Introduction
Due to global environmental difficulties and the depletion of fossil resources, sustainable energy generation is one of the most important tasks of this century [
1]. Hydrogen is a viable alternative energy carrier because of its stability, abundance on Earth’s surface, and lack of greenhouse gas emissions [
2,
3,
4,
5,
6,
7]. Due to its renewability, the use of solar energy to make hydrogen is an appropriate technique for meeting the energy demand [
4,
8,
9,
10,
11,
12,
13]. Photoreforming of organics in the aqueous phase [
14,
15,
16] is an emerging technique able to combine wastewater treatment and energy production. The creation of visible–light active and stable photocatalytic/photoelectrocatalytic materials is now the greatest obstacle for many researchers, despite a large number of photocatalysts suggested for hydrogen production [
17,
18,
19]. Among the techniques to improve the photocatalytic activity of the photocatalysts, many researchers have proposed the combination of semiconductors, such as TiO
2, with other materials capable of extending the visible light absorption to the visible range [
20,
21,
22,
23]. The heterojunction formation by combining TiO
2 and Cu
2O is a viable route to improve solar light utilization [
24,
25,
26,
27]. Among the different techniques reported in literature findings to prepare Cu
2O/TiO
2 composite material, a simple and industrially practicable ball milling procedure to dryness was selected as an alternate strategy for preparing photocatalysts in our recent study [
28]. The results of the previous study allowed the selection of the best photocatalyst for hydrogen generation prepared by mixing Cu
2O and TiO
2 (i.e., 1%wt. Cu
2O in TiO
2 photocatalyst material prepared through ball milling method at a rotation rate of 200 rpm and milling time of 1 min). To optimize the photocatalytic hydrogen production in presence of the best-performing photocatalyst, the effect of the following variables on the hydrogen production is herein investigated:
- ⮚
Concentration of the sacrificial species;
- ⮚
Type of sacrificial species used;
- ⮚
Temperature and pH of the system;
- ⮚
Photoefficiency under direct sunlight irradiation.
The quantum yield and the light-to-chemical energy are estimated under the optimal conditions seen based on the data.
Material and Methods
1.1. Preparation of the Photocatalyst
The synthesis of the photocatalyst is performed by following the procedure reported by Muscetta et al. [
28], in which the catalyst is completely characterized. A fixed amount of TiO
2 (1500 mg) is mixed in the agate milling tank (PM100, RETSCH), with agate balls and a fixed amount of Cu
2O (150 mg), fixing the rotation rate to 200 rpm and the milling time to 1 minute. In the following sections, the photocatalytic material used will be named (1%wt) Cu
2O/TiO
2.
1.2. Photocatalytic Tests and Analytical Determination
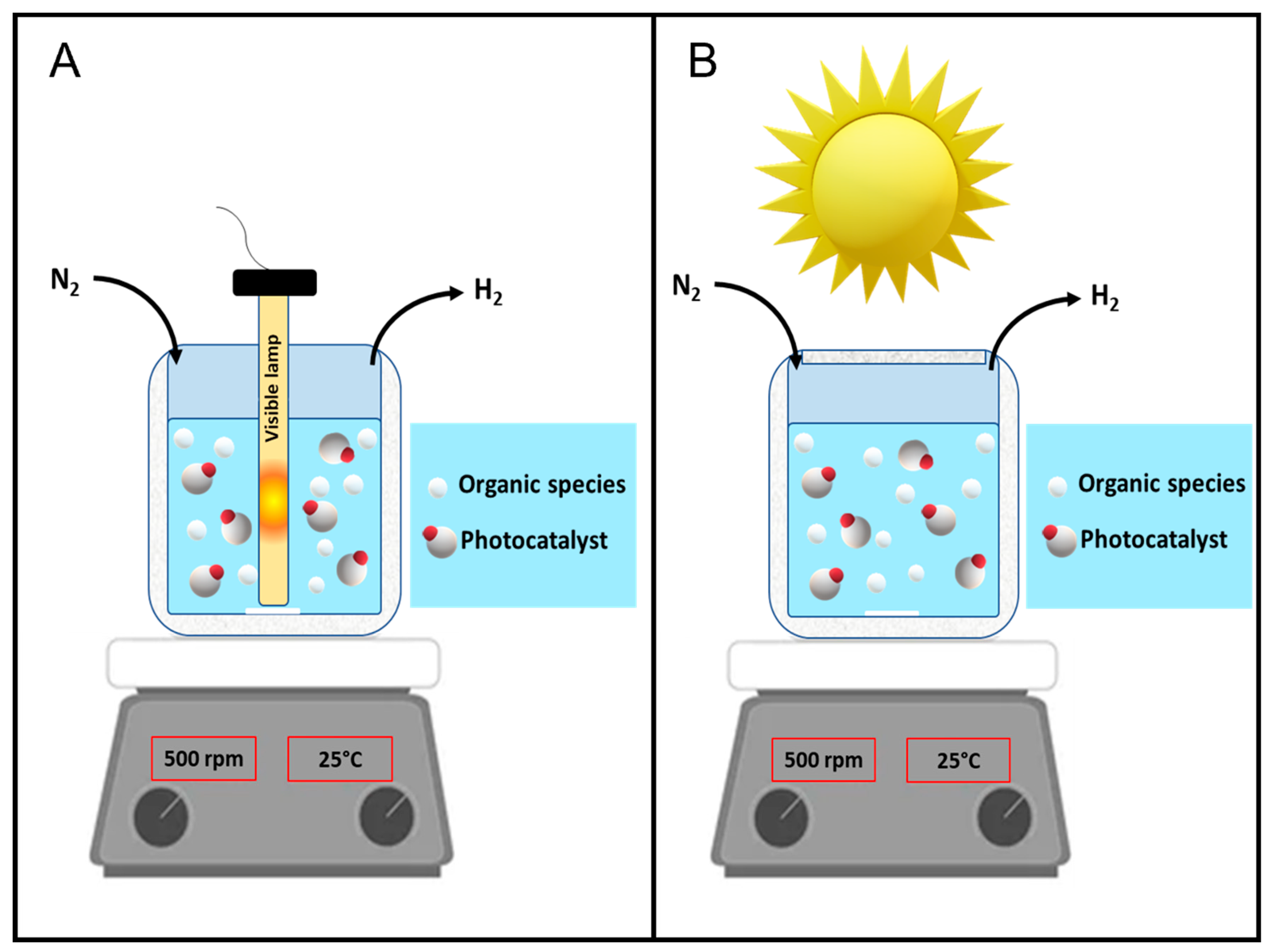
All the materials used in the present investigation are reported in the Supplementary materials. Photocatalytic tests are performed as in [
28] (see
Figure 1A,B for the schematic illustration of the experimental setup). The Supplemental materials also include a full explanation of the reactor. A UV cutoff solution (NaNO
2 0.5 M) is pumped through the quartz sleeve to allow the investigation of the photocatalytic activity under visible light conditions (λ>400 nm), as reported by others [
29]. To perform a typical experimental run, 210 mg of Cu
2O-TiO
2catalyst are suspended in a doubly distilled aqueous solution (V=0.3 L), at fixed pH and concentration of the sacrificial species (0 ÷ 2.5 M). A N
2 stream is bubbled into the solution starting 40 minutes before inserting the photocatalyst to prevent the interaction between dissolved oxygen and copper species or photogenerated electrons. The effect of the temperature was assessed by increasing the temperature of the system up to 80°C (at this temperature a condenser was located on the reactor to prevent the evaporation of the organic species). To test the effect of pH, in some runs the solution pH is corrected to selected values by adding a diluted solution of KOH or HClO
4.
The suspension, containing a fixed concentration of the sacrificial agent (2.5 M) and a fixed amount of the photocatalytic material (700 ppm), was exposed to solar radiation (See the
Figure 1B for the schematic illustration of the experimental set – up) to evaluate the photoefficiency of the system under solar light irradiation in selected runs. These runs were carried out in early September 2021 in Naples (40° 50′ 0″ N, 14° 15′ 0″ E), between the hours 11.00 and 13.00 under clear sky conditions. To evaluate the separate contribution of the UV and visible radiation ranges in hydrogen production, some photocatalytic runs are carried out by employing a cut-off filter to eliminate radiations with wavelengths less than 400 nm.
Figure 1.
Schematic illustration of the annular batch reactor experimental set-up for the experiments conducted (A) under artificial UV and/or Visible light source and (B) under solar irradiation.
Figure 1.
Schematic illustration of the annular batch reactor experimental set-up for the experiments conducted (A) under artificial UV and/or Visible light source and (B) under solar irradiation.
Hydrogen estimation is carried out as reported elsewhere [
28,
30] The pH of the solution is measured using an Orion 420 p pH–meter (Thermo).
