Submitted:
15 February 2023
Posted:
16 February 2023
Read the latest preprint version here
Abstract
Keywords:
Introduction
Applicatıon and Mechanisms of ICIT
The Bottlenecks of ICIT, the Complexity and Effective Management
Pathogenesis of irAEs, Management Burden and Strategies
Cancer Pain Management throughout ICIT
Discussion
- How soon the regression starts after the initial infusion
- What the rate of regression is
- How soon the remission is reached after the initial infusion
- The general personal immune strength
- The compatibility of baseline biomarkers with the ICIT
- Number of days without any irAE after the initial infusion
| Step# | Description of Step |
|---|---|
| 1 | ICIT continues |
| 2 | Check whether the patient is psychologically doing well and GO TO step 3 if YES, otherwise WITHHOLD ICIT until improvement |
| 3 | Check whether the patient is symptomatically doing well and GO TO step 4 if YES, otherwise WITHHOLD ICIT until improvement |
| 4 | Check whether the patient has developed any irAE or toxicity and GO TO step 5 if NO, otherwise REFER to management algorithms presented in the previous section |
| 5 | Check whether MRNn+1 > MRNn (n є ℕ) and GO TO step 6 if NO, otherwise DISCONTINUE ICIT permanently |
| 6 | Check whether NTn+1 > NTn+2 (n є ℕ) and GO TO step 7 if NO, otherwise DISCONTINUE ICIT permanently |
| 7 | Check whether VTn+1 > 1.15xVTn (n є ℕ) and GO TO step 8 if NO, otherwise DISCONTINUE ICIT permanently |
| 8 | CONTINUE ICIT |
| 9 | GO TO step 1 and MAKE n=n+1 |
Conclusions
Funding
IRB Approval
Clinical Trials
Patient Consent
Conflicts of Interest
References
- Mahoney DJ, Stojdl DF, Laird G (2014) Virus therapy for cancer. Sci Am 311(5):54-9. [CrossRef]
- Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565-70. [CrossRef]
- Postow MA, Callahan MK, Wolchok JD (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33:1974–82. [CrossRef]
- Champiat S, Lambotte O, Barreau E et al (2016) Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann Oncol 27(4):559-74. [CrossRef]
- Chiou VL, Burotto M (2015) Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 33(31):3541-3. [CrossRef]
- Abril-Rodriguez G, Ribas A (2017) SnapShot: immune checkpoint inhibitors. Cancer Cell 31(6):848-848.e1. [CrossRef]
- Linsley PS, Wallace PM, Johnson J et al (1992) Immunosuppression in vivo by a soluble form of the CTLA-4 T cell activation molecule. Science 257(5071):792-5. [CrossRef]
- Egen JG, Allison JP (2002) Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16(1):23-35. [CrossRef]
- Leach DR, Krummel MF, Allison JP (1996) Enhancement of antitumor immunity by CTLA-4 blockade. Science 271(5256):1734-6. [CrossRef]
- Han Y, Liu D, Li L (2020) PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 10(3):727-42.
- Dong H, Zhu G, Tamada K, Chen L (1999) B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med 5(12):1365-9. [CrossRef]
- Mohsenzadegan M, Bavandpour P, Nowroozi MR et al (2021) The potential of T cell immunoglobulin and mucin-domain containing-3 (Tim-3) in designing novel immunotherapy for bladder cancer. Endocr Metab Immune Disord Drug Targets (12):2131-46. [CrossRef]
- Sprangers B (2019) Pembrolizumab-related renal toxicities: diagnosis first, treatment later. Clin Kidney J 12(1):78-80. [CrossRef]
