetate (Chemsolute 8694-1KG); sodium carbonate (AppliChem, AP141648.1211); sodium chloride (Acros Organics, 446212500); sodium hydroxide (Sigma-Aldrich, 30620-1KG-R); sodium thiosulfate (Roth, HN25.1); Triton X-100 (Sigma-Aldrich, T8787-50ML), 37470.01). Water was purified by an Ultra-Pure Water System from Millipore Co., with a resistivity of 18.2 MΩ·cm.
Antibodies: Anti-rabbit antibody (Origene, R1364P); anti-mouse antibody (Jackson ImmunoResearch (111-005-008); HRP-anti-mouse antibody (115-035-003); HRP-anti-rabbit antibody (Jackson ImmunoResearch (111-035-003)
Preparation of the monolithic raw column: The sintered core monolith (Por. 5, ultrafine, pore size 1-1.6 µm, borosilicate glass 3.3) was obtained from ROBU Glasfilter-Geräte GmbH, Hattert, Germany. The monolithic cylinder is prepared by custom order with the designation VitraPOR 5, diameter 8 mm, length 35 mm, front surfaces finely sawn. The raw cylinder is glued into a titanium tube with the outer dimensions of 35 mm x 12 mm and a wall thickness of 1 mm using silicon F liquid, obtainable from Weicon (13200310). A detailed description of the construction and manufacturing of the column and the column holder is shown in previous work [
37]. In this work, the monolith length was increased from 15 mm to 35 mm.
2.1. Generation of a peptide binder for CCMV
2.1.1. Experimental peptide binder generation
For the screening, a one-bead-one-compound (OBOC) library with a ladder sequence was synthesized as previously reported [
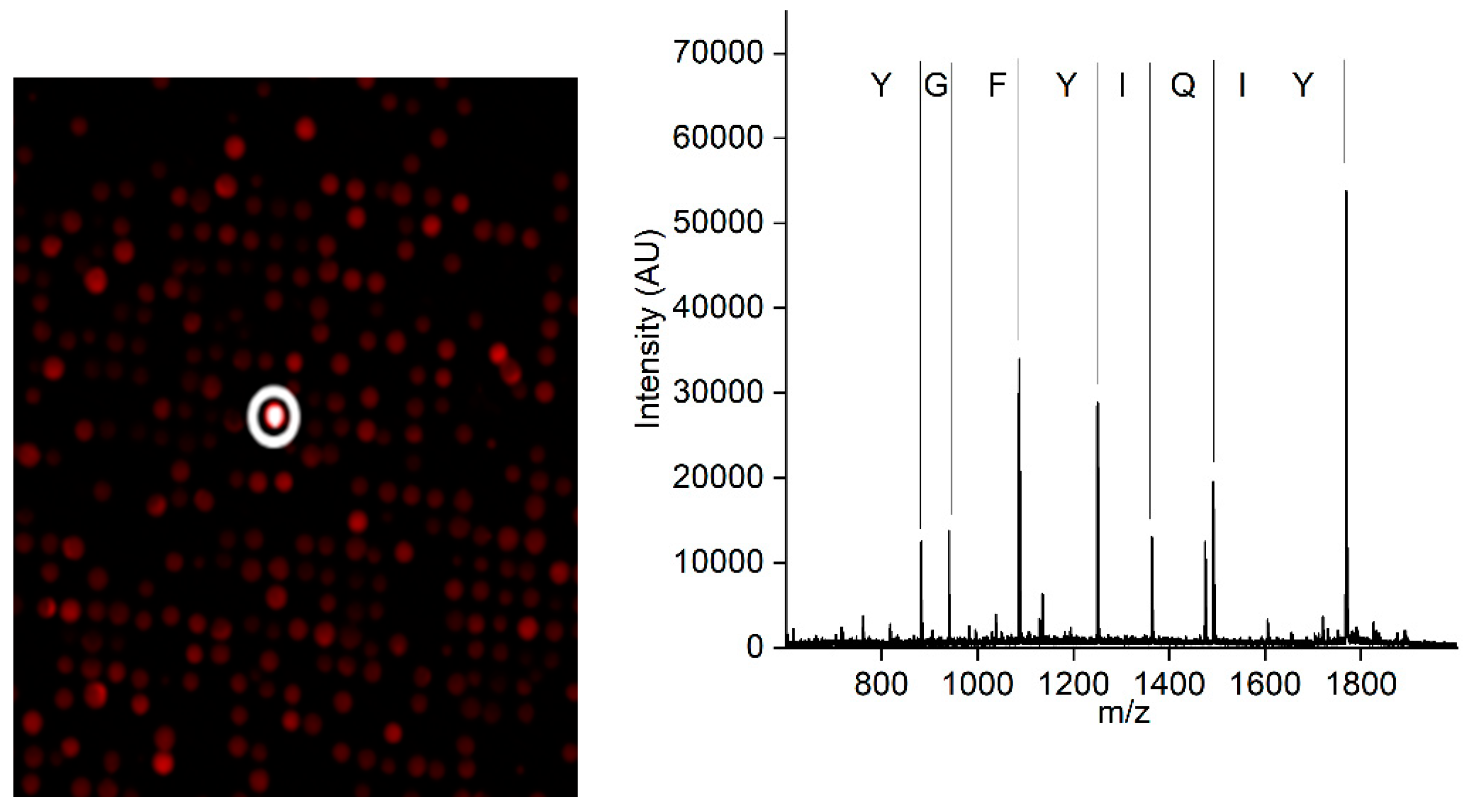
38]. The peptide library was synthesized by attaching the 4-hydroxybenzoic acid linker to TentaGel-resin. A short spacer containing (Gly-Gly-Thr-Glu-Arg-Ser-Gly-Gly) was added. On the spacer, octamer peptides with a ladder sequence were coupled via split-and-mix-method. The library contained glycine, serine, proline, tyrosine, glutamine, isoleucine, phenylalanine, tryptophane, histidine, glutamic acid, and arginine. The beads were immobilized with an electrically conductive, double-sided adhesive tape attached to a glass microscope slide to create the chip.
Fluorescently labeled CCMV was made by mixing 95 µL of a solution of CCMV in PBS (1 g/L) and 5 µL of NHS-Dy-654 (5 g/L) under dark conditions for 1 h at 22 °C. The solution was purified by using a Vivaspin500 filter. The solution was mixed with 400 µl acetate buffer (0.2 M, pH 4.8) and centrifuged for 5 min at 12,000 g. 450 µL of the acetate buffer was added, and the solution was again centrifuged for 5 min at 12,000 g. The step was repeated two times. The coupling success was examined via UV-spectroscopy and MALDI-TOF-MS.
The chip was pre-incubated in 15 ml acetate buffer for 1 h, and the buffer was removed. Afterward, a virus-free plant extract (300 mg of mashed leaves in 2 mL 0.2 M sodium-acetate buffer pH 4.8; centrifuged at 25.000 x g at 4 °C and filtered by 0.2 µm) was added and incubated for 2 h. The solution was removed, and the chip was washed three times with acetate buffer, dried for 30 min under air, and scanned with a microarray scanner Axon GenePix 4300A (Axon Instruments, Molecular Devices, LLC, California, USA) at 635 nm. After this pre-scan, the chip was again incubated in acetate buffer for 1 h. Following that, 5.17 µL of the CCMV-Dy654 conjugate was mixed with 15 mL of the plant extract, and the chip was incubated under dark conditions overnight. The solution was removed, and the chip was washed three times with acetate buffer, dried for 30 min under air, and scanned again with the microarray scanner at 635 nm.
The plant matrix was removed from the chip by incubation of 6 M guanidine HCl solution for 20 min. The chip was washed with 0.1% TFA in double deionized water, acetate buffer, 0.3% ammonium dodecyl sulfate in double deionized water, double deionized water, double deionized water:EtOH:TFA (94,9%:5%:0,1%), and double deionized water. The peptides were cleaved in a humid ammonia gas atmosphere overnight, and a 2,5-DHAP matrix was applied via an airbrush system, as previously reported [
38]. The coordinates of the positive particles on the chip from the fluorescence scan were used for aiming with the laser in the MALDI-TOF-MS (Bruker autoflex max smartbeam2) measurement.
The found peptide was resynthesized by using the Titan 357 peptide synthesizer (AAPPTec, Kentucky, USA). For this, 100 mg of Fmoc-Rink Amide aminomethyl resin (Iris Biotech GmbH, Germany, 0.59 mmol/g) was placed into a reaction vessel. The Fmoc protecting group was cleaved by applying 2.5 ml of cleaving solution (20% piperidine in DMF) and shaking for 5 min. The solution was removed, and another 2.5 mL cleaving solution was added for 10 min shaking. The resin was washed five times with 2 ml DMF for 1 min each. After removal of the last washing solution, 1 mL of amino acid with oxyma (both 0.4 M in DMF) and 1 mL of DIC (0.4 m in DMF) were added to the resin and shaken for 1 h. The coupling solution was removed, and the resin was washed once with 2 mL DMF for 1 min. Again, the amino acid/oxyma solution was added (1 mL) for the second coupling round. This time 1 mL HCTU (0.4 M in DMF) and 1 mL of DIPEA (1 M in DMF) were added to the resin-amino-acid-slurry and shaken for 1 h. The solution was removed, and the resin was washed three times with 2 mL DMF for 1 min each. Again, Fmoc was removed, as described before. Finally, the resin was washed two times with EtOH (2 mL), and the particles were placed in a syringe reactor. 4 mL of agent K (83% TFA, 5% double deionized water, 5% 1,2-ethanedithiol, 5% thioanisol, 2% triisopropylsilane) were added to the resin for 3 h. The solution was poured into cold diethyl ether, and the precipitate was collected by centrifugation at 3280 x g. The precipitate was washed once with diethyl ether and dissolved with DMSO. After lyophilization, a product of 75 mg was obtained. The crude peptide was used without further purification.