Results and Discussions
Effect of Organic Concentration
The effect of the different parameters affecting the preparation stage of the photocatalyst in the presence of methanol was previously discussed [
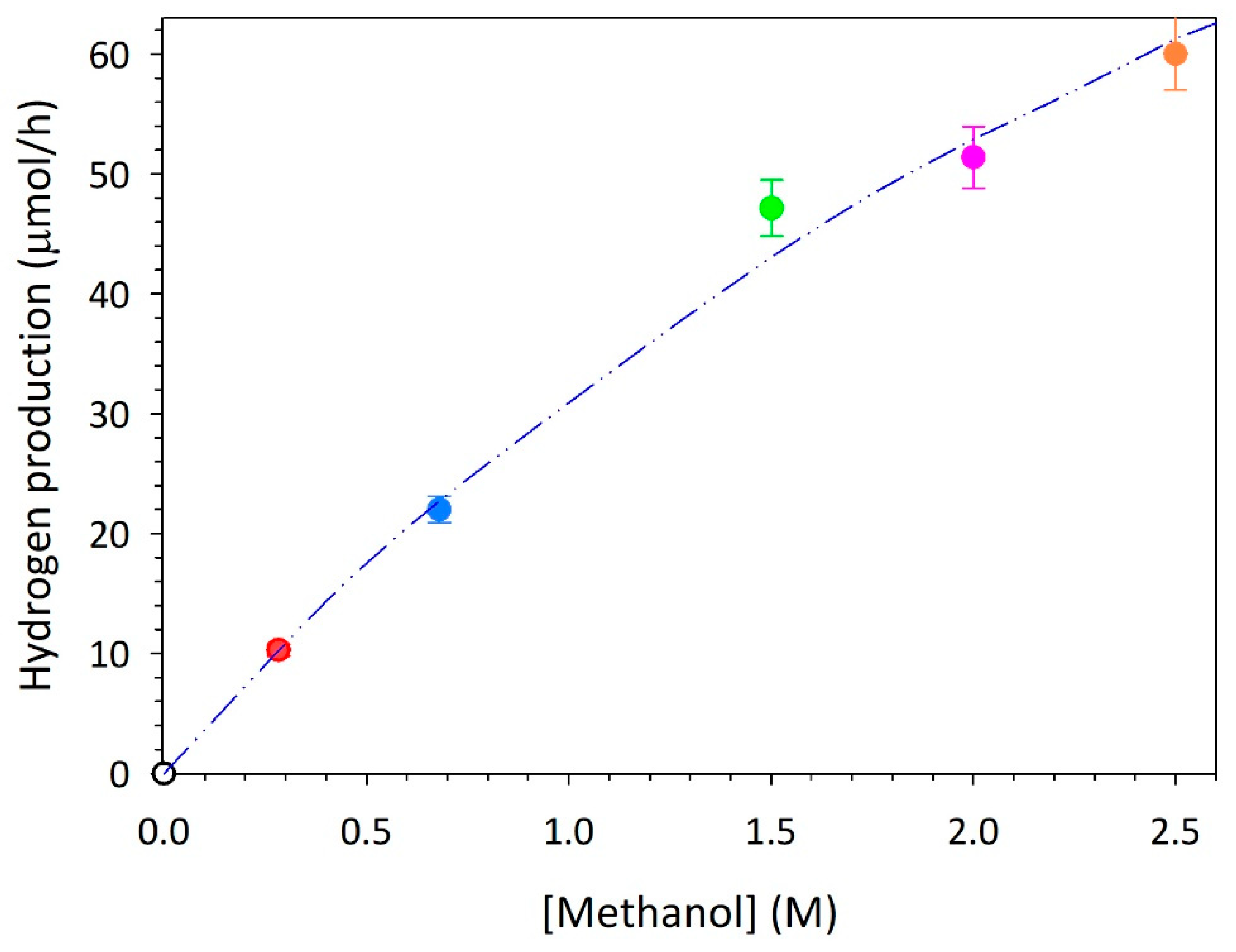
28]. The influence of methanol concentration on the photocatalytic generation of hydrogen is herein explored. The main results are reported in
Figure 2. The reaction rate observed during the photocatalytic runs at varying organic concentrations is properly described by a Langmuir–Hinshelwood model. The adsorbed species concentration may be thus calculated through the equilibrium reaction r.1 and the equation eq.1, in which K
ads (M
-1) is the adsorption equilibrium constant and
is the concentration of free active sites on the catalyst surface:
To obtain a value of the adsorption constant (K
ads) for methanol over the Cu
2O/TiO
2 composite photocatalyst, the following Langmuir–Hinshelwood–type model describing hydrogen generation rate is adopted (eq.2):
where
=
(
being the kinetic constant) and
are the apparent kinetic constant of substrate oxidation and the organic adsorption constant, respectively.
(M) is the concentration of total active site on the catalyst surface for a fixed catalyst load q(g/L). The term
is calculated through the following equation (eq. 3):
where N is the total moles of active sites per unit mass of catalyst (mol/g).
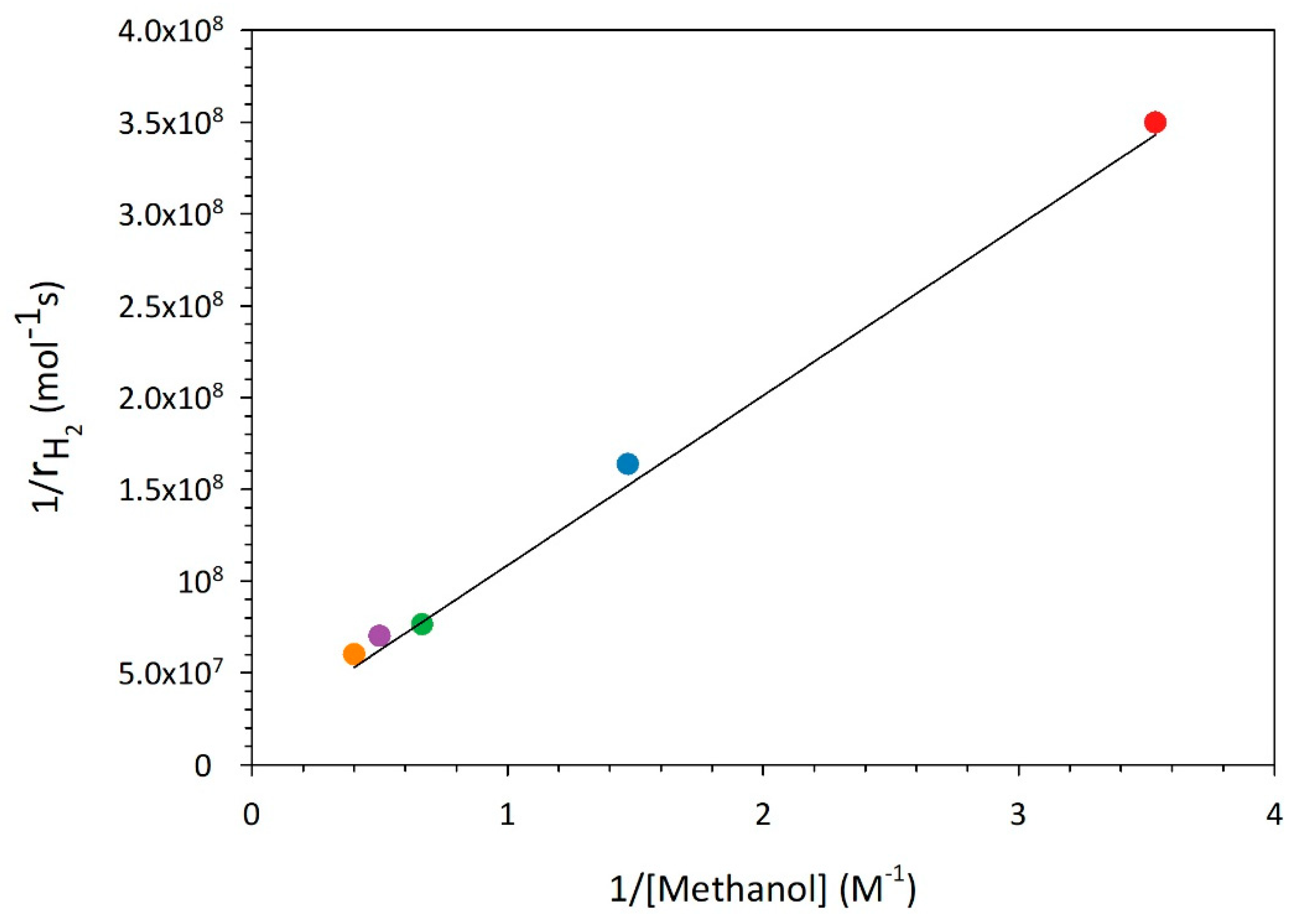
Starting from eq.3 and plotting the reciprocal of the hydrogen reaction rate versus the reciprocal of the organic concentration (
Figure 3), a linear trend is observed, from which it was possible to obtain a suitable value for
. By following the optimization procedure reported elsewhere [
30], a value of 0.22 M
-1 is obtained in presence of the best photocatalyst tested when methanol is used as a scavenger. This value resulted in accordance with those reported in literature for similar systems in presence of the same organic compound [
31].