- Cortazar FB, Marrone KA, Troxell ML et al (2016) Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 90(3):638-47. [CrossRef]
- Hanna RM, Selamet U, Bui P et al (2018) Acute kidney injury after pembrolizumab-induced adrenalitis and adrenal insufficiency. Case Rep Nephrol Dial 8(2):171-77. [CrossRef]
- Dumoulin DW, Visser S, Cornelissen R et al (2020) Renal toxicity from pemetrexed and pembrolizumab in the era of combination therapy in patients with metastatic nonsquamous cell NSCLC. J Thorac Oncol 15(9):1472-83. [CrossRef]
- Izzedine H, Mathian A, Champiat S et al (2019) Renal toxicities associated with pembrolizumab. Clin Kidney J 12(1):81-8. [CrossRef]
- Warner BM, Baer AN, Lipson EJ et al (2019) Sicca syndrome associated with immune checkpoint inhibitor therapy. Oncologist 24(9):1259-69. [CrossRef]
- Som A, Mandaliya R, Alsaadi D et al (2019) Immune checkpoint inhibitor-induced colitis: a comprehensive review. World J Clin Cases 7(4):405-18. [CrossRef]
- El Sabbagh R, Azar NS, Eid AA, Azar ST (2020) Thyroid dysfunctions due to immune checkpoint inhibitors: a review. Int J Gen Med 13:1003-9. [CrossRef]
- Johnson DB, McDonnell WJ, Gonzalez-Ericsson PI et al (2019) A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat Med 25(8):1243-50. [CrossRef]
- Liu X, Liang X, Liang J, Li Y, Wang J (2020) Immune thrombocytopenia induced by immune checkpoint inhibitors in solid cancer: case report and literature review. Front Oncol 10:530478. [CrossRef]
- Salinas N, Nowak E, Etienne M et al (2021) Causes of pruritus in patients treated with immune checkpoint inhibitors for melanomas or skin carcinomas. Front Med (Lausanne) 8:632683. [CrossRef]