2.1.2. Molecular dynamics simulations of the peptide with GROMACS
GROMACS v.2022, a molecular dynamics (MD) simulation tool, was used to model the equilibrium properties of the aptamer peptide in solution [
39]. The following outlines the modeling pipeline: As a starting point, an initial model of the peptide and linker was generated with Alphafold2 (AF2) [
40]. The modeled peptide of
rank 1, was then processed for MD simulations to obtain an equilibrium structure in solution. The peptide's coordinates were solvated in a dodecahedron box of TIP3P water molecules using periodic boundary conditions. A minimum distance between the solute and the boundary was set to 1 nm, and the AMBER99SB forcefield [
41] was applied to the peptide. GROMACS took the protonation state of the peptide free in a solvent at pH 7, which should be indifferent from that at pH 4.8. No ion was added to the system as the dilute buffer ions were unlikely to interact with the peptide. The system was energy minimized with 500 steps of the steepest descent algorithm. Then heavy peptide atoms were position restrained with harmonic force constants of 1000 kJ mol
−1 nm
−2, while water molecules were allowed to equilibrate in an NVT ensemble for 200 ps followed by an NPT equilibration for a further 200 ps using the Leap-Frog algorithm [
42]. The mean temperature of 300 K was achieved using the velocity-rescaling thermostat [
43] with a time constant of 0.1 ps. The pressure was maintained at 1 bar using the Parrinello-Rahman barostat [
44] with a time constant of 2 ps. Short-range nonbonded interactions were truncated using a Verlet cut-off scheme at 10 Å. The smooth particle-mesh Ewald [
45] was used to calculate long-range electrostatics. The production simulation consisted of 5 parallel 1 μs simulations using a timestep of 2 fs, with frames saved every 40 ps. All input files for the simulations can be found at:
https://github.com/meyresearch/CCMV_aptamer_msm.
2.1.3. Markov modeling to obtain the dominant equilibrium peptide structure in solution
In order to analyze the 5 μs of trajectory data, a Markov State model (MSM) was built using pyEMMA v2.5.7 [
46] following the pyEMMA tutorial [
47] and similar approaches [
48,
49]. The trajectories were featured using backbone torsion angles and distances between α-carbon atoms. Time-lagged independent component analysis (TICA) [
50] was performed to reduce the dimension of the feature space, which then produced a 64-dimensional subspace using a 95% kinetic variance cut-off. The TICA space was discretized using a
k-means algorithm with 300 cluster centers. The maximum likelihood MSM was estimated from the discrete trajectories using a lag time of 1 ns. The PCCA+ algorithm [
51] was used to coarse-grain the MSM into two metastable states. The most likely state was identified as the dominant equilibrium peptide structure. The final visualisation of the equlibrium structure rendered using VMD v.1.9.3 [
52], was obtained by drawing random samples from the most populated PCCA state. A Jupyter notebook containing the MSM analysis is provided at:
https://github.com/meyresearch/CCMV_aptamer_msm.
2.2. Preparation of the affinity column with CCMV-binding peptide
An assembled column system consisting of the monolithic column [
37] built in a 3D-printed column holder was connected to a syringe pump (Standard Infusion Only Pump 11 Elite from Harvard Apparatus, USA), and several reagents and washing solutions were pumped through the column. If not indicated otherwise, a flow rate of 1 mL/min was used, and the reactions took place at room temperature.
To clean the glass surface, at 1 mL/min 10 mL of Mucasol (1%), NaOH (10%), and HCl (10%), were pumped through the column subsequently. Afterward, the column was washed with 10 mL of PBS at 1 mL/min to obtain a neutral pH before the column was equilibrated with 10 mL of toluene. In the first reaction step, 10 mL of (3-glycidyloxypropyl)trimethoxysilane (10% in toluene) was pumped through the column within 10 min. The column was sealed on both sides and incubated for 1 h at room temperature. After the reaction took place, the column was manually flushed with an air-filled syringe to remove the remaining silane solution from the column. The monolithic column was then removed from its column holder and placed in a drying oven (UNB 200, Memmert) to incubate overnight at 130 °C. Then, the column was allowed to cool down for 20 minutes and put back in the column holder. After flushing the reassembled column with 10 mL of toluene at 1 mL/min to remove the left-over silane, the column was again flushed with air to remove any left toluene. The column surface was now presenting epoxide groups and was ready for coupling the column with the target peptide via its thiol group.
For the peptide coupling, 12 mg of the CCMV-binding peptide was dissolved in 7.2 mL of DMSO. While gently vortexing the peptide solution, 4.8 mL of a Tris buffer (100 mM, pH 9.0) was added slowly. The acquired 12 mL of ready-to-couple peptide solution was now pumped through the column within 1 h at room temperature. To block the remaining epoxy groups, the column was flushed with 10 mL of a cysteine solution (100 mM in Tris buffer (100 mM, pH 9.0)) within 1 h. Next, the column was flushed at a flowrate of 1 mL/min with 10 mL of a Tris buffer (100 mM, pH 9.0), 10 mL of H2O and eventually washed with 10 mL of EtOH (20% in H2O) and stored in a fridge until further use.
2.3. Optimized protocol for the purification of CCMV
CCMV was isolated from leaves of Vigna unguiculata (cowpeas, black-eyed peas). For the germination of the seeds and growth of the plants, a hydroponic system with in-built and automated LED illumination from iDOO (Model: ID-IG301) was used. Before the plants reached the 3-leaf stage, cotyledons (seed leaves) were inoculated with a 50 µL suspension of 0.1 mg/mL CCMV in 0.05 M sodium acetate buffer and 1 mM Na2EDTA, pH 4.8 and a spatula tip of silicon carbide powder (carborundum, 600 grit) each, by gentle but firm rubbing of the leaves surface. After 3 minutes, excess silicon carbide was rinsed off with lab water. After 7-14 days, symptomatic leaves were harvested, weighed, and stored at -20 °C in a plastic bag. Any further symptomatic regrown leaves were also harvested and stored at -20 °C in a plastic bag.
The infected plant material was homogenized with a standard household blender (Bosch VitaBoost MMBH6P6BDE). For every gram of leaves, 6 mL of extraction buffer (0.2 M sodium acetate buffer with 10 mM Na2-EDTA and 0.1% (w/v) ascorbic acid, pH 4.8) was used. After 1 min of blending, 1 droplet of defoamer (neodisher Entschäumer S, article number: 430148) was added and mixed with the extract for 3 s. The extract was transferred into 50 mL falcon tubes and centrifuged at 15,000 x g and 4 °C for 20 min, then filtered with a 0.2 µm syringe filter. To the filtrate, a final concentration of 10% (w/v) PEG 8000 was added and thoroughly vortexed. Afterward, the solution was placed in an overhead shaker and allowed to incubate at 4 °C overnight. After incubation, the solution was centrifuged at 15000 x g and 4 °C for 25 min to obtain a yellow pellet. The supernatant was discarded. The pellet was completely resuspended in binding buffer (0.05 M sodium acetate buffer with 1 mM Na2-EDTA, pH 4.8) using a quarter of the initial volume by thorough vortexing. A few seconds of ultrasonication can help in the resuspension process. After resuspension, a pellet is again formed by centrifuging at 15000 x g and 4 °C for 15 min. This pellet does not contain CCMV. The supernatant is filtered with a 0.2 µm syringe filter and used for the affinity extraction performed on an FPLC system (ÄKTA pure 25 L, GE Healthcare Life Sciences) using the peptide aptamer column described in section 2.2.
Before each run on the FPLC, the column was washed with 20 mL of 1% Triton X-100 solution, followed by washing with elution buffer (6 mM acetic acid, 1 mM Na
2-EDTA, pH 3.6), and a subsequent equilibration with lab water and then binding buffer (0.05 M sodium acetate buffer with 1 mM Na
2EDTA, pH 4.8). The fractions of the fraction collector were filled each with 10 µL of neutralization buffer (292 mM acetic acid, 584 mM sodium acetate, pH 4.5). Then, the sample loop (10 mL) was filled with the sample. During the injection, a flow of 2 mL/min was applied. Afterward, a washing step with 10 mL binding buffer and a flow rate of 10 mL/min was used. Finally, CCMV was eluted with the elution buffer (6 mM acetic acid, 1 mM Na
2EDTA, pH 3.6) at a flow rate of 6 mL/min and collected in 200 µL fractions in the fraction collector. The neutralized fractions, now at 0.05 M sodium acetate buffer with 1 mM Na
2EDTA, pH 4.8, were pooled so that ~95% of the peak area was saved without unnecessary dilution of the sample and stored at 4 °C for later analysis. An aliquot was used to determine the concentration of the CCMV by UV absorbance, using the extinction coefficient of CCMV at 260 nm: ε
260=5.87 mg
-1 mL cm
-1 [
53]. The column was re-equilibrated with binding buffer and stored at 20% ethanol at 4 °C.
2.4. Characterization of purification steps with silver-stained SDS-PAGE
From all samples shown in
Figure 7, 10 µL aliquots were taken and each supplemented with 10 µL of 2x SDS loading buffer, mixed, and heated to 95 °C in a ThermoMixer C (Eppendorf, 5382000015) for 15 min. The samples were mixed again, and subsequently, 10 µL of each sample was loaded onto the gel (Novex™ WedgeWell™ 8-16%, Tris-Glycine, 1.0 mm, Mini Protein Gel, Invitrogen, XP08160BOX), which was run with 900 mL of SDS running buffer in a fridge at 4 °C with a XCell SureLock Mini-Cell Electrophoresis System from Invitrogen (EI0001). Run times: 20 min at 80 V + 25 min at 200 V. The following silver-staining protocol was applied to visualize protein bands: The gel was washed twice in lab water for 2 min each. Afterward, the gel was incubated in sodium thiosulfate Na
2S
2O
3 (1.3 mM) for 1 min and then washed with water for 10 s. The gel was then transferred into a staining container containing freshly prepared AgNO
3 (5.9 mM) and incubated for 25 min. The gel was washed twice with water for 10 s each and then incubated for 30 s with sodium carbonate Na
2CO
3 (236 mM). In the next step, the gel was incubated in a solution of Na
2CO
3 (236 mM) and formaldehyde (2.5 mM) for 7 min. After the completed staining, the gel was washed twice with water and then incubated in Na
2EDTA (50 mM) for 10 min. Lastly, the gel was washed twice with water, and a photograph of the final gel was taken.