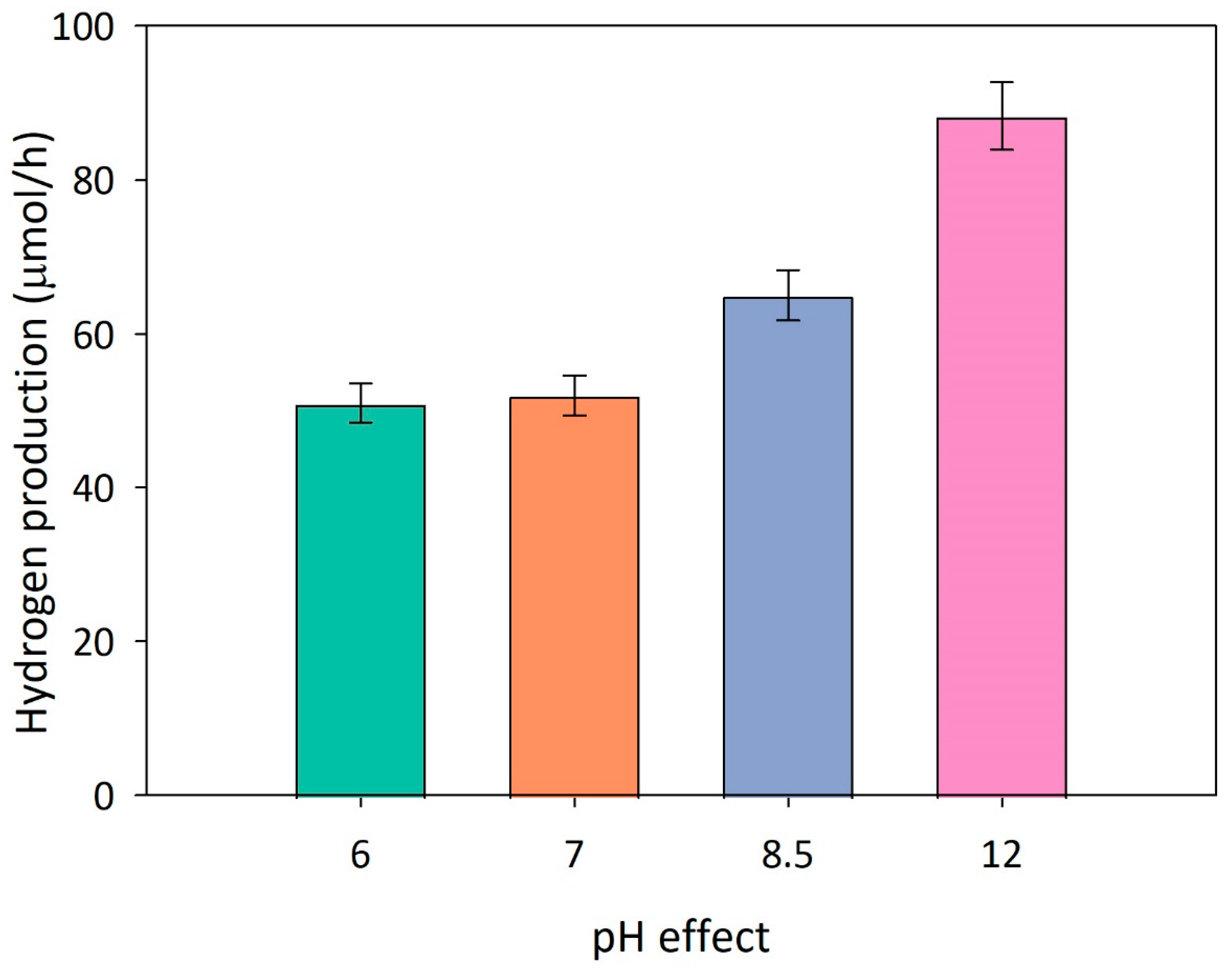
Effect of pH
Some authors have reported a considerable effect of the suspension pH on the photocatalytic hydrogen generation for different photocatalytic materials and scavengers [
27,
30,
32,
33]. To evaluate the effect of this variable on the photoactivity, several runs are carried out at varying the solution pH.
Figure 4 reports the mean hydrogen production rate collected at different pH values. A higher reactivity may be recognized under alkaline conditions, whereas negligible activity is recorded at acidic conditions (data not shown). Despite the great number of studies in the literature survey focusing on the effect of pH, only few authors have attempted to explain the correlation between solution pH and photocatalytic activity [
30,
34,
35,
36]. The surface properties of the photocatalyst, its stability in the aqueous solution, the shift of the band gap, and the molecular properties of the sacrificial agent should be considered to understand the phenomenon.
The following remarks should be considered to underpin the present photocatalytic outcomes:
- (i)
The stability of the copper species on the surface of TiO
2 particles is affected by the pH. In particular, lower stability is recorded by several authors under acidic conditions [
34,
37,
38].
- (ii)
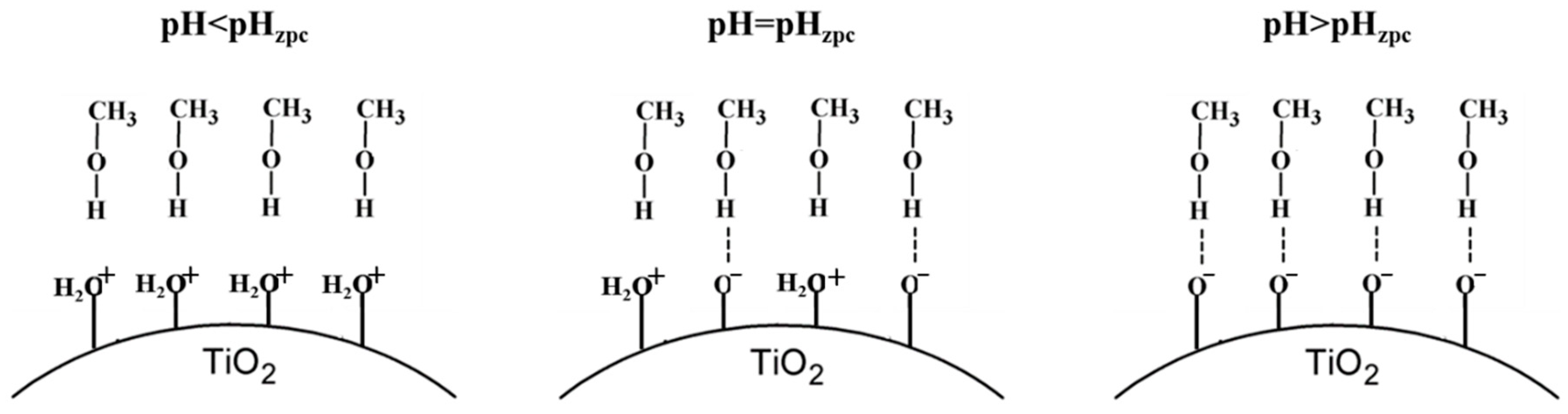
About Methanol (i.e., the sacrificial agent), has a pK
a value of 15.0 [
39]. Thus, methanol is present in non-dissociated form for all the pH values tested (See the
Figure 5).
- (iii)
The pH
zpc of TiO
2 is about 6.25 [
40], Thus, a high concentration of negative charges is recorded on the catalyst surface under alkaline conditions (See the
Figure 5) able to promote the interaction between substrate and catalyst surface.
- (iv)
Negative charges on the catalyst surface (under alkaline conditions) reduce particle agglomeration [
41].
Figure 4.
Hydrogen evolution rate at varying the pH of the suspension. Experimental conditions: V=0.30 L; P=1 atm; T=35°C; [Methanol] = 2.5 M; C(1%wt.)Cu2O/TiO2 = 700 ppm; visible range.
Figure 4.
Hydrogen evolution rate at varying the pH of the suspension. Experimental conditions: V=0.30 L; P=1 atm; T=35°C; [Methanol] = 2.5 M; C(1%wt.)Cu2O/TiO2 = 700 ppm; visible range.
Figure 5.
Schematic illustration of adsorption conditions of methanol on the photocatalytic Titania surface at different pH values.
Figure 5.
Schematic illustration of adsorption conditions of methanol on the photocatalytic Titania surface at different pH values.
Based on the above considerations, the greater photocatalytic activity in alkaline conditions may be attributed to (i) the presence of stable copper species involved in visible light-induced hydrogen generation and (ii) the more efficient adsorption of the organic compounds.