- Ghoraba H, Or C, Karaca I et al (20229 Immunotherapy-induced retinopathy mimicking cancer associated retinopathy. Am J Ophthalmol Case Rep 26:101449. [CrossRef]
- Takatsuki K, Yanagihara T, Egashira A et al (2021) A rare case of pembrolizumab-induced dermatomyositis in a patient with cancer of unknown primary origin. Am J Case Rep 22:e930286. [CrossRef]
- Rai M, Go M (2020) Nivolumab induced adrenal insufficiency: rare side-effect of a new anti-cancer therapy - immune-checkpoint inhibitors. Cureus 12(4):e7625. [CrossRef]
- Hercun J, Vincent C, Bilodeau M, Lapierre P (2022) Immune-mediated hepatitis during immune checkpoint inhibitor cancer immunotherapy: lessons from autoimmune hepatitis and liver immunology. Front Immunol 13:907591. [CrossRef]
- Weber JS, Kähler KC, Hauschild A (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30(21):2691-7. [CrossRef]
- Hassel JC, Heinzerling L, Aberle J et al (2017) Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): evaluation and management of adverse drug reactions. Cancer Treat Rev 57:36-49. [CrossRef]
- Martins F, Sofiya L, Sykiotis GP et al (2019) Adverse effects of immune-checkpoint inhibitors: epidemiology, management and surveillance. Nat Rev Clin Oncol 16(9):563-80. [CrossRef]
- Wang DY, Salem JE, Cohen JV et al (2018) Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 4(12):1721-28. Erratum in: JAMA Oncol 4(12):1792. [CrossRef]
- Oble DA, Mino-Kenudson M, Goldsmith J et al (2008) Alpha-CTLA-4 mAb-associated panenteritis: a histologic and immunohistochemical analysis. Am J Surg Pathol 32(8):1130-7. [CrossRef]
- Read S, Malmström V, Powrie F (2000) Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med 192(2):295-302. [CrossRef]