2.5. Characterization of purification steps with size exclusion chromatography (SEC)
Size exclusion chromatography was performed on an FPLC (ÄKTA pure 25 L, GE Healthcare Life Sciences); 280 nm was used as the detection wavelength. A flow rate of 2.6 mL/min was used during washing steps and 0.5 mL/min during separations. The experiment was performed with samples 1, 3, 4, and 5, subsequently. To each of the four samples, 150 mM sodium chloride was added. The CCMV eluate (sample 5) was diluted in SEC running buffer to a concentration of 100 µg/mL. The obtained samples were filtered with a 0.22 µm syringe filter. The column (Hi Prep 26/60 Sephacryl S-300-HR, from Cytiva, 17119601) was connected to the FPLC system and then equilibrated with 1.5 column volumes (CV) water and SEC running buffer, subsequently. During the applied purification protocol, the column was first equilibrated with 0.1 CV of SEC running buffer before the 1 mL sample was injected through the 2 mL sample loop using 10 mL of SEC running buffer. Afterward, the column was eluted with 1.5 CV of SEC running buffer, where the separation took place.
2.6. Characterization of purification steps with MALDI-TOF-MS
Samples 1, 3, 4, and 5 were desalted using Zeba Spin 7K MWCO size-exclusion desalting columns (75 µL) according to the manufacturer's protocol. 1 µL of the eluted solution was pipetted on a target spot of the MALDI plate, and 1 µL of α-cyano-4-hydroxycinnamic acid (10 mg/mL in 50% H2O, 49.9% ACN, 0.1% TFA (v/v/v)) was added. The droplet was mixed by pipetting up and down several times and then allowed to dry. MALDI-TOF-MS was performed on a Bruker Autoflex maX in linear mode. The obtained spectra were averaged over 5000 laser shots.
2.7. Purity determination by reversed-phase HPLC
The eluate of the affinity extraction (sample 5) was diluted with lab water to a concentration of 0.3 mg/mL and filtered with a 0.1 µm syringe filter into an HPLC vial. A high-performance liquid chromatography system with a diode array detector (HPLC-DAD; Agilent Technologies 1260 Infinity II series) was used. The injection volume was 30 µL. A C8 column (AdvanceBio RP-mAb SB-C8, 2.1 x 150 mm, 3,5 µm, Agilent Technologies) was used as the stationary phase. Separation was performed with A=0.2% TFA in H2O and B=0.16% TFA in acetonitrile with the following gradient: 0-3 minutes 99% A and 1% B, 3-23 minutes 30% A and 70% B, 23-40 minutes 1% A and 99% B at a constant flow rate of 0.8 mL/min. Baseline subtraction, peak finding, and integration were performed using Chromeleon chromatographic data analysis software (version 7.2.10.23925).
2.8. Determination of integrity and monodispersity
Transmission electron microscopy (TEM) images were obtained in a Talos F200S Microscope (Thermo Fisher Scientific) by using a 200 kV microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimens were prepared by negative staining the CCMV with a 3% solution (w/v) of phosphotungstic acid in ultrapure water onto a 3 mm copper grid (lacey, 400 mesh, TED PELLA, 01824) using the side-blot method.
Dynamic light scattering experiments were performed at a Zetasizer Malvern Instrument with disposable cuvettes made from polystyrene (10 mm) purchased from Th. Geyer GmbH & Co. KG (Renningen, Germany). Measurements were performed in forward scatter mode and the advanced cumulant model. A 1 mg/mL CCMV solution in 0.05 M acetate buffer containing 1 mM EDTA, pH 4.8, was freshly prepared and measured.
Atomic force microscopy (AFM) measurements were performed on a HORIBA OmegaScope with LabRAM HR evolution instrument, using tapping mode (AC mode) with ATEC-NC (frequency 335 kHz, spring constant 45 N/m) tips. The AC mode automatically controls all the parameters with an exception for the scan rate which was set to 1 Hz. A 1 µL 0.1 mg/mL CCMV solution was added to 9 µL 1x Tris-acetate-EDTA buffer containing 15 mM MgCl2 and incubated on a plasma-treated silicon chip for 10 mins. The chip was then washed two times with a mixture of ethanol and water (1:1) and blow dried with compressed air. The chip was taped on a magnetic disc and inserted in the AFM instrument for imaging.
2.9. Quantification of CCMV by ELISA
For the immunoassay, antiserum (BAM-CCMV-rab-pAb01) from rabbits after immunization with CCMV was purified via protein G affinity chromatography. The experiment was performed on an ÄKTA pure 25 L from GE Healthcare Life Sciences, and 280 nm was used as the detection wavelength. A flow rate of 4 mL/min was used if not mentioned otherwise. The rabbit serum was diluted 1:10 in a phosphate binding buffer (pH 7.4). The obtained serum solution (500 µL) was filtered using a 0.22 µm syringe filter and then filled into the 2 mL sample loop of the FPLC system. The column (HiTrap 1 mL Protein G HP, Cytiva, 29048581) was connected to the system and then washed with water, phosphate elution buffer, and phosphate binding buffer subsequently. During the applied method, the column was first equilibrated with 10 mL of binding buffer before the sample was injected with 6 mL of phosphate binding buffer at a flow rate of 0.5 mL/min through the sample loop. After a washing step with 15 mL of phosphate binding buffer to remove loosely bound components, IgG was eluted with 5 mL of phosphate elution buffer (pH 2.3). Finally, the column was re-equilibrated with 10 mL of phosphate binding buffer to a pH of 7.4. An IgG solution with 0.5 mg/mL in 600 µL was obtained, determined with UV absorption at 280 nm, and rabbit IgG as a reference in a photometer (NanoPhotometer NP80, Implen).
For the determination of CCMV with an enzyme-linked immunosorbent assay (ELISA), the protein G purified polyclonal antibody was used, as well as an anti-CCMV monoclonal antibody (BAM-CCMV-29-81) and secondary antibodies (polyclonal anti-mouse antibody and polyclonal peroxidase-conjugated anti-rabbit antibody). All steps were performed at room temperature, washing was done in triplicate using 300 µL of washing buffer (KH2PO4 1.25 mM; Na2HPO4∙2H2O 6.67 mM; Tween 20 0.3 mM), and incubation was performed on a plate shaker. The high-binding microplate was coated in 100 µL of 1x PBS, pH 7.4, containing 2.6 µg/mL of anti-mouse antibody and incubated overnight. The next morning, the plate was washed, and 100 µL of the monoclonal anti-CCMV antibody at a concentration of 0.72 µg/mL in 1x PBS, pH 7.4, was added to the wells of the microplate. After one hour, the plate was washed and then incubated with 250 µL of the blocking solution (5% skim milk powder in 1x PBS, pH 7.4) for another hour. Following the next washing, the wells were treated with 100 µL of the sample dilutions (usually ranging from 1:100 to 1:50,000, depending on the supposed concentration) and the calibration solutions in PBS-T for an hour. 100 µL of polyclonal anti-CCMV antibody (0,34 µg/mL) in PBS-T was added after washing, and the plate was again placed on the shaker for one hour. The plate was washed, and 100 µL of the peroxidase-conjugated anti-rabbit antibody in PBS-T was added at a concentration of 2.67 ng/mL and incubated for 30 minutes. Then a final wash step was performed. For detection, 100 µL TMB substrate was added to the wells and incubated for 15 minutes in darkness. In the last step, 100 µL of stop solution (250 mM H2SO4) was added, and the absorbance at 450 nm was measured using the plate reader.
4. Discussion
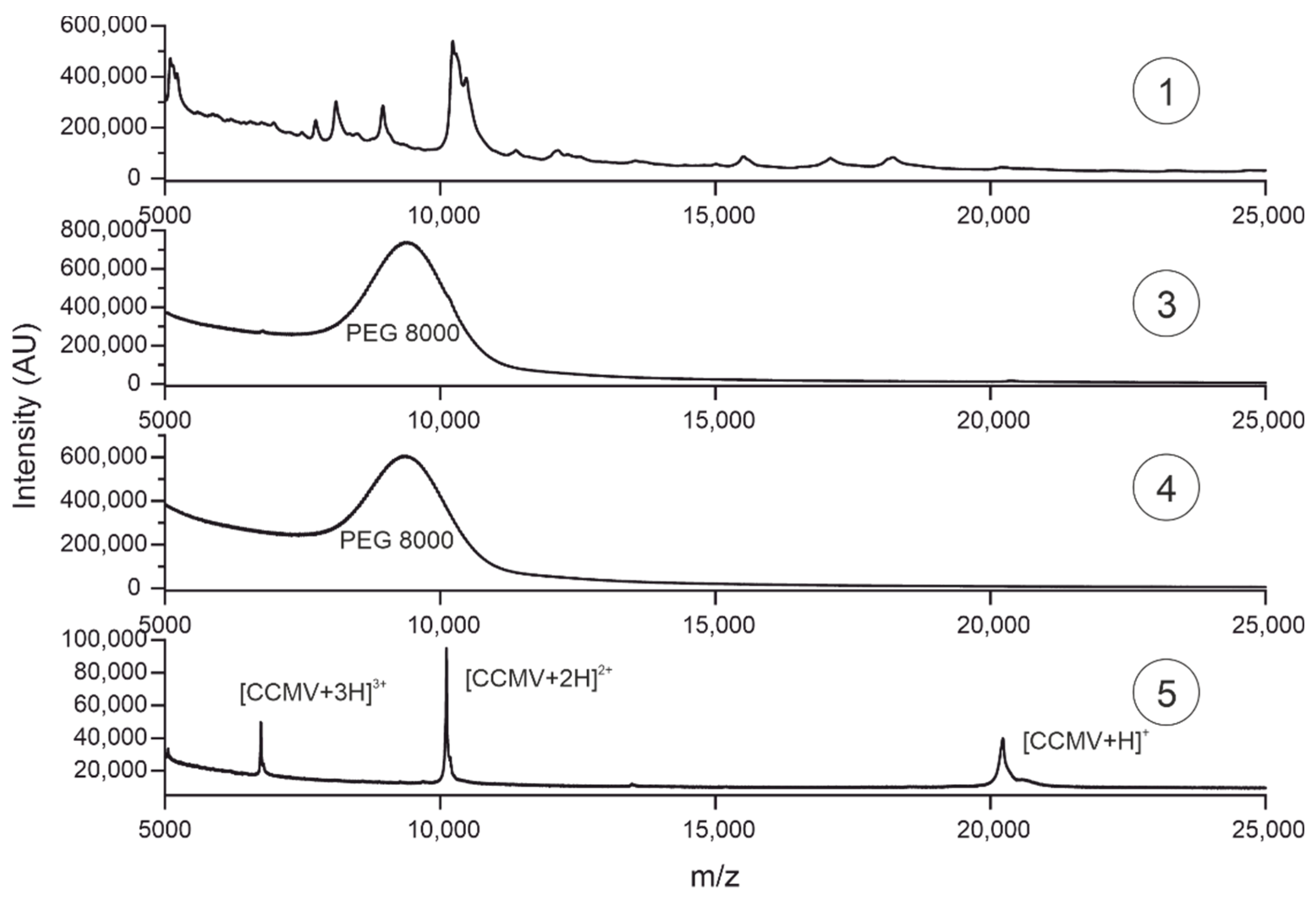
The results of this work highlight the power of affinity extraction as a polishing step for the purification of CCMV from crude plant extracts. As SDS-PAGE (
Figure 8) and SEC (
Figure 9) analysis show, the precipitation of CCMV with PEG is not sufficient to separate the virus from other proteins of the plant matrix. Even worse, PEG remains as an impurity after resuspension of this pellet, as can be seen in the MALDI-TOF-MS (
Figure 10). Therefore, the final step of every plant virus purification protocol involving precipitation with PEG should also remove this contaminant. So far, many established protocols use density gradient centrifugation for this purpose. However, the ultracentrifuges needed for this procedure are expensive and limit the upscaling of the process. Furthermore, whether the PEG is removed completely at this step remains questionable. Other contaminants may be introduced by sucrose or CsCl gradients. The new purification method by affinity extraction using a novel peptide aptamer can address previous shortcomings of virus purity. No expensive equipment is needed for the workflow, which enables more laboratories to explore plant viruses like CCMV as a potential nanotechnological platform. In the future, we expect that using peptide aptamer-based purification protocols will pave the way for future applications of CCMV and other plant viruses and make sure that high purity requirements can be routinely reached. Affinity columns also show promise for the purification of the CCMV coat proteins from culture supernatants from recombinant expression (data not shown). In that case, a His-Tag would not be needed to purify the coat proteins. The novel peptide aptamer found in this study might also be explored for selective cargo loading and "plug-n-play functionalization" as analogously described for the related plant virus
cowpea mosaic virus (CPMV) [
58]. Further modeling of the interaction between the peptide and CCMV is of interest to determine the exact binding site.