Effect of Temperature
Photocatalytic processes are generally reported to be poorly influenced by the temperature, as the electron-hole generation mainly depends on the radiation intensity [
42]. However, the temperature may improve the activity of the system, being able to (
i) increase the reaction rate of the hydrogen generation and (ii) improve the product desorption from the catalyst [
43]. Thus, temperature helps hydrogen generation reaction compete more effectively with charge carries recombination, as reported by different authors [
44,
45,
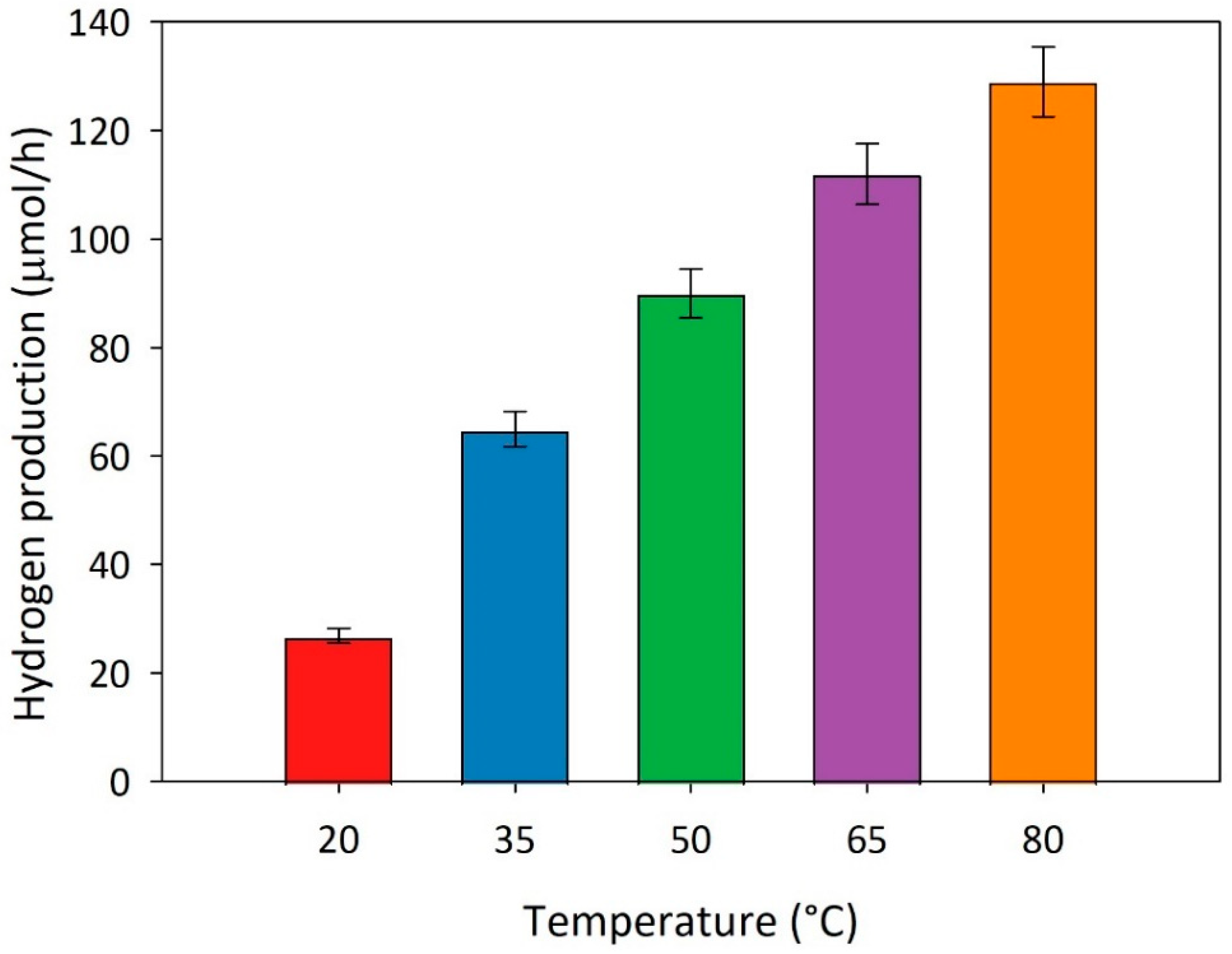
46]. For this reason, some experimental runs are performed varying the temperature of the suspension between 20°C and 80°C, in presence of methanol as a scavenger. The main results are reported in
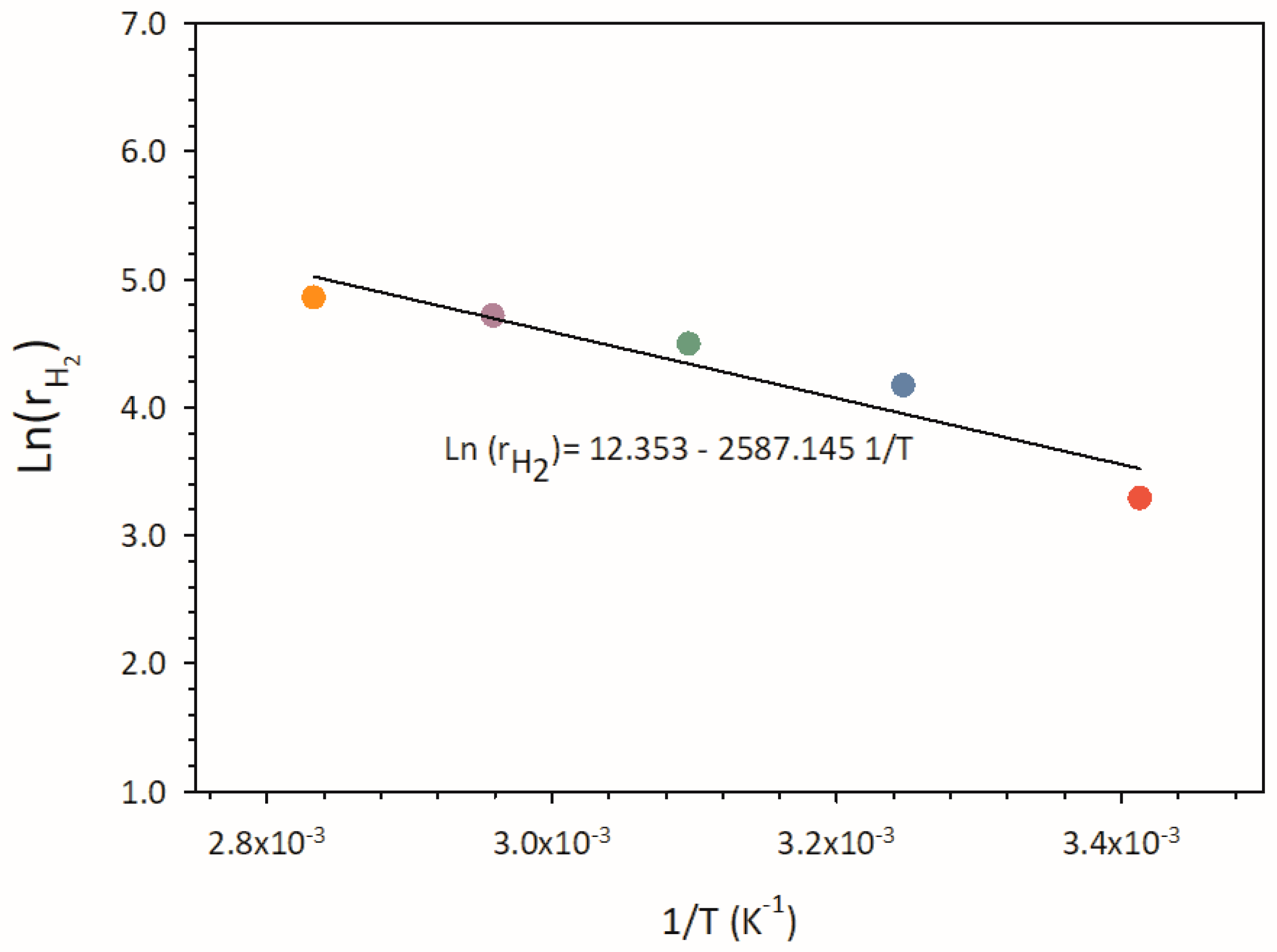
Figure 6. As clearly sown by the diagram, when the temperature increases from 20°C to 80°C, the hydrogen production rate achieves a value about 4.5 times higher than that obtained at the lowest temperature, thus proving the beneficial effect of temperature on the system behaviour.
By considering that the photocatalytic hydrogen production follows a pseudo-first-order law for all the temperatures tested, it is possible to plot the Arrhenius-type equation from 293 K to 353 K (See
Figure 7). A value of 21.51 KJ/mol is easily estimated for the apparent activation energy E
a from the slope of the Arrhenius plot. Such a value is consistent with those reported in the literature survey for similar systems [
46,
47].