- Troxell ML, Higgins JP, Kambham N (2016) Antineoplastic treatment and renal injury: an update on renal pathology due to cytotoxic and targeted therapies. Adv Anat Pathol 23(5):310-29. [CrossRef]
- Shirali AC, Perazella MA, Gettinger S (2016) Association of acute interstitial nephritis with programmed cell death 1 inhibitor therapy in lung cancer patients. Am J Kidney Dis 68(2):287-91. [CrossRef]
- Caturegli P, Di Dalmazi G, Lombardi M et al (2016) Hypophysitis secondary to cytotoxic T-lymphocyte-associated protein 4 blockade: insights into pathogenesis from an autopsy series. Am J Pathol 186(12):3225-35. [CrossRef]
- Matson DR, Accola MA, Rehrauer WM, Corliss RF (2018) Fatal myocarditis following treatment with the PD-1 inhibitor nivolumab. J Forensic Sci 63(3):954-57. [CrossRef]
- Zha H, Han X, Zhu Y et al (2017) Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. Oncoimmunology 6(10):e1349587. [CrossRef]
- Moller DR (1999) Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 16(1):24-31.
- Facco M, Cabrelle A, Teramo A et al (2011) Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 66(2):144-50. [CrossRef]
- Lomax AJ, McGuire HM, McNeil C et al (2017) Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheum Dis 20(9):1277-85. [CrossRef]
- Yamauchi I, Sakane Y, Fukuda Y et al (2017) Clinical features of nivolumab-induced thyroiditis: a case series study. Thyroid 27(7):894-901. [CrossRef]
- Quirk SK, Shure AK, Agrawal DK (2015) Immune-mediated adverse events of anticytotoxic T lymphocyte-associated antigen 4 antibody therapy in metastatic melanoma. Transl Res 166(5):412-24. [CrossRef]