The purity of CCMV isolates was previously given as an absorbance ratio of A
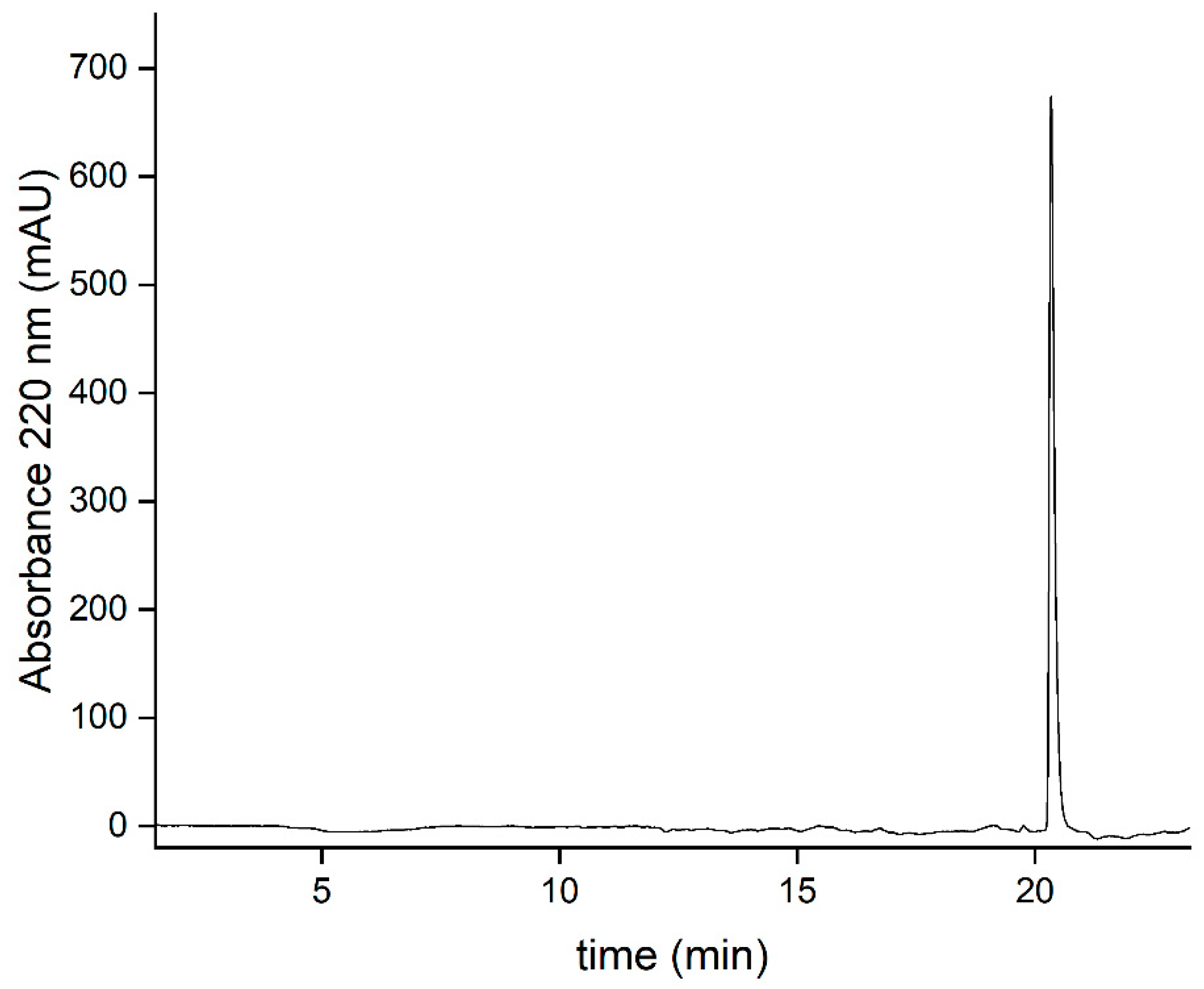
260/280, which should be between 1.5-1.7. Indeed, this ratio is useful to quickly assess the correct RNA and capsid protein ratio for relatively pure samples. However, today, it seems outdated and too imprecise to be considered a valid method for protein purity assessment. Instead, we propose to use a standard reversed-phase chromatographic separation with detection at 220 nm (see
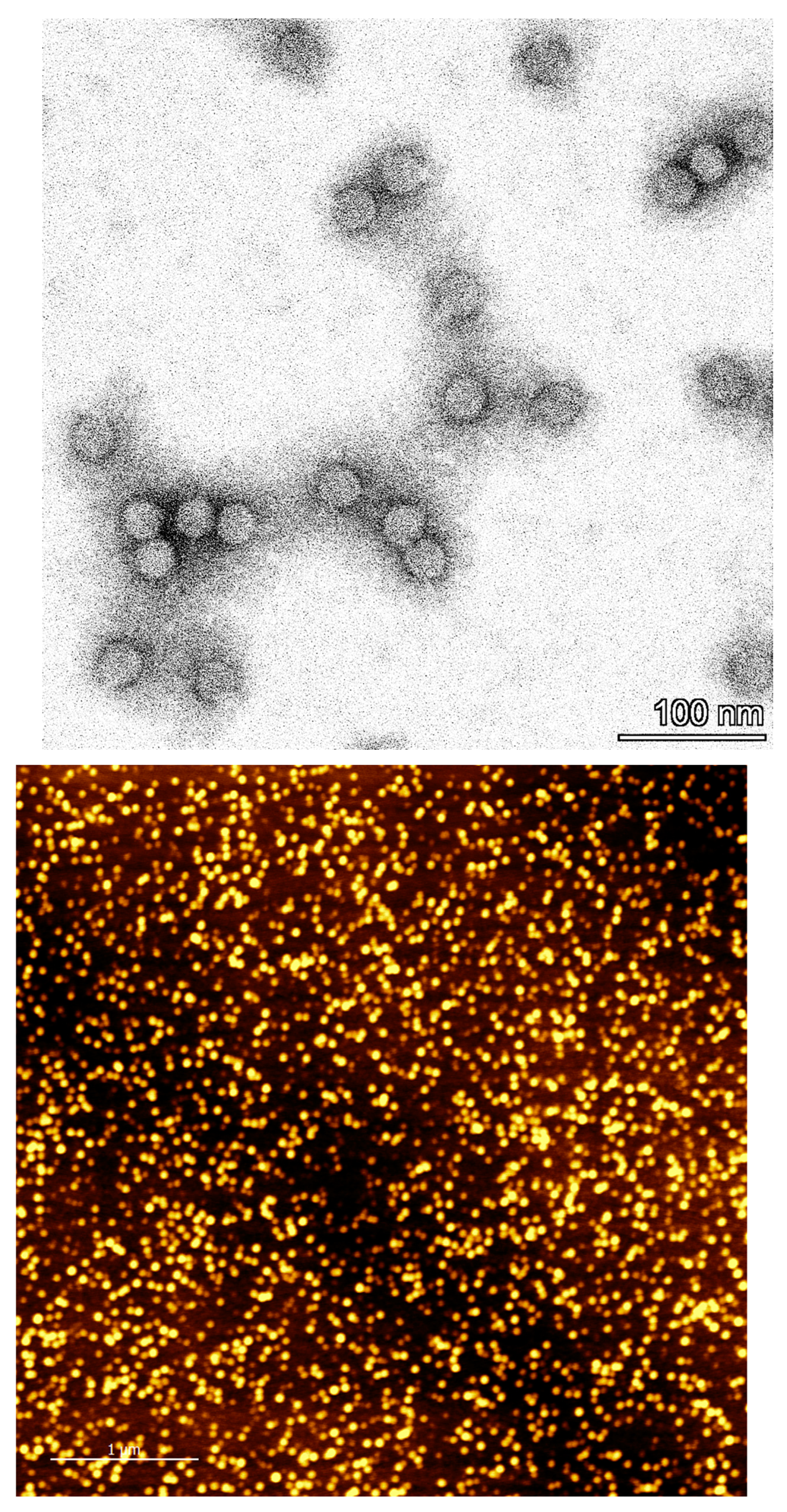
Figure 11) to obtain a purity based on the relative peak ratios of the capsid protein and any contaminants. The correct integrity of the particles should then be checked using more methods for particle analysis, such as TEM, AFM, or DLS (see
Figure 12).