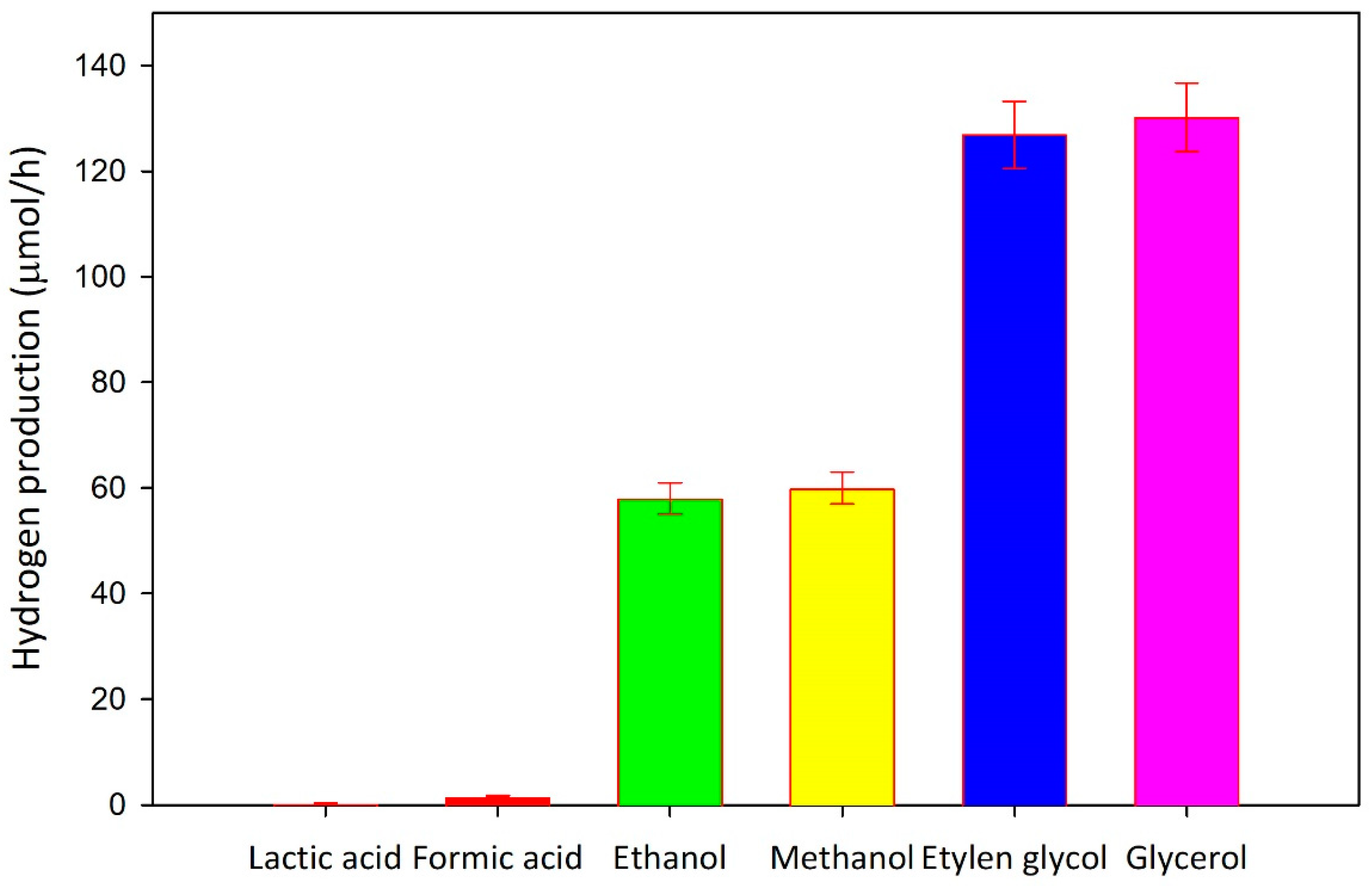
Effect of the Sacrificial Agent
Many researchers proposed hydrogen production through photoreforming of organics in aqueous phase [
14,
15,
16]. Organic species may derive from renewable sources or organic-rich effluents [
48,
49,
50]. Due to (i) the possibility of linking hydrogen generation with water treatment and (ii) the cheap cost of the feedstock, the potential use of organic contaminants found in wastewater is particularly appealing [
48,
51,
52,
53]. Evaluating the effect of the scavenger used on photocatalytic hydrogen production is thus necessary. To this aim, some experimental runs are carried out at varying the sacrificial species (i.e., alcohols or carboxylic acids). The main results collected during this investigation are reported in the
Figure 8. Glycerol and 1,2–Ethandiol result the greatest options for hydrogen generation in presence of Cu
2O/TiO
2 photocatalyst, according with literature findings in which Cu-based TiO
2 was used to generate hydrogen through photoreforming [
37]. On the contrary, no hydrogen production is herein detected in case of pure water and seawater. Both the nucleophilicity of the groups in the molecular structure of the sacrificial agents and the capacity of the organics to adsorb on the catalyst surface affect the reactivity of the organics with photogenerated hole and can be used to explain these findings. The four species exhibiting the higher photoefficiency for hydrogen evolution (i.e., methanol, ethanol, glycerol and 1,2-ethanediol) have similar values of nucleophilicity, as reported the
Table 1. As regards the adsorption phenomenon, the development of the bidentate (and tridentate) complexes can be proposed for glycerol and 1,2-ethanediol. On the other hand, the creation of weaker monodentate complexes can be supposed for ethanol and methanol, according to their structures.
By contrast, the almost negligible photo efficiency for hydrogen evolution recorded in the presence of formic acid and lactic acid can be related to the following phenomena: (i) acidic conditions (See
Table 1) reduce the stability of copper species, as previously discussed; (ii) under acidic conditions, the photocatalyst surface is positively charged, thus exhibiting a weak interaction with the organic compounds. Based on these considerations, glycerol is the best sacrificial agent tested in this study. In addition, a beneficial effect of the temperature on hydrogen evolution is observed also in the case of glycerol. Indeed, when glycerol is used as a scavenger and the system temperature of the system is set at 80°C, the hydrogen generation rate achieves a value of 260 µmol·h
-1.
Table 1.
pKa values of several compounds used in the experiments.
Table 1.
pKa values of several compounds used in the experiments.
| Organic species |
pKa value |
Natural pH of the solution |
Reference |
| Ethanol |
15.9 |
7.0 |
[54] |
| Methanol |
15.3 |
8.5 |
[39] |
| 1,2-ethanediol |
15.1 |
6.0 |
[39] |
| Glycerol |
14.4 |
6.5 |
[39] |
| Lactic acid |
3.86 |
2.0 |
[55] |
| Formic acid |
3.75 |
3.0 |
[54] |
Efficiency Calculation
To easily compare the present photocatalytic outcomes with previous findings in the literature survey, the apparent quantum efficiency (
) and the light–to–chemical energy efficiency (
) are determined from the best photocatalytic data obtained according to the following equations (eq.4 – eq.5):
where:
- -
(mol·s-1) is the hydrogen generation rate;
- -
is the standard change in enthalpy for the combustion of hydrogen and oxygen (282.0 103 J/mol);
- -
I (W·cm-2) is the light source’s specific irradiance;
- -
S () is the illuminated area.
In
Figure 9 the most significant outcomes are reported. When a high–pressure Hg lamp is used, along with a (1%wt) Cu
2O/TiO
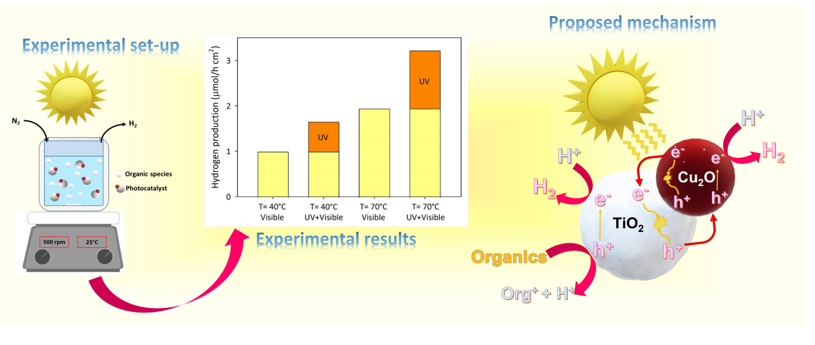
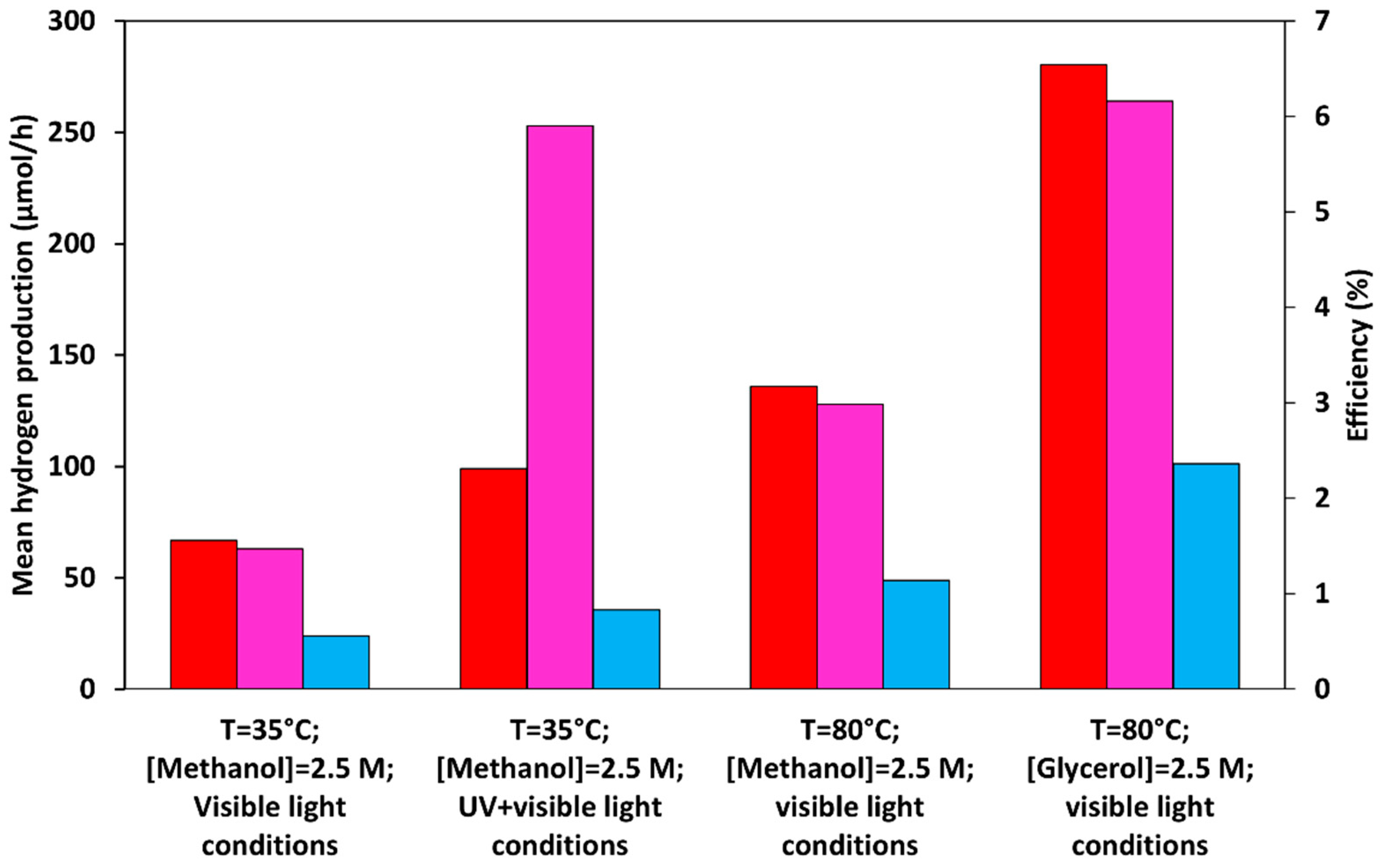
2 composite photocatalyst prepared through the ball milling method (200 rpm, 1 min) at a system temperature of 80°C, maximum AQE values of 3.17% and 6.54% are calculated under visible light radiation in presence of methanol and glycerol, respectively. Under identical circumstances, the maximum light–to–chemical energy efficiencies values of 1.16% and 2.36 % are obtained for methanol and glycerol, respectively.