- Polakos NK, Cornejo JC, Murray DA et al (2006) Kupffer cell-dependent hepatitis occurs during influenza infection. Am J Pathol 168(4):1169-78; quiz 1404-5. [CrossRef]
- Teulings HE, Limpens J, Jansen SN et al (2015) Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 33(7):773-81. [CrossRef]
- Freeman-Keller M, Kim Y, Cronin H et al (2016) Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 22(4):886-94. [CrossRef]
- Wang Y, Abu-Sbeih H, Mao E et al (2018) Endoscopic and histologic features of immune checkpoint inhibitor-related colitis. Inflamm Bowel Dis 24(8):1695-1705. [CrossRef]
- Faje AT, Sullivan R, Lawrence D et al (2014) Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab 99(11):4078-85. [CrossRef]
- Spain L, Diem S, Larkin J (2016) Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev 44:51-60. [CrossRef]
- González-Rodríguez E, Rodríguez-Abreu D; Spanish Group for Cancer Immuno-Biotherapy (GETICA) (2016) Immune checkpoint inhibitors: review and management of endocrine adverse events. Oncologist 21(7):804-16. [CrossRef]
- Kumar V, Chaudhary N, Garg M et al (2017) Current diagnosis and management of immune related adverse events (irAEs) induced by immune checkpoint inhibitor therapy. Front Pharmacol 8:49. [CrossRef]
- Friedman CF, Proverbs-Singh TA, Postow MA (2016) Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA Oncol 2(10):1346-53. [CrossRef]
- Torino F, Barnabei A, De Vecchis L et al (2012) Hypophysitis induced by monoclonal antibodies to cytotoxic T lymphocyte antigen 4: challenges from a new cause of a rare disease. Oncologist 17(4):525-35. [CrossRef]
- Linardou H, Gogas H (2016) Toxicity management of immunotherapy for patients with metastatic melanoma. Ann Transl Med 4(14):272. [CrossRef]