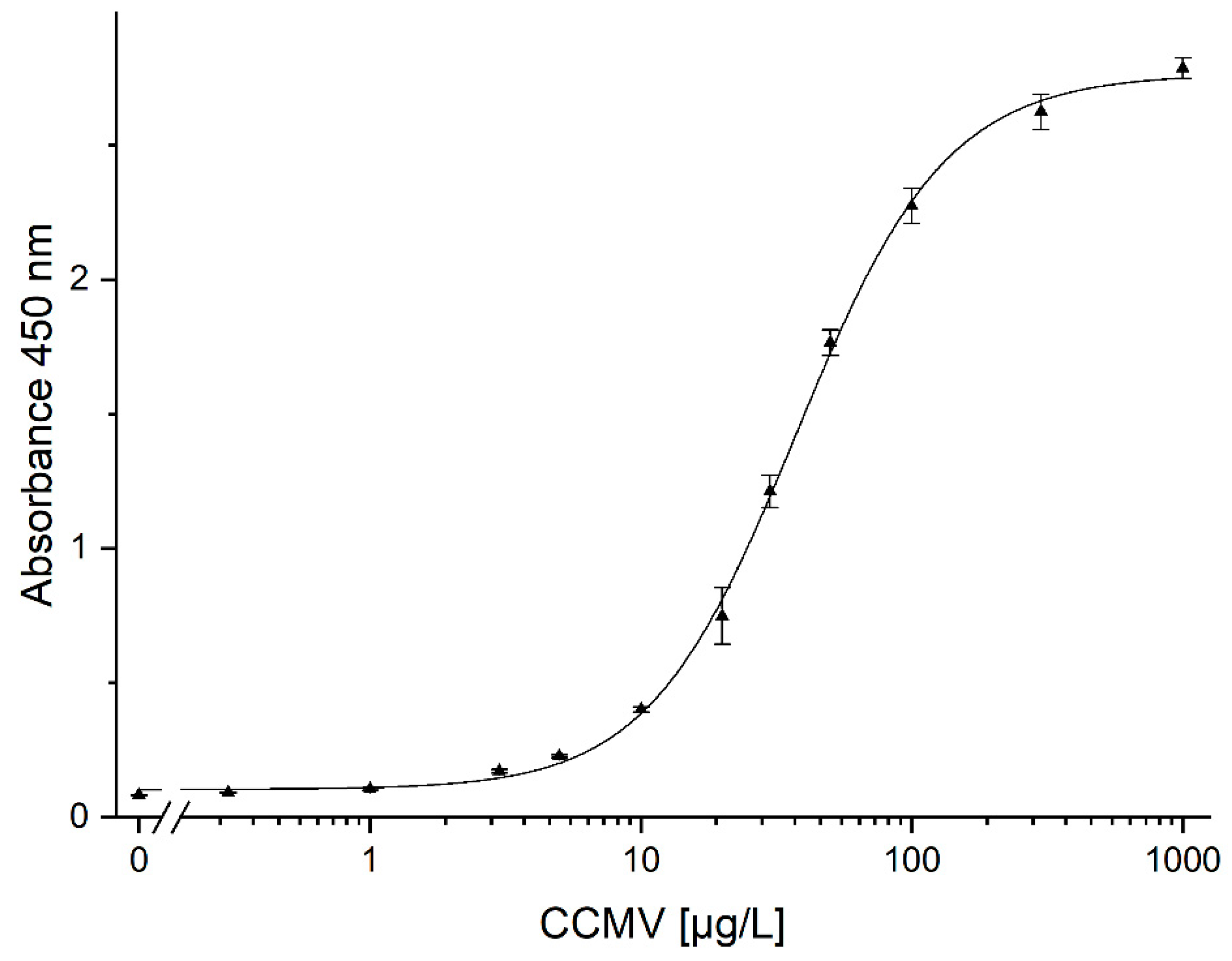
Quantification of CCMV in complex matrices can be achieved using immunoassays. This work presents a new ELISA for CCMV, using both polyclonal and monoclonal antibodies (
Figure 13). This assay can be used to determine the concentration of CCMV in field samples. In addition, yields of CCMV for other purification protocols can be evaluated. The monoclonal antibody BAM-CCMV-29-81 presented here might also be a good starting point for developing a lateral flow test against CCMV in plant samples, which can be envisioned to reduce crop failures substantially.
Author Contributions
Conceptualization, M.G.W., and G.T.; methodology, G.T. and M.G.W.; validation, G.T., M.P., and C.R.; formal analysis, G.T., M.P., C.R., A.S.J.S.M., Y.Z.; investigation, M.P., G.T., C.R., P.S.W., R.H., Y.Z., A.M., J.L.V., C.P., H.B., K.N., R.Z., P.A., and T.S.; data curation, G.T., M.P., A.S.J.S.M., Y.Z., C.R., and G.B.; writing—original draft preparation, G.T., C.R., M.P., A.S.J.S.M., Y.Z., and J.O.K.; writing—review and editing, G.T., M.G.W., A.S.J.S.M., J.O.K., and I.B.; visualization, G.T., A.S.J.S.M., Y.Z., M.P., C.R., R.H.; supervision, M.G.W.; project administration, M.G.W.; funding acquisition, M.G.W. All authors have read and agreed to the published version of the manuscript.
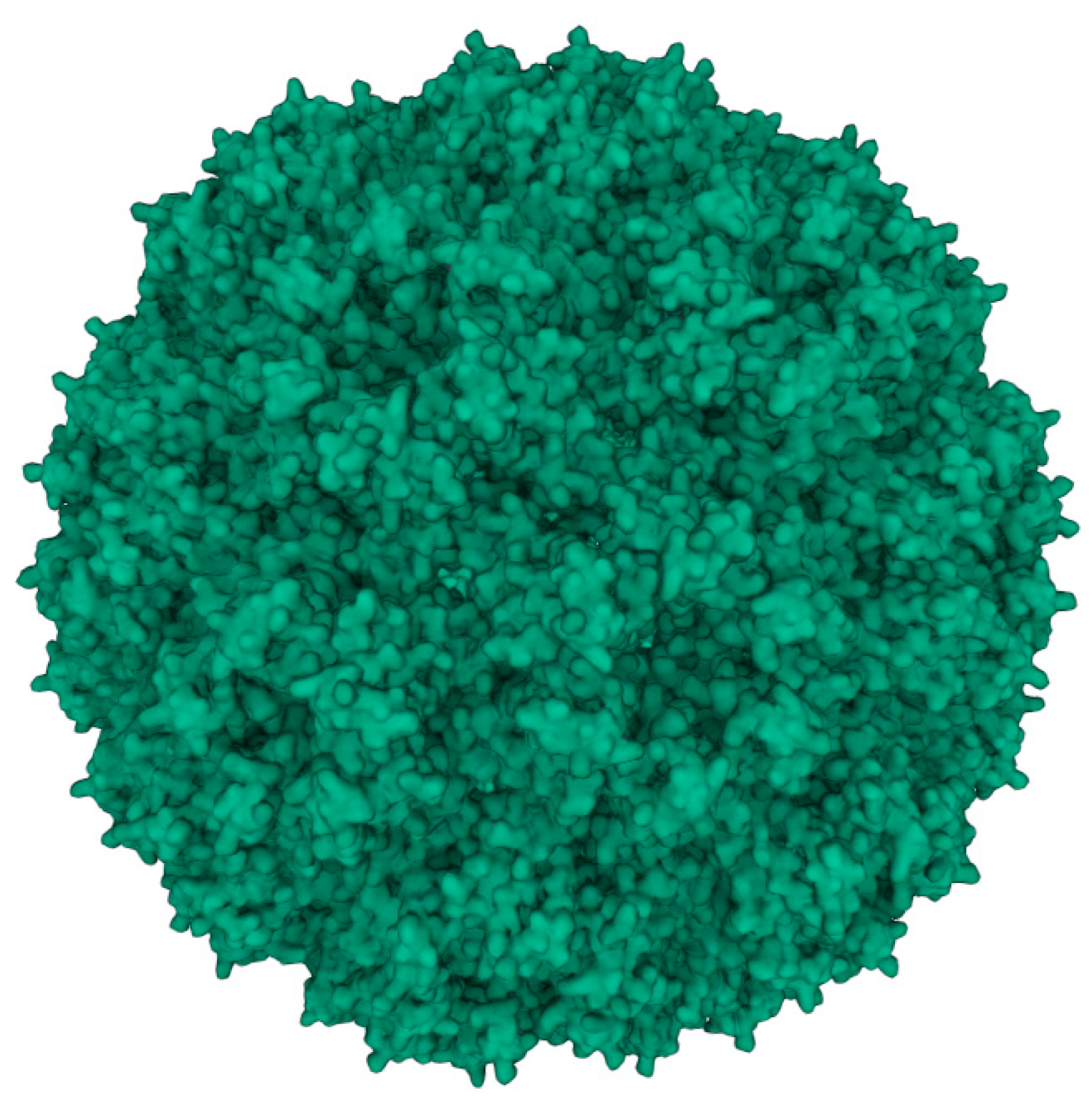
Figure 1.
3D model of the capsid protein of CCMV (PDB ID: 1CWP) generated with X-ray crystallography and cryo-electron microscopy data from the RCSB protein data bank [
20], created with Mol*viewer [
21].
Figure 1.
3D model of the capsid protein of CCMV (PDB ID: 1CWP) generated with X-ray crystallography and cryo-electron microscopy data from the RCSB protein data bank [
20], created with Mol*viewer [
21].