Additionally, the efficiency values obtained in the visible light spectrum (AQE=1.56%, η=0.56) are only slightly lower than those collected under identical circumstances (T=35°C, methanol as a scavenger) by using UVA+Visible light radiation (AQE=2.31%, η=0.83). In fact, as reported in the figure, despite the huge discrepancy in hydrogen generation in the presence and absence of UVA radiation, the different light intensities induce comparable activities in terms of quantum yield and light–to–chemical energy efficiency.
Figure 9.
Mean hydrogen generation rate (∎), AQE (∎), and (∎) obtained under visible light irradiation (λ>400 nm) and UV-A + Visible light conditions.
Figure 9.
Mean hydrogen generation rate (∎), AQE (∎), and (∎) obtained under visible light irradiation (λ>400 nm) and UV-A + Visible light conditions.
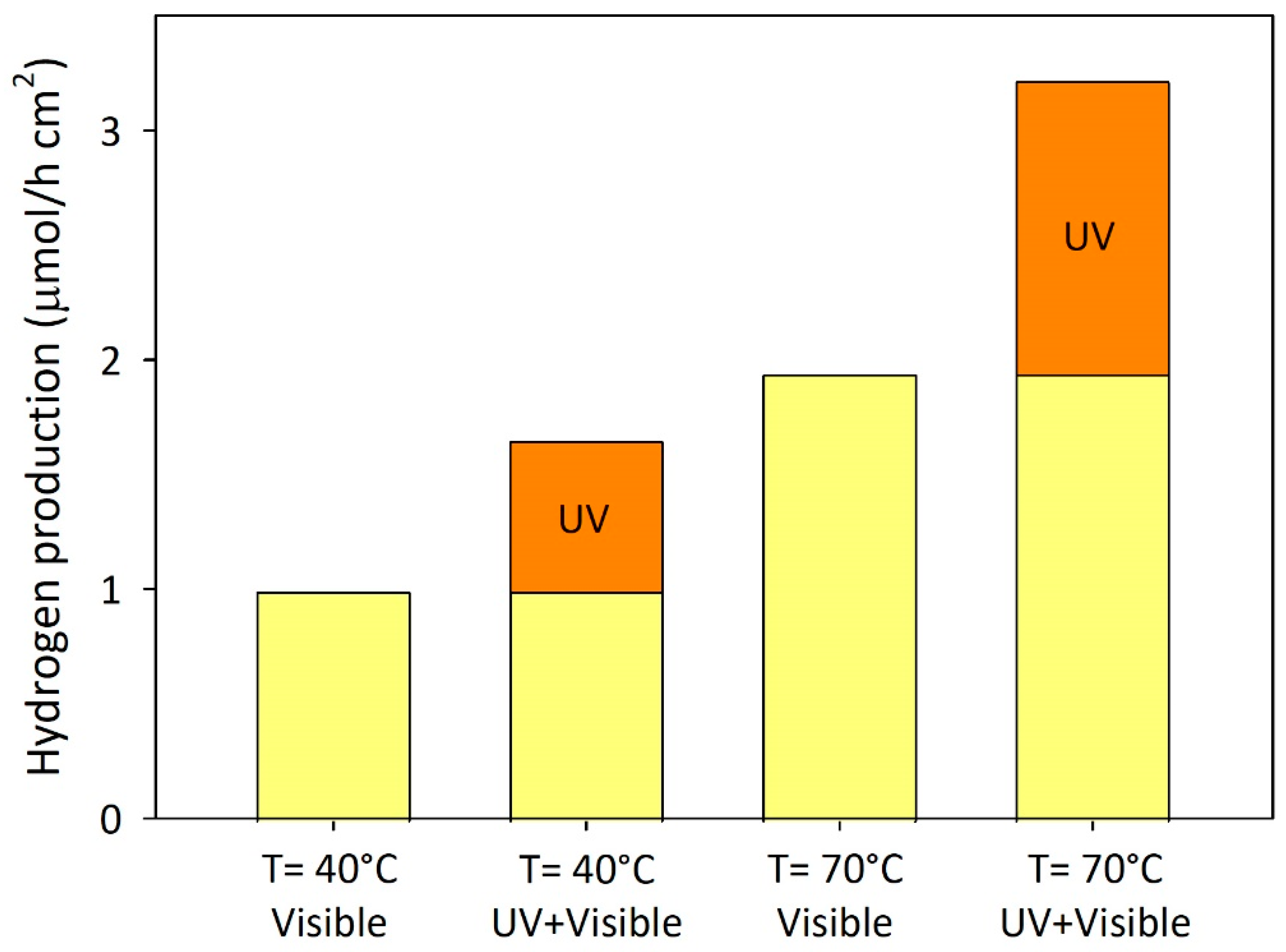
Photocatalytic Activity under Solar Conditions
To conclude the experimental campaign of the present study, a set of experiments are performed under direct solar irradiation by using the apparatus schematically represented in the Material and methods section (
Figure 1B).
Figure 10 reports the main results from the photocatalytic runs conducted under direct solar irradiation.
Hydrogen production rate is about 1 μmol⸱h
-1⸱cm
-2 at the temperature of 40°C and in the presence of visible light irradiation only. Hydrogen generation increases by about 1.6 times when all wavelengths of the solar spectrum are used. The result indicates that the contribution of the visible light portion of the solar spectrum supports about 60% of hydrogen production under sunlight radiation, whereas the improvement in hydrogen generation obtained without any UV cut-off filter maybe ascribed to the UV component. Furthermore, as the temperature rises to 70°C, the hydrogen generation rate increases to around 3 μmol⸱h
-1⸱cm
-2, demonstrating the favourable influence of temperature on photocatalytic activity. The efficiencies of the catalytic system adopted in the present investigation for hydrogen generation are calculated by using the irradiance values data collected during the experimental runs. The ranges of irradiation considered are 315–400 nm and 380–550 nm, where the mean values of the specific irradiances are about 15.56 W·m
-2 and 95.75 W·m
-2, respectively.
Table 2 reports the values of the light to chemical energy efficiency. A value of about 6.5% is obtained considering the contribution of the UV range at a system temperature of 70°C. As regards the light-to-chemical energy efficiency in the solar range, the low calculated values may result from an overestimation of the specific irradiance collected under the visible range (380 – 550 nm). Indeed, wavelengths higher than about 420 nm are completely useless for the activation of the adopted photocatalyst due to its bandgap value (i.e., 3 eV).
Activity under the Best Conditions
Eventually, the light-to-chemical energy efficiency is also estimated under the best conditions recorded in the previous sections. In particular, some photocatalytic experiments are carried out under direct sunlight radiation by fixing (i) the temperature of the system at 80°C, (ii) the pH of the suspension at 12.0, and (iii) glycerol as a scavenger. Under these conditions, η reached a value of 8.15% in the UV range, and 2.81% in the visible light range only.