- Weber JS, Yang JC, Atkins MB, Disis ML (2015) Toxicities of immunotherapy for the practitioner. J Clin Oncol 33(18):2092-9. [CrossRef]
- Zhang HC, Luo W, Wang Y (2019) Acute liver injury in the context of immune checkpoint inhibitor-related colitis treated with infliximab. J Immunother Cancer 7(1):47. [CrossRef]
- Calvo R (2019) Hematological side effects of immune checkpoint inhibitors: the example of immune-related thrombocytopenia. Front Pharmacol 10:454. [CrossRef]
- Menzies AM, Johnson DB, Ramanujam S et al (2017) Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann Oncol 28(2):368-76. [CrossRef]
- Johnson DB, Sullivan RJ, Ott PA et al (2016) Ipilimumab therapy in patients with advanced melanoma and preexisting autoimmune disorders. JAMA Oncol 2(2):234-40. [CrossRef]
- Abdel-Wahab N, Shah M, Lopez-Olivo MA, Suarez-Almazor ME (2018) Use of immune checkpoint inhibitors in the treatment of patients with cancer and preexisting autoimmune disease: a systematic review. Ann Intern Med 168(2):121-30. [CrossRef]
- Horvat TZ, Adel NG, Dang TO et al (2015) Immune-related adverse events, need for systemic immunosuppression, and effects on survival and time to treatment failure in patients with melanoma treated with ipilimumab at Memorial Sloan Kettering Cancer Center. J Clin Oncol 33(28):3193-8. [CrossRef]
- Bessede A, Marabelle A, Guégan JP et al (2022) Impact of acetaminophen on the efficacy of immunotherapy in cancer patients. Ann Oncol 33(9):909-15. [CrossRef]
- Lissoni P, Mandalà M, Brivio F (2000) Abrogation of the negative influence of opioids on IL-2 immunotherapy of renal cell cancer by melatonin. Eur Urol 38(1):115-8. [CrossRef]