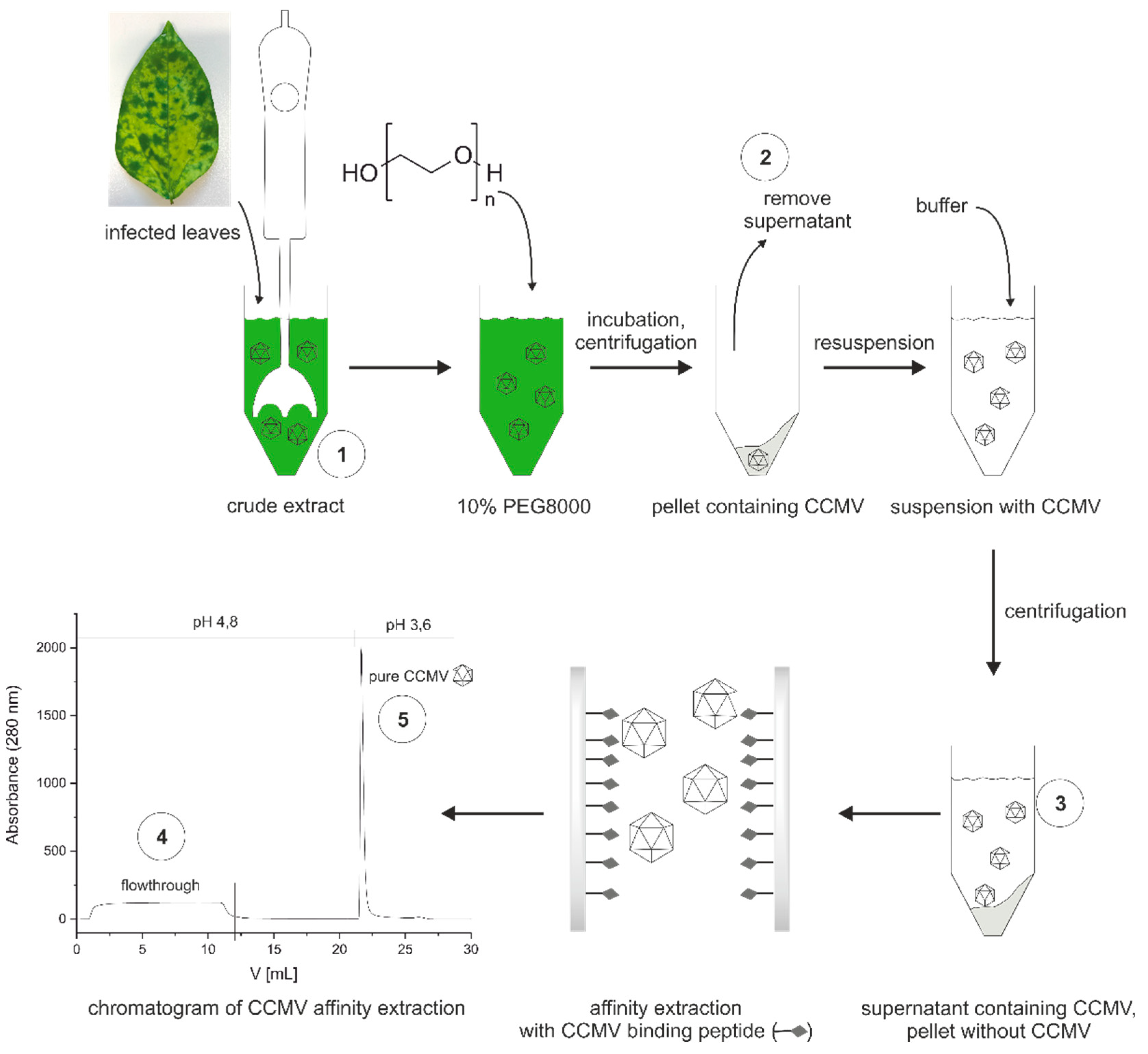
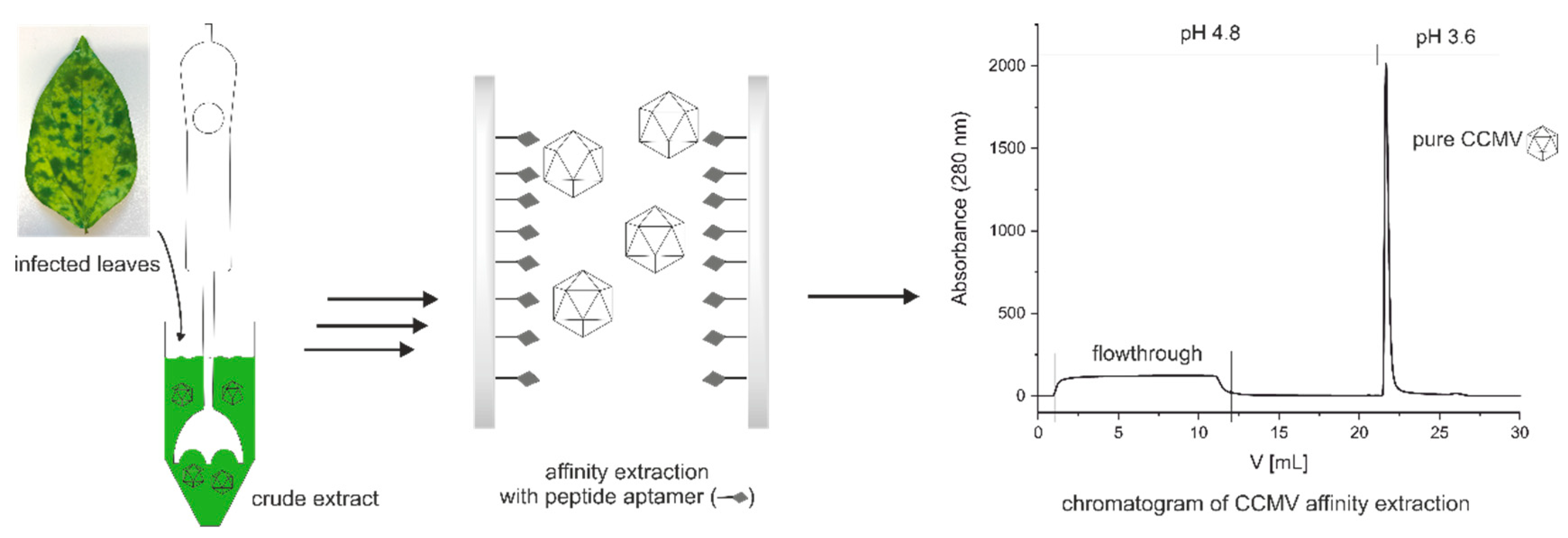
Figure 2.
Simplified workflow for the purification of CCMV using a novel peptide aptamer.
Figure 2.
Simplified workflow for the purification of CCMV using a novel peptide aptamer.
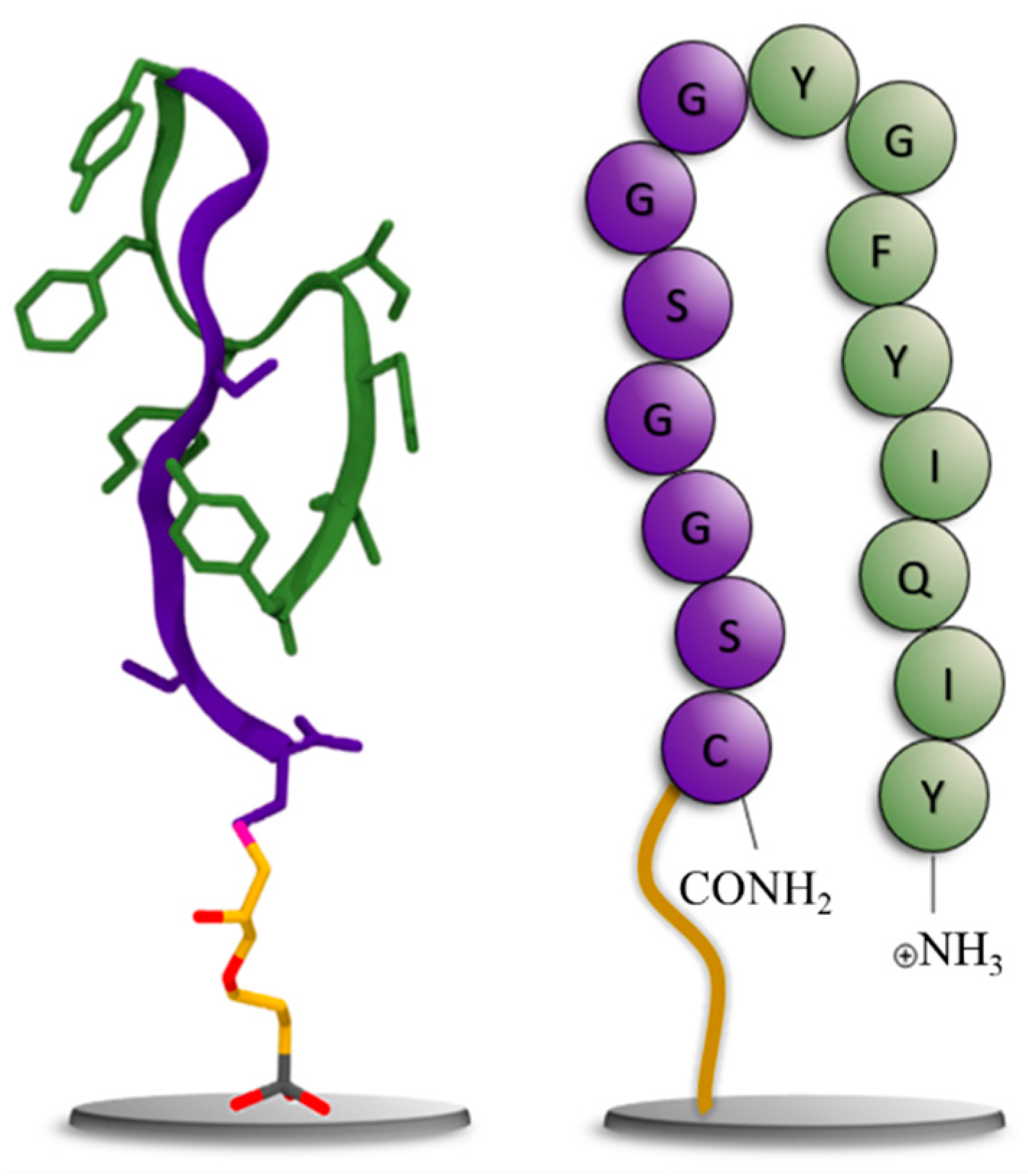
Figure 4.