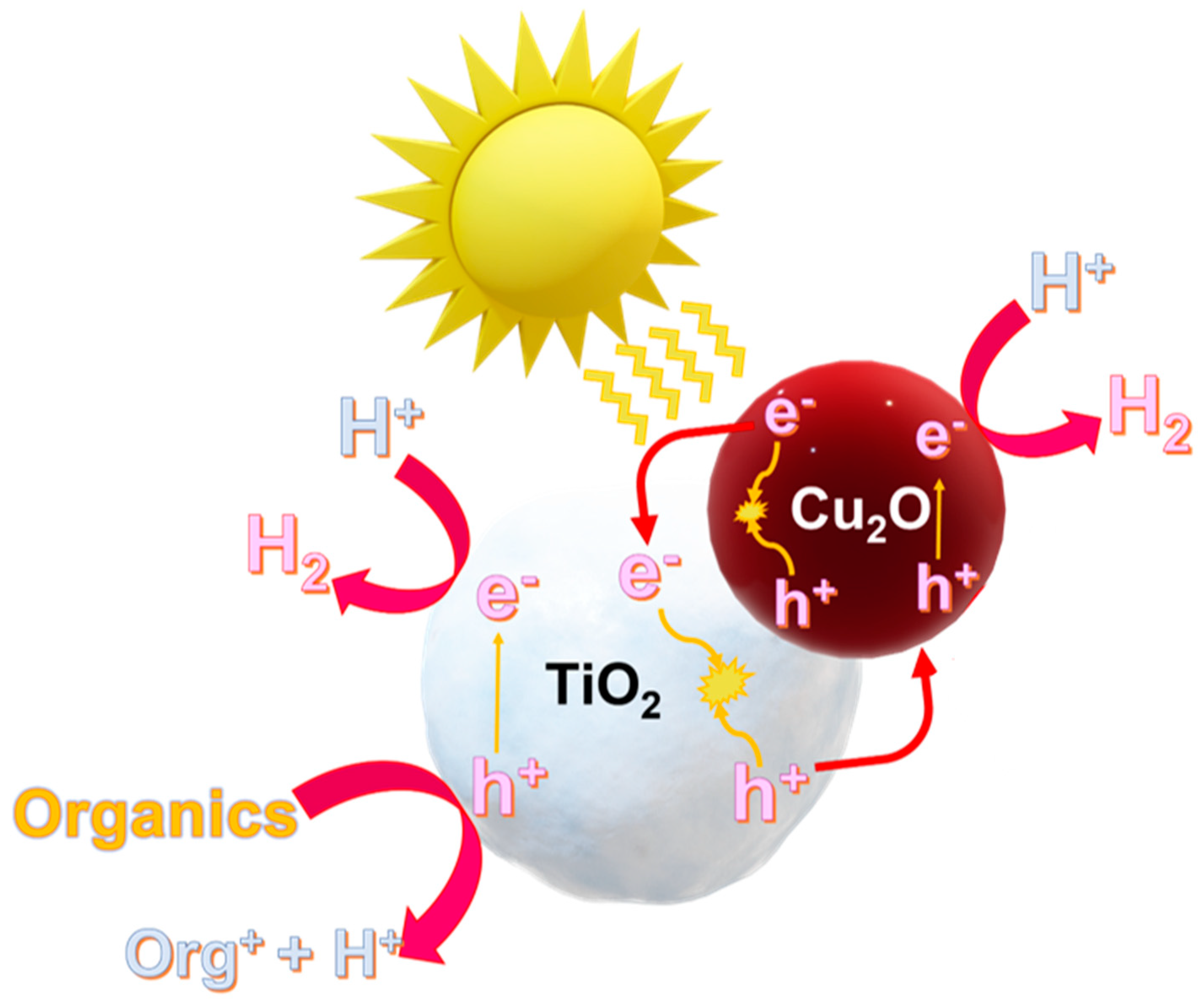
2. Proposed Mechanism under Solar Radiation
An attempt to explain the mechanism of hydrogen generation is herein proposed and discussed (See
Figure 11). As previously reported, cuprous oxide in the p–n heterojunction systems based Cu
2O/TiO
2 photocatalysts acts as an energy antenna [
27], being able to absorb energy within the visible spectrum. Under direct sunlight radiation, in which the UV and the visible components are present, the activation of both semiconductors (i.e., Cu
2O and TiO
2) can be considered (See the reactions r. 2 and r.3):
After charge carrier generation, an electric field from TiO
2 to Cu
2O and band bending are established. Positive holes can react with the adsorbed organic species (mainly on the titania surface), following the reactions r.4 and r.5, thus forming byproducts and protons, which in turn can react with the photogenerated electrons to form
(through the reaction r.6).
Obviously, electron-hole pairs may recombine to produce heat and light (through reactions r.7 and r.8), despite the p-n heterojunction formation boosts photogenerated charge carrier separation, resulting in better photocatalytic efficiencies with respect to those observed with bare materials.
Conclusions
The evaluation of the photocatalytic activity for hydrogen generation of the best performing photocatalyst at varying organic concentration allows the identification of an adsorption equilibrium constant of 0.22 M-1. A beneficial effect effect is recorded with raising the system temperature, with an increase in hydrogen productivity of nearly 4.5 times with respect to the lowest temperature. Such a temperature effect is related to the higher reaction rate of hydrogen generation and the easier desorption of the products from the catalyst. As regards the effect of solution pH, alkaline conditions exert beneficial effect on the photocatalytic hydrogen generation. Under the best conditions tested (i.e., glycerol as scavenger, pH=12.0, T=80°C, and direct sunlight irradiation), a value of light-to-chemical energy efficiency of 8% in the UV range is observed. Therefore, the simple photocatalytic system is suited to the visible light driven hydrogen generation. Despite the good performances detected under visible light irradiation only, the UV component in the solar spectrum is able to rise by 40% the photocatalytic hydrogen generation with respect to the visible light irradiation only.
References
- Ford, A.; Gillich, A.; Mirzania, P. Sustainable Energy and Energy Ef Fi Cient Technologies. 2020, 611–630. [CrossRef]
- Li, X.; Liu, B.; Chen, Y.; Fan, X.; Li, Y.; Zhang, F.; Zhang, G.; Peng, W. Decoration of Cu2O Photocathode with Protective TiO2 and Active WS2 Layers for Enhanced Photoelectrochemical Hydrogen Evolution. Nanotechnology 2018, 29. [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen Production from Renewable and Sustainable Energy Resources: Promising Green Energy Carrier for Clean Development. Renewable and Sustainable Energy Reviews 2016, 57, 850–866. [CrossRef]
- Hosseini, S.E. Hydrogen from Solar Energy , a Clean Energy Carrier from a Sustainable Source of Energy. 2019, 1–22. [CrossRef]
- Møller, K.T.; Jensen, T.R.; Akiba, E. Progress in Natural Science : Materials International Hydrogen - A Sustainable Energy Carrier. Progress in Natural Science: Materials International 2017, 27, 34–40. [CrossRef]
- Policastro, G.; Cesaro, A.; Fabbricino, M. Photo-Fermentative Hydrogen Production from Cheese Whey: Engineering of a Mixed Culture Process in a Semi-Continuous, Tubular Photo-Bioreactor. Int J Hydrogen Energy 2022. [CrossRef]
- Policastro, G.; Carraturo, F.; Compagnone, M.; Guida, M.; Fabbricino, M. Enhancing Hydrogen Production from Winery Wastewater through Fermentative Microbial Culture Selection. Bioresour Technol Rep 2022, 19. [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl Catal B 2019, 244, 1021–1064. [CrossRef]
- Clarizia, L. Hydrogen Production through Photoreforming of Oxygenated Organic Substrates over Cu/TiO2 Catalysts. 2017, 1–154.
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M.; Shen, S.; Liu, G.; Hu, X.; Zhang, X.; et al. Efficient Solar Hydrogen Production by Photocatalytic Water Splitting: From Fundamental Study to Pilot Demonstration. Int J Hydrogen Energy 2010, 35, 7087–7097. [CrossRef]
- Gong, J.; Li, C.; Wasielewski, M.R. Advances in Solar Energy Conversion. Chem Soc Rev 2019, 48, 1862–1864. [CrossRef]
- Kar, S.K.; Sharma, A.; Roy, B. Solar Energy Market Developments in India. Renewable and Sustainable Energy Reviews 2016, 62, 121–133. [CrossRef]
- Shaner, M.R.; Atwater, H.A.; Lewis, N.S.; McFarland, E.W. A Comparative Technoeconomic Analysis of Renewable Hydrogen Production Using Solar Energy. Energy Environ Sci 2016, 9, 2354–2371. [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [CrossRef]
- Lucchetti, R.; Onotri, L.; Clarizia, L.; Natale, F. di; Somma, I. di; Andreozzi, R.; Marotta, R. Removal of Nitrate and Simultaneous Hydrogen Generation through Photocatalytic Reforming of Glycerol over “in Situ” Prepared Zero-Valent Nano Copper/P25. Appl Catal B 2017, 202, 539–549. [CrossRef]
- Miwa, T.; Kaneco, S.; Katsumata, H.; Suzuki, T.; Ohta, K.; Chand Verma, S.; Sugihara, K. Photocatalytic Hydrogen Production from Aqueous Methanol Solution with CuO/Al2O3/TiO2 Nanocomposite. Int J Hydrogen Energy 2010, 35, 6554–6560. [CrossRef]
- Yang, G.; Jiang, Z.; Shi, H.; Xiao, T.; Yan, Z. Preparation of Highly Visible-Light Active N-Doped TiO2 Photocatalyst. J Mater Chem 2010, 20, 5301–5309. [CrossRef]
- Rehman, S.; Ullah, R.; Butt, A.M.; Gohar, N.D. Strategies of Making TiO2 and ZnO Visible Light Active. J Hazard Mater 2009, 170, 560–569. [CrossRef]
- Xu, S.; Feng, D.; Shangguan, W. Preparations and Photocatalytic Properties of Visible-Light-Active Zinc Ferrite-Doped TiO2 Photocatalyst. The Journal of Physical Chemistry C 2009, 113, 2463–2467. [CrossRef]
- Al-Azri, Z.H.N.; Chen, W.T.; Chan, A.; Jovic, V.; Ina, T.; Idriss, H.; Waterhouse, G.I.N. The Roles of Metal Co-Catalysts and Reaction Media in Photocatalytic Hydrogen Production: Performance Evaluation of M/TiO2 Photocatalysts (M = Pd, Pt, Au) in Different Alcohol-Water Mixtures. J Catal 2015, 329, 355–367. [CrossRef]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl Catal B 2019, 244, 1021–1064. [CrossRef]
- Humayun, M.; Raziq, F.; Khan, A.; Luo, W. Modification Strategies of TiO2 for Potential Applications in Photocatalysis: A Critical Review. Green Chem Lett Rev 2018, 11, 86–102. [CrossRef]
- Muscetta, M.; Russo, D. Photocatalytic Applications in Wastewater and Air Treatment: A Patent Review (2010–2020). Catalysts 2021, 11.