- Prasetya RA, Metselaar-Albers M, Engels F (2021) Concomitant use of analgesics and immune checkpoint inhibitors in non-small cell lung cancer: a pharmacodynamics perspective. Eur J Pharmacol 906:174284. [CrossRef]
- Kato S, Goodman A, Walavalkar V et al (2017) Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 23(15):4242-50. [CrossRef]
- Saâda-Bouzid E, Defaucheux C, Karabajakian A et al (2017) Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann Oncol 28(7):1605-11. [CrossRef]
- Freixinos VR, Garcia A, Fasani R et al (2018) Immune profile and outcomes of patients (pts) with gynecological malignancies (GYN) enrolled in early phases immunotherapy (IO) trials. J Clin Oncol 36(15_suppl):5595. [CrossRef]
- Matos I, Martin-Liberal J, Hierro C et al (2018) Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J Clin Oncol 36(15_suppl):3032. [CrossRef]
- Arnold CE, Rajnicek AM, Hoare JI et al (2019) Physiological strength electric fields modulate human T cell activation and polarisation. Sci Rep 9(1):17604. [CrossRef]
- Salerno S, La Mendola C, La Manna MP et al (2009) Reversible effect of magnetic fields on human lymphocyte activation patterns: different sensitivity of naive and memory lymphocyte subsets. Radiat Res 172(4):444-50. [CrossRef]
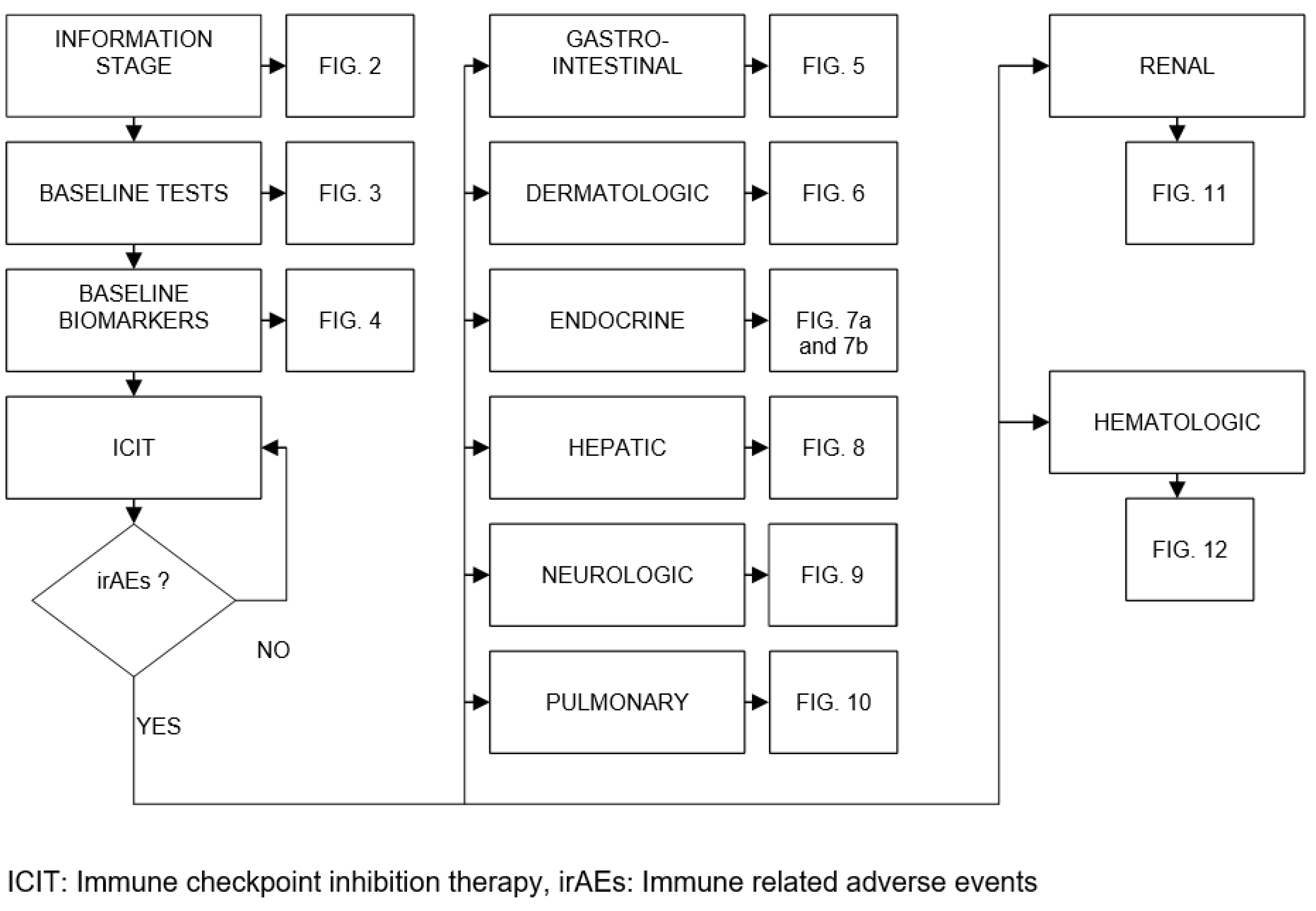
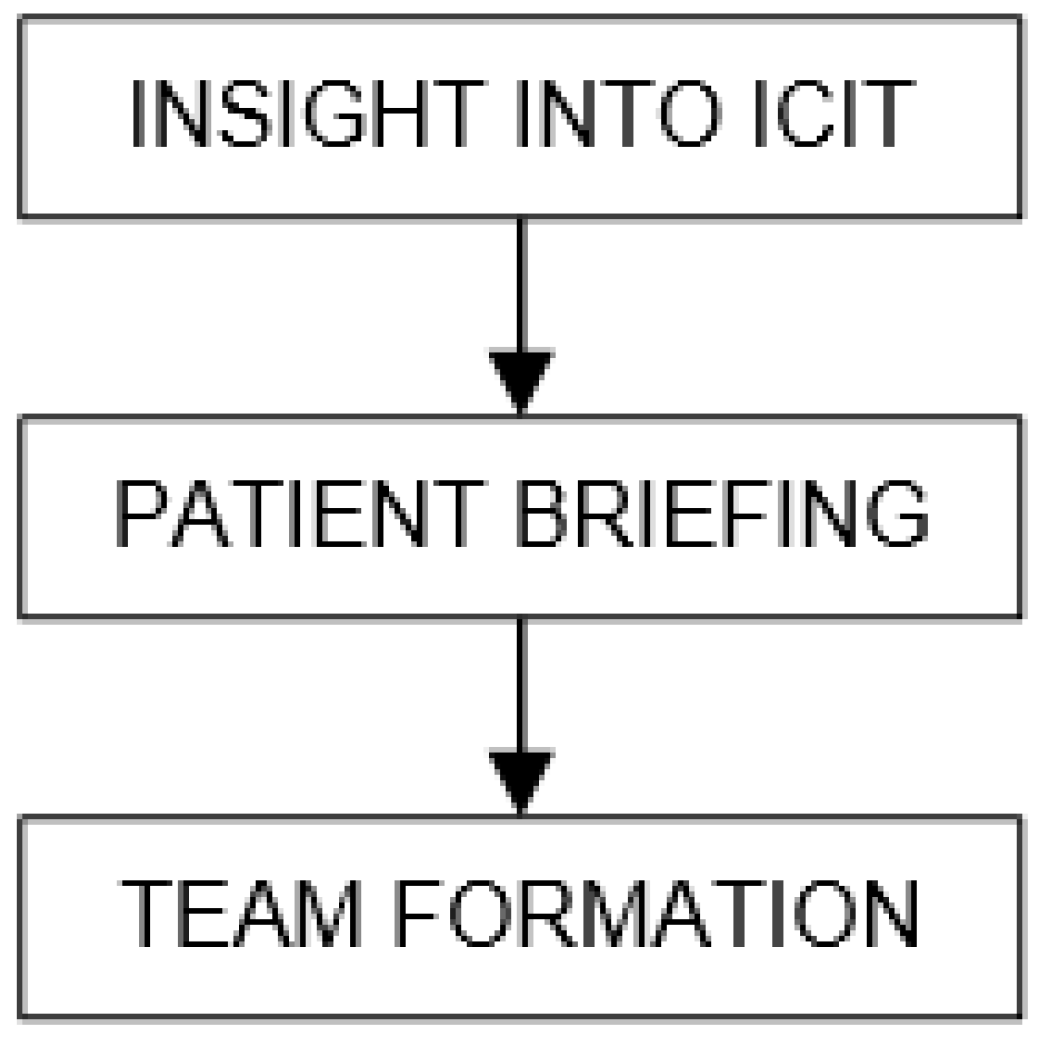
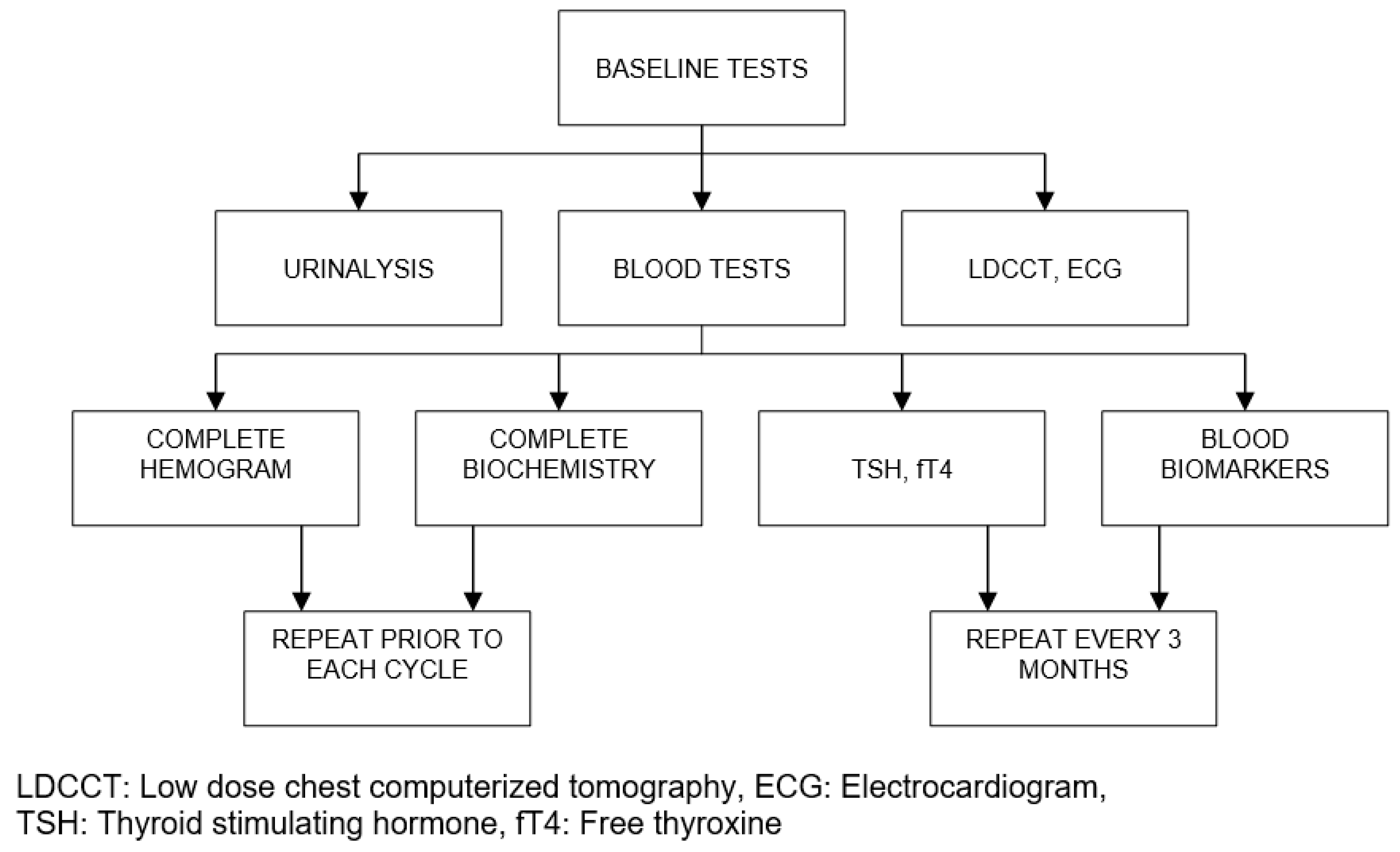
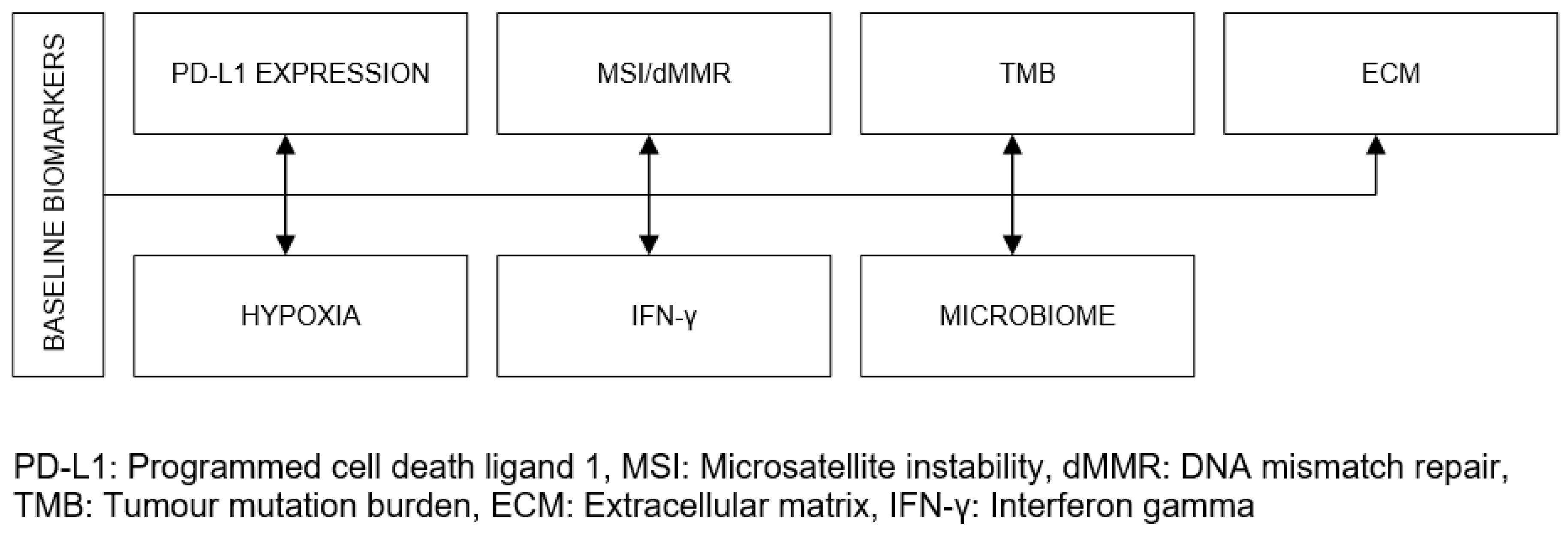
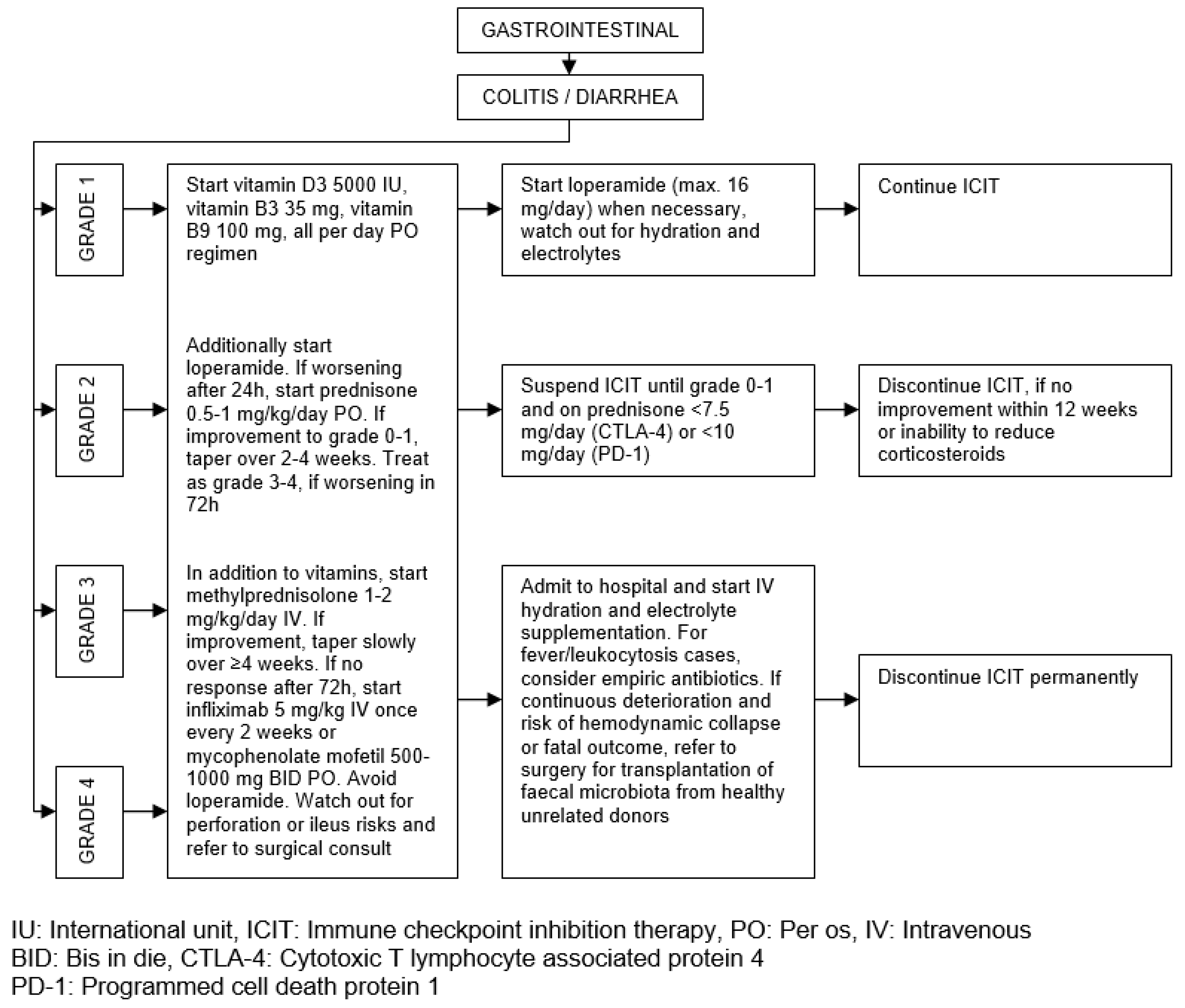
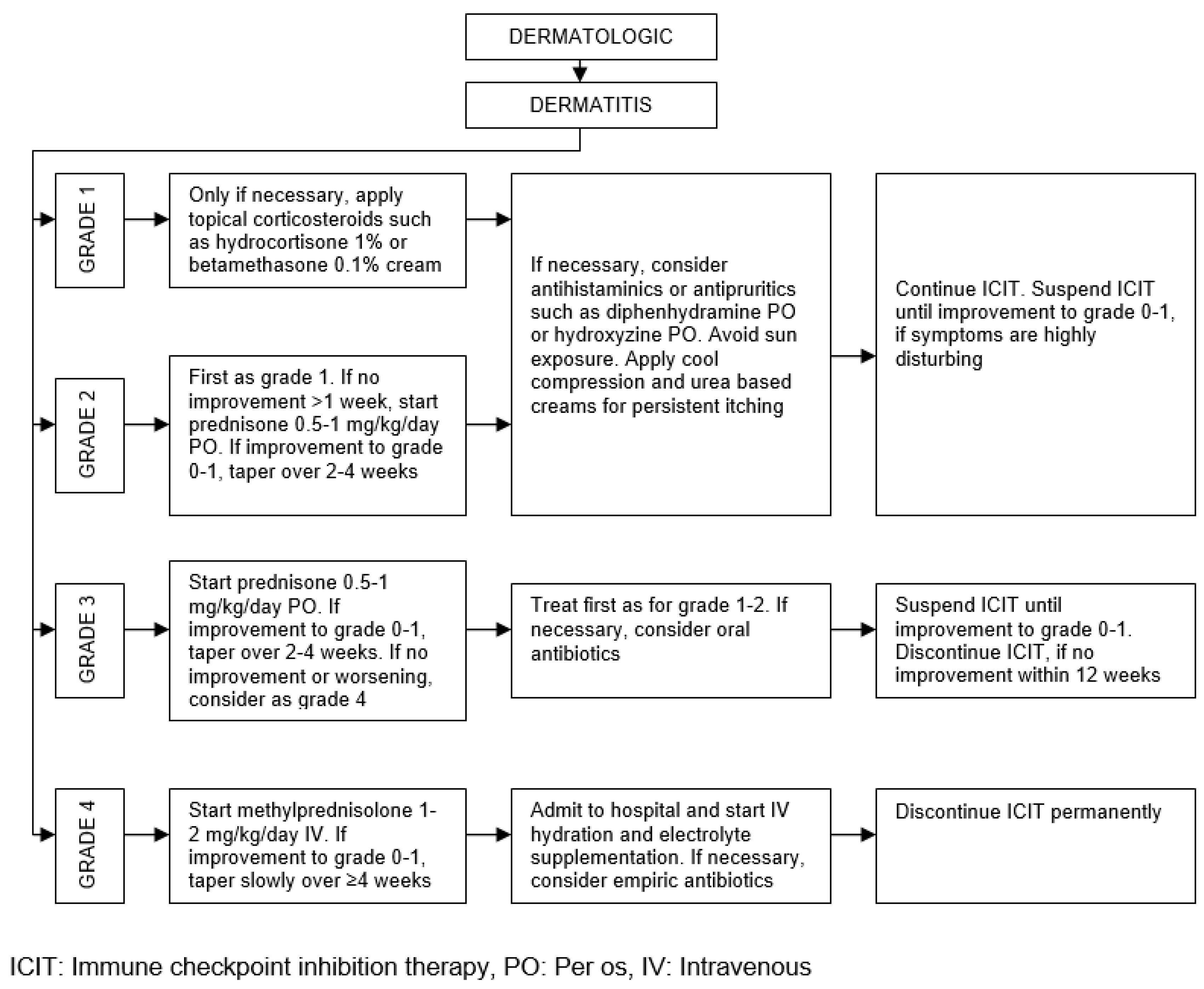
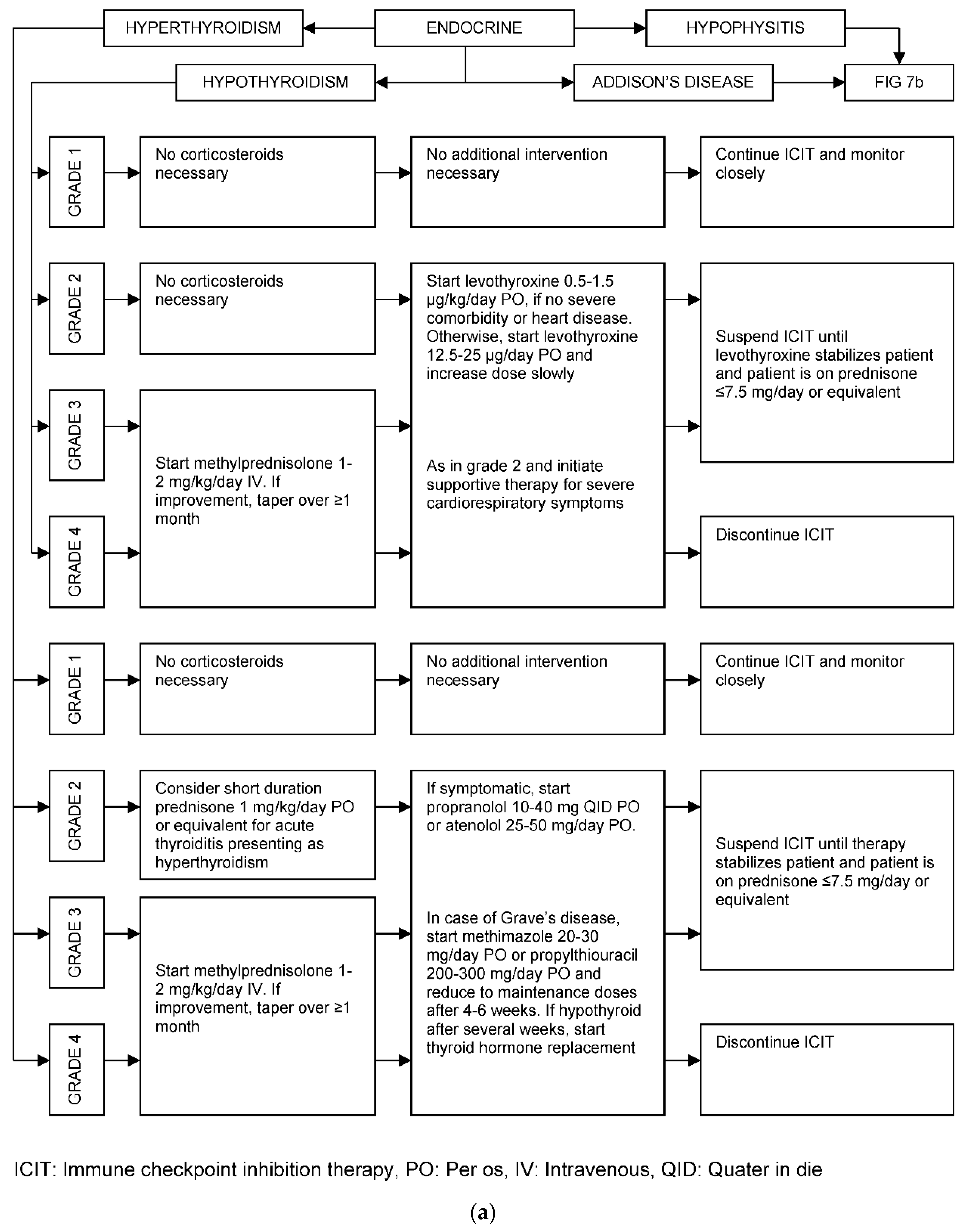
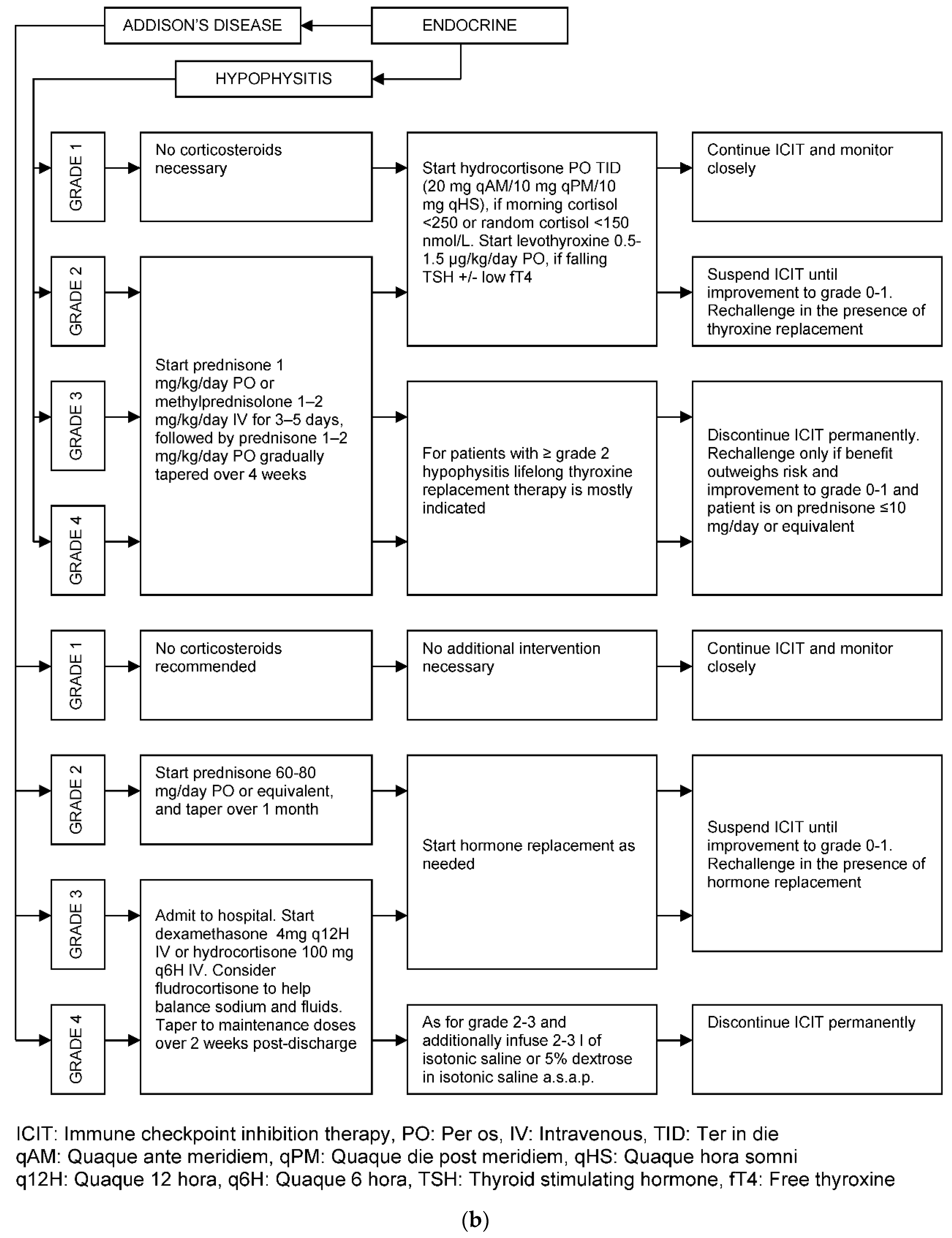
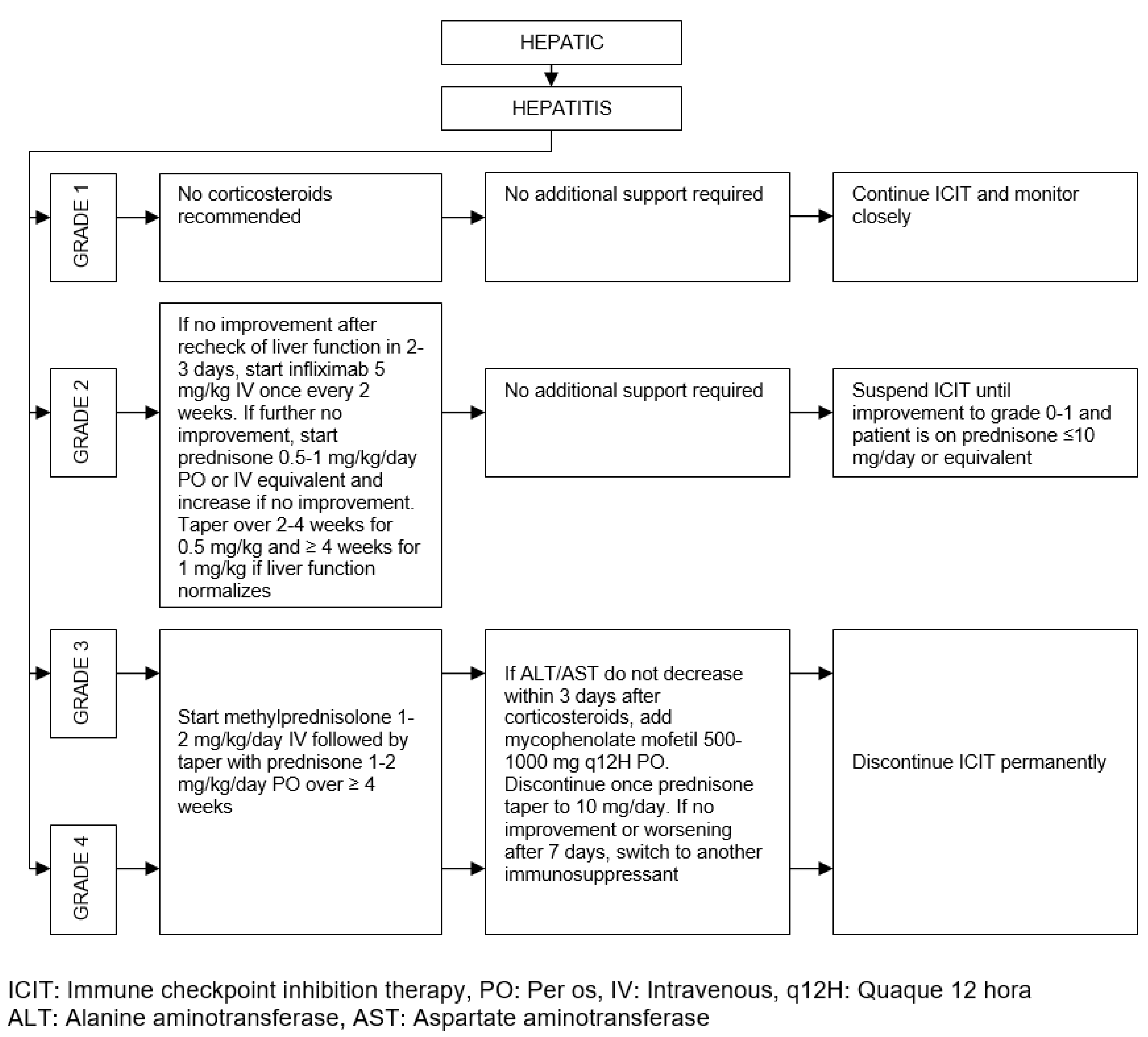
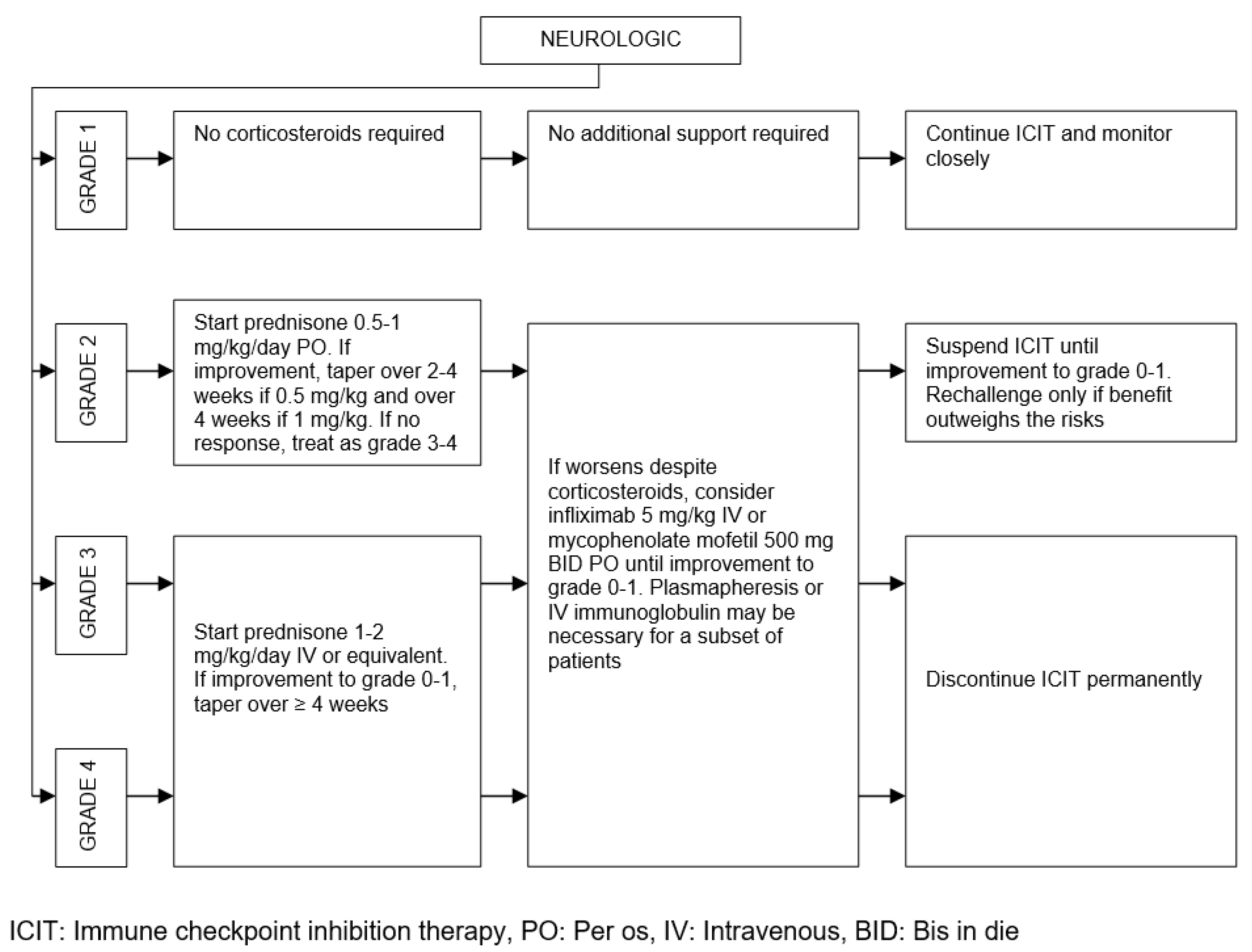