Left: Representative structure of the equilibrium-folded structure of the peptide; the glycine-serine linker is shown in purple, and the aptamer in green, with the silane linker shown in licorice representation. Right: Cartoon representation of the peptide for comparison using the same color scheme.
Figure 4.
Left: Representative structure of the equilibrium-folded structure of the peptide; the glycine-serine linker is shown in purple, and the aptamer in green, with the silane linker shown in licorice representation. Right: Cartoon representation of the peptide for comparison using the same color scheme.
Figure 5.
Scheme for preparing the affinity column for CCMV purification: Surface functionalization of a sintered glass monolith with epoxy silane and coupling of the thiol-containing CCMV-binding peptide aptamer via ring-opening of the epoxide. Please note: In this figure, the N-terminus of the peptide NH2-Tyr-Ile-Gln-Ile-Tyr-Phe-Gly-Tyr-Gly-Gly-Ser-Gly-Gly-Ser-Cys-NH2 is located on the right-hand side, and the C-terminus is amidated. Due to the specific synthesis and subsequent screening procedure, the peptide must be oriented so that the C-terminus is directed to the solid phase.
Figure 5.
Scheme for preparing the affinity column for CCMV purification: Surface functionalization of a sintered glass monolith with epoxy silane and coupling of the thiol-containing CCMV-binding peptide aptamer via ring-opening of the epoxide. Please note: In this figure, the N-terminus of the peptide NH2-Tyr-Ile-Gln-Ile-Tyr-Phe-Gly-Tyr-Gly-Gly-Ser-Gly-Gly-Ser-Cys-NH2 is located on the right-hand side, and the C-terminus is amidated. Due to the specific synthesis and subsequent screening procedure, the peptide must be oriented so that the C-terminus is directed to the solid phase.
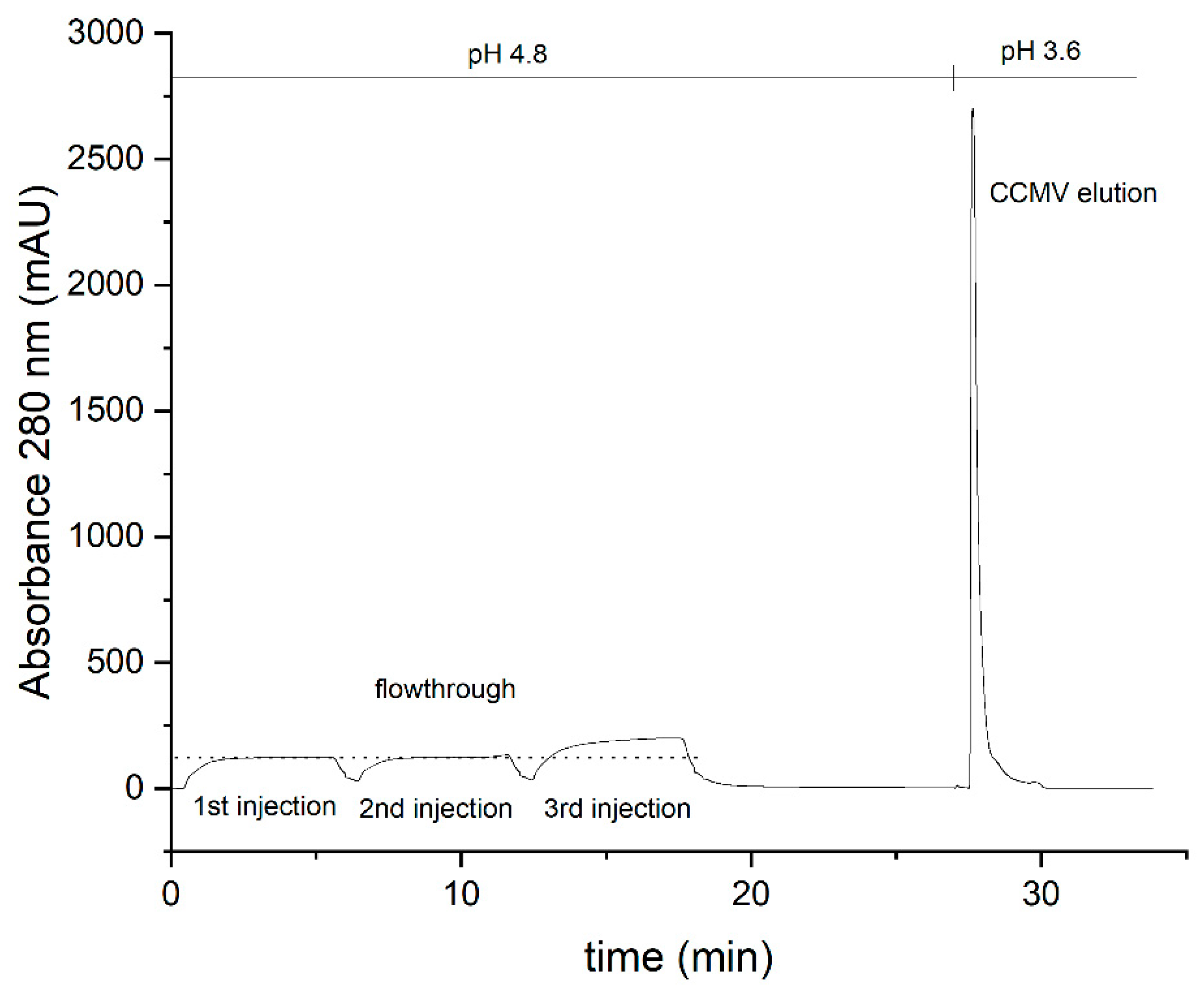
Figure 6.
Column capacity for the binding of CCMV. Three 10 mL injections of a resuspended PEG-precipitate solution containing 0.1 mg/mL CCMV were made in succession. The flowthrough of the 1st and 2nd injection is free of any CCMV as determined with ELISA. It contains impurities from the plant matrix and PEG8000. After the third injection, the column capacity is exceeded, and flowthrough peak is increased, as indicated by the dashed line. The column capacity was determined as approximately 2 mg of CCMV. After washing with binding buffer and elution at pH 3.6, pure CCMV was obtained as a narrow peak. The eluting CCMV is immediately neutralized to approximately 50 mM sodium acetate pH 4.8 with a small volume of concentrated neutralization buffer present in the fraction collector.
Figure 6.
Column capacity for the binding of CCMV. Three 10 mL injections of a resuspended PEG-precipitate solution containing 0.1 mg/mL CCMV were made in succession. The flowthrough of the 1st and 2nd injection is free of any CCMV as determined with ELISA. It contains impurities from the plant matrix and PEG8000. After the third injection, the column capacity is exceeded, and flowthrough peak is increased, as indicated by the dashed line. The column capacity was determined as approximately 2 mg of CCMV. After washing with binding buffer and elution at pH 3.6, pure CCMV was obtained as a narrow peak. The eluting CCMV is immediately neutralized to approximately 50 mM sodium acetate pH 4.8 with a small volume of concentrated neutralization buffer present in the fraction collector.
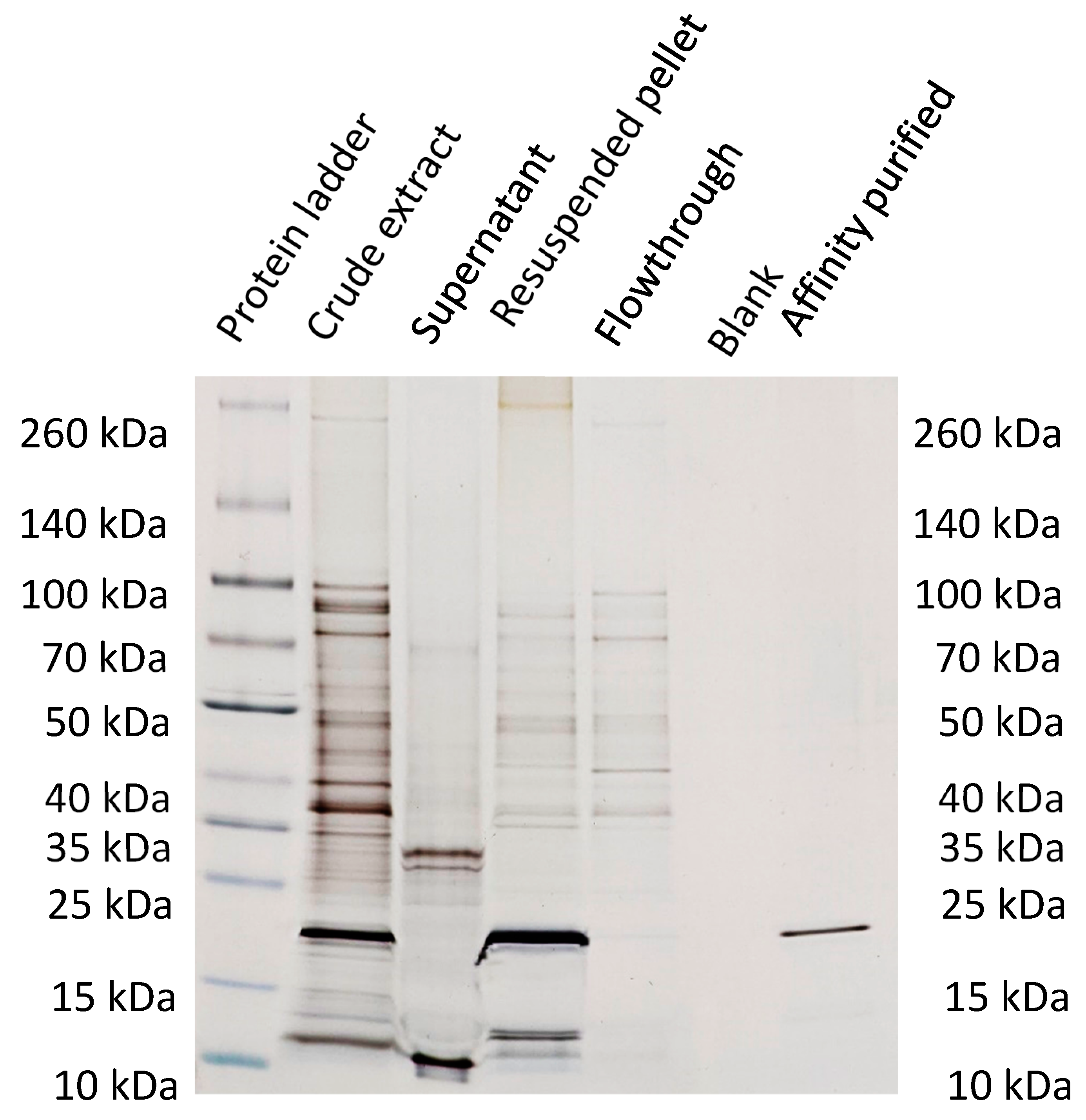
Figure 8.