- Niu, W.; Moehl, T.; Cui, W.; Wick-Joliat, R.; Zhu, L.; Tilley, S.D. Extended Light Harvesting with Dual Cu2O-Based Photocathodes for High Efficiency Water Splitting. Adv Energy Mater 2018, 8, 1–8. [CrossRef]
- Yan, L.; Yang, F.; Tao, C.Y.; Luo, X.; Zhang, L. Highly Efficient and Stable Cu2O–TiO2 Intermediate Photocatalytic Water Splitting. Ceram Int 2020, 46, 9455–9463. [CrossRef]
- Le, L.; Wu, Y.; Zhou, Z.; Wang, H.; Xiong, R.; Shi, J. Cu2O Clusters Decorated on Flower-like TiO2 Nanorod Array Film for Enhanced Hydrogen Production under Solar Light Irradiation. J Photochem Photobiol A Chem 2018, 351, 78–86. [CrossRef]
- Muscetta, M.; Andreozzi, R.; Clarizia, L.; di Somma, I.; Marotta, R. Hydrogen Production through Photoreforming Processes over Cu2O/TiO2 Composite Materials: A Mini-Review. Int J Hydrogen Energy 2020. [CrossRef]
- Muscetta, M.; Jitan, S. al; Palmisano, G.; Andreozzi, R.; Marotta, R.; Cimino, S.; di Somma, I. Visible Light – Driven Photocatalytic Hydrogen Production Using Cu2O/TiO2 Composites Prepared by Facile Mechanochemical Synthesis. J Environ Chem Eng 2022, 10, 107735. [CrossRef]
- Cheng, P.; Li, W.; Zhou, T.; Jin, Y.; Gu, M. Physical and Photocatalytic Properties of Zinc Ferrite Doped Titania under Visible Light Irradiation. J Photochem Photobiol A Chem 2004, 168, 97–101. [CrossRef]
- Muscetta, M.; Clarizia, L.; Garlisi, C.; Palmisano, G.; Marotta, R.; Andreozzi, R.; Ii, F.; Chimica, I.; Industriale, P. Hydrogen Production upon UV-Light Irradiation of Cu / TiO 2 Photocatalyst in the Presence of Alkanol- Amines. Int J Hydrogen Energy 2020. [CrossRef]
- Clarizia, L.; Somma, I. Di; Onotri, L.; Andreozzi, R.; Marotta, R. Kinetic Modeling of Hydrogen Generation over Nano-Cu(s)/TiO2 Catalyst through Photoreforming of Alcohols. Catal Today 2017, 281, 117–123. [CrossRef]
- Badawy, M.I.; Ghaly, M.Y.; Ali, M.E.M. Photocatalytic Hydrogen Production over Nanostructured Mesoporous Titania from Olive Mill Wastewater. Desalination 2011, 267, 250–255. [CrossRef]
- Lin, W.C.; Yang, W.D.; Huang, I.L.; Wu, T.S.; Chung, Z.J. Hydrogen Production from Methanol/Water Photocatalytic Decomposition Using Pt/Tio2-Xnx Catalyst. Energy and Fuels 2009, 23, 2192–2196. [CrossRef]
- Wu, Y.; Lu, G.; Li, S. The Role of Cu(I) Species for Photocatalytic Hydrogen Generation over CuO x /TiO2. Catal Letters 2009, 133, 97–105. [CrossRef]
- Karimi Estahbanati, M.R.; Mahinpey, N.; Feilizadeh, M.; Attar, F.; Iliuta, M.C. Kinetic Study of the Effects of PH on the Photocatalytic Hydrogen Production from Alcohols. Int J Hydrogen Energy 2019, 44, 32030–32041. [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive Review and Future Perspectives on the Photocatalytic Hydrogen Production. Journal of Chemical Technology and Biotechnology 2019, 94, 3049–3063.
- Clarizia, L.; Spasiano, D.; di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper Modified-TiO2 Catalysts for Hydrogen Generation through Photoreforming of Organics. A Short Review. Int J Hydrogen Energy 2014, 39, 16812–16831. [CrossRef]
- Xu, S.; Ng, J.; Zhang, X.; Bai, H.; Sun, D.D. Fabrication and Comparison of Highly Efficient Cu Incorporated TiO 2 Photocatalyst for Hydrogen Generation from Water. Int J Hydrogen Energy 2010, 35, 5254–5261. [CrossRef]
- Serjeant, E.P.; Dempsey, Boyd. Ionisation Constants of Organic Acids in Aqueous Solution; International Union of Pure and Applied Chemistry. Commission on Equilibrium Data. IUPAC chemical data series ; no. 23.; Pergamon Press: Oxford ;, 1979; ISBN 0080223397.
- Kosmulski, M. Isoelectric Points and Points of Zero Charge of Metal (Hydr)Oxides: 50 Years after Parks’ Review. Adv Colloid Interface Sci 2016, 238, 1–61. [CrossRef]
- Hu, J.; Wang, J.; Liu, S.; Zhang, Z.; Zhang, H.; Cai, X.; Pan, J.; Liu, J. Effect of TiO2 Nanoparticle Aggregation on Marine Microalgae Isochrysis Galbana. J Environ Sci (China) 2018, 66, 208–215. [CrossRef]
- Gao, M.; Zhang, T.; Ho, G.W. Advances of Photothermal Chemistry in Photocatalysis, Thermocatalysis, and Synergetic Photothermocatalysis for Solar-to-Fuel Generation. Nano Res 2022.
- Reza, M.S.; Ahmad, N.B.H.; Afroze, S.; Taweekun, J.; Sharifpur, M.; Azad, A.K. Hydrogen Production from Water Splitting through Photocatalytic Activity of Carbon-Based Materials. Chem Eng Technol 2022.
- Huaxu, L.; Fuqiang, W.; Ziming, C.; Shengpeng, H.; Bing, X.; Xiangtao, G.; bo, L.; Jianyu, T.; Xiangzheng, L.; Ruiyang, C.; et al. Analyzing the Effects of Reaction Temperature on Photo-Thermo Chemical Synergetic Catalytic Water Splitting under Full-Spectrum Solar Irradiation: An Experimental and Thermodynamic Investigation. Int J Hydrogen Energy 2017, 42, 12133–12142. [CrossRef]
- Zhang, Z.; Maggard, P.A. Investigation of Photocatalytically-Active Hydrated Forms of Amorphous Titania, TiO2·nH2O. J Photochem Photobiol A Chem 2007, 186, 8–13. [CrossRef]
- Song, R.; Luo, B.; Geng, J.; Song, D.; Jing, D. Photothermocatalytic Hydrogen Evolution over Ni2P/TiO2 for Full-Spectrum Solar Energy Conversion. Ind Eng Chem Res 2018, 57, 7846–7854. [CrossRef]
- Velázquez, J.J.; Fernández-González, R.; Díaz, L.; Pulido Melián, E.; Rodríguez, V.D.; Núñez, P. Effect of Reaction Temperature and Sacrificial Agent on the Photocatalytic H2-Production of Pt-TiO2. J Alloys Compd 2017, 721, 405–410. [CrossRef]
- Rico-Oller, B.; Boudjemaa, A.; Bahruji, H.; Kebir, M.; Prashar, S.; Bachari, K.; Fajardo, M.; Gómez-Ruiz, S. Photodegradation of Organic Pollutants in Water and Green Hydrogen Production via Methanol Photoreforming of Doped Titanium Oxide Nanoparticles. Science of the Total Environment 2016, 563–564, 921–932. [CrossRef]
- Munusamy, T.D.; Chin, S.Y.; Tarek, M.; Khan, M.M.R. Sustainable Hydrogen Production by CdO/Exfoliated g-C3N4 via Photoreforming of Formaldehyde Containing Wastewater. Int J Hydrogen Energy 2021, 46, 30988–30999. [CrossRef]
- Koca, A.; Ahin, M.S. Photocatalytic Hydrogen Production by Direct Sun Light from Sulfide=sulfite Solution; 2002; Vol. 27;
- Wei, Z.; Liu, J.; Shangguan, W. A Review on Photocatalysis in Antibiotic Wastewater: Pollutant Degradation and Hydrogen Production. Chinese Journal of Catalysis 2020, 41, 1440–1450. [CrossRef]
- Chowdhury, P.; Malekshoar, G.; Ray, M.B.; Zhu, J.; Ray, A.K. Sacrificial Hydrogen Generation from Formaldehyde with Pt/TiO2 Photocatalyst in Solar Radiation. Ind Eng Chem Res 2013, 52, 5023–5029. [CrossRef]
- Zhang, S.; Wang, L.; Liu, C.; Luo, J.; Crittenden, J.; Liu, X.; Cai, T.; Yuan, J.; Pei, Y.; Liu, Y. Photocatalytic Wastewater Purification with Simultaneous Hydrogen Production Using MoS2 QD-Decorated Hierarchical Assembly of ZnIn2S4 on Reduced Graphene Oxide Photocatalyst. Water Res 2017, 121, 11–19. [CrossRef]
- Riddick, J.A.; Bunger, W.B.; Sakano, T.K. Organic Solvents: Physical Properties and Methods of Purification.; 4th ed.; Wiley-Interscience: New York, 1986;
- Williams, M. The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; 15th ed.; Royal Society of Chemistry: Cambridge, UK, 2013;
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).