Silver-stained SDS-PAGE analysis of each purification step and the final eluate. Lane 1: Protein ladder; Lane 2: Crude extract; Lane 3: Supernatant after precipitation with PEG 8000; Lane 4: Supernatant of resuspended pellet; Lane 5: Flowthrough of affinity column; Lane 6: Binding buffer as blank; Lane 7: CCMV eluate obtained by affinity purification (final product, diluted to approx. 20 µg/mL).
Figure 8.
Silver-stained SDS-PAGE analysis of each purification step and the final eluate. Lane 1: Protein ladder; Lane 2: Crude extract; Lane 3: Supernatant after precipitation with PEG 8000; Lane 4: Supernatant of resuspended pellet; Lane 5: Flowthrough of affinity column; Lane 6: Binding buffer as blank; Lane 7: CCMV eluate obtained by affinity purification (final product, diluted to approx. 20 µg/mL).
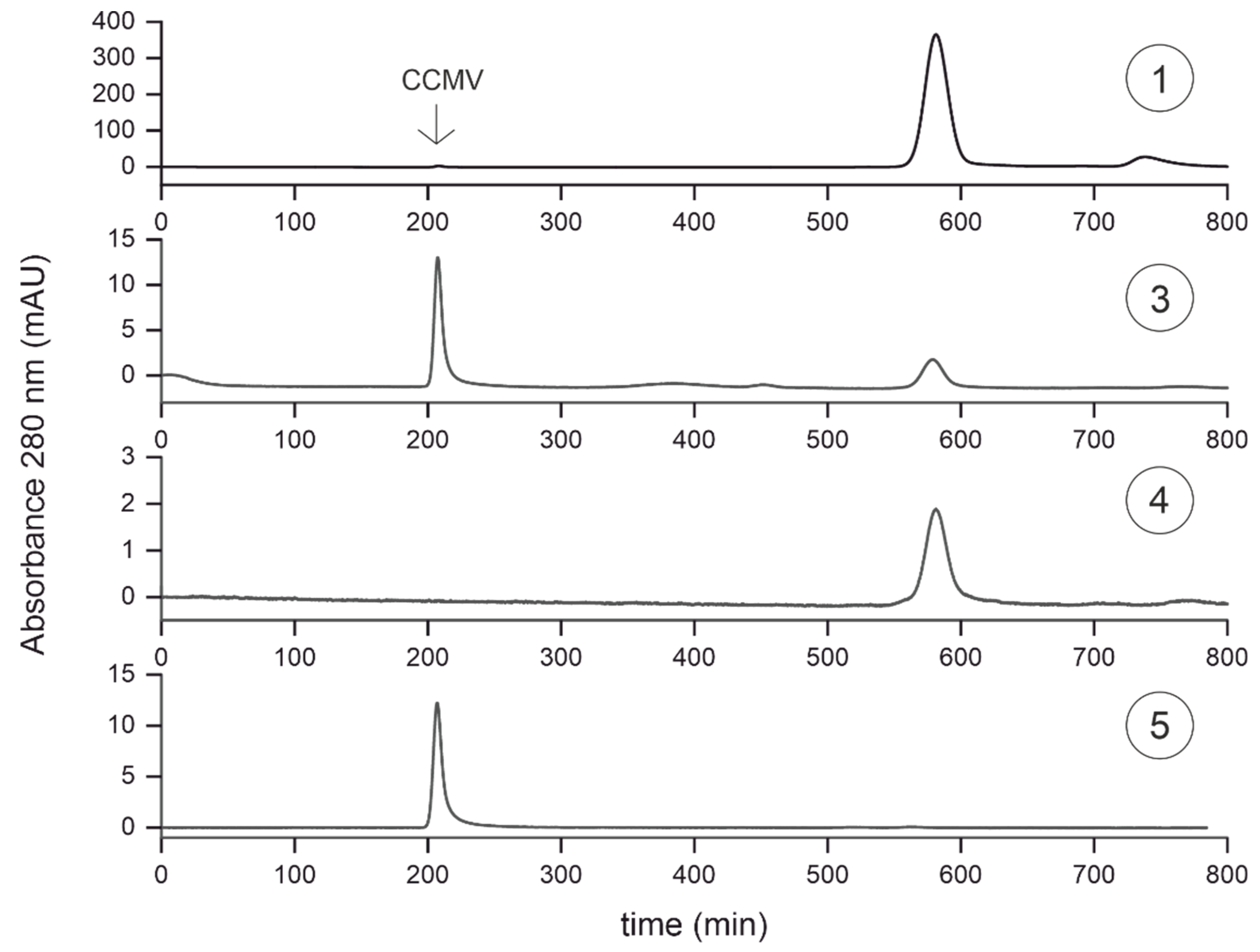
Figure 9.
Size exclusion chromatograms (SEC) of samples taken at different steps of the purification protocol are shown in
Figure 7: ➀ crude extract, ➂ filtered supernatant after pelleting with PEG 8000, ➃ flowthrough of the affinity extraction, ➄ eluate of the affinity extraction. SEC was performed with a Hi Prep 26/60 Sephacryl S-300-HR, Cytiva (17119601); the running buffer was 0.05 M sodium acetate buffer, 0.15 M NaCl and 1 mM Na
2EDTA, pH 4.8 with a flow rate of 0.5 mL/min. .
Figure 9.
Size exclusion chromatograms (SEC) of samples taken at different steps of the purification protocol are shown in
Figure 7: ➀ crude extract, ➂ filtered supernatant after pelleting with PEG 8000, ➃ flowthrough of the affinity extraction, ➄ eluate of the affinity extraction. SEC was performed with a Hi Prep 26/60 Sephacryl S-300-HR, Cytiva (17119601); the running buffer was 0.05 M sodium acetate buffer, 0.15 M NaCl and 1 mM Na
2EDTA, pH 4.8 with a flow rate of 0.5 mL/min. .
Figure 10.
MALDI-TOF mass spectra of samples taken at different steps of the purification protocol are shown in
Figure 7: ➀ Crude extract, ➂ filtered supernatant after pelleting with PEG 8000, ➃ flowthrough of the affinity extraction, ➄ eluate of the affinity extraction.
Figure 10.
MALDI-TOF mass spectra of samples taken at different steps of the purification protocol are shown in
Figure 7: ➀ Crude extract, ➂ filtered supernatant after pelleting with PEG 8000, ➃ flowthrough of the affinity extraction, ➄ eluate of the affinity extraction.
Figure 11.
Reversed-phase high-performance liquid chromatography (RP-HPLC) of CCMV after purification by affinity chromatography. 9 µg CCMV was injected and subsequently separated using the following gradient: 0-3 minutes 99% A (H2O with 0.2% TFA) and 1% B (ACN with 0.16% TFA), 3-23 minutes 30% A and 70% B, 23-40 minutes 1% A and 99% B at a constant flow rate of 0.8 mL/min.
Figure 11.
Reversed-phase high-performance liquid chromatography (RP-HPLC) of CCMV after purification by affinity chromatography. 9 µg CCMV was injected and subsequently separated using the following gradient: 0-3 minutes 99% A (H2O with 0.2% TFA) and 1% B (ACN with 0.16% TFA), 3-23 minutes 30% A and 70% B, 23-40 minutes 1% A and 99% B at a constant flow rate of 0.8 mL/min.
Figure 12.
Detection of intact CCMV nanoparticles after affinity extraction. Top: Negative staining TEM image showing virions with a diameter of 28 nm, Bottom: AFM image.
Figure 12.
Detection of intact CCMV nanoparticles after affinity extraction. Top: Negative staining TEM image showing virions with a diameter of 28 nm, Bottom: AFM image.
Figure 13.
Calibration curve for the determination of CCMV with a sandwich enzyme-linked immunosorbent assay (ELISA). Error bars correspond to the standard deviation of quadruplicates. LOD and LOQ were calculated to be 0.25 µg/L, and 0.79 µg/L, respectively. No matrix effect was observed by diluting the samples to the working range of this assay (3-200 µg/L).
Figure 13.
Calibration curve for the determination of CCMV with a sandwich enzyme-linked immunosorbent assay (ELISA). Error bars correspond to the standard deviation of quadruplicates. LOD and LOQ were calculated to be 0.25 µg/L, and 0.79 µg/L, respectively. No matrix effect was observed by diluting the samples to the working range of this assay (3-200 µg/L).
Table 1.
The yield of CCMV after each purification step; absolute values in mg, relative values in percent relative to 100% determined for the crude extract. Errors were taken from quadruplicate absorbances, calculating the concentrations from the calibration function and determining their standard deviation.
Table 1.
The yield of CCMV after each purification step; absolute values in mg, relative values in percent relative to 100% determined for the crude extract. Errors were taken from quadruplicate absorbances, calculating the concentrations from the calibration function and determining their standard deviation.
| Purification step |
Absolute yield [mg] |
Relative yield [%] |
| 1 - Crude extract |
3.52 ± 0.27 |
100 (def.) |
| 2 - Supernatant of 1 |
0.040 ± 0.001 |
(1.1) |
| 3 – Resuspended pellet |
2.91 ± 0.04 |
83 |
| 4 – Flowthrough of affinity column |
< 0.001 |
(0) |
| 5 – Eluate of affinity step |
1.57 ± 0.02 |
45 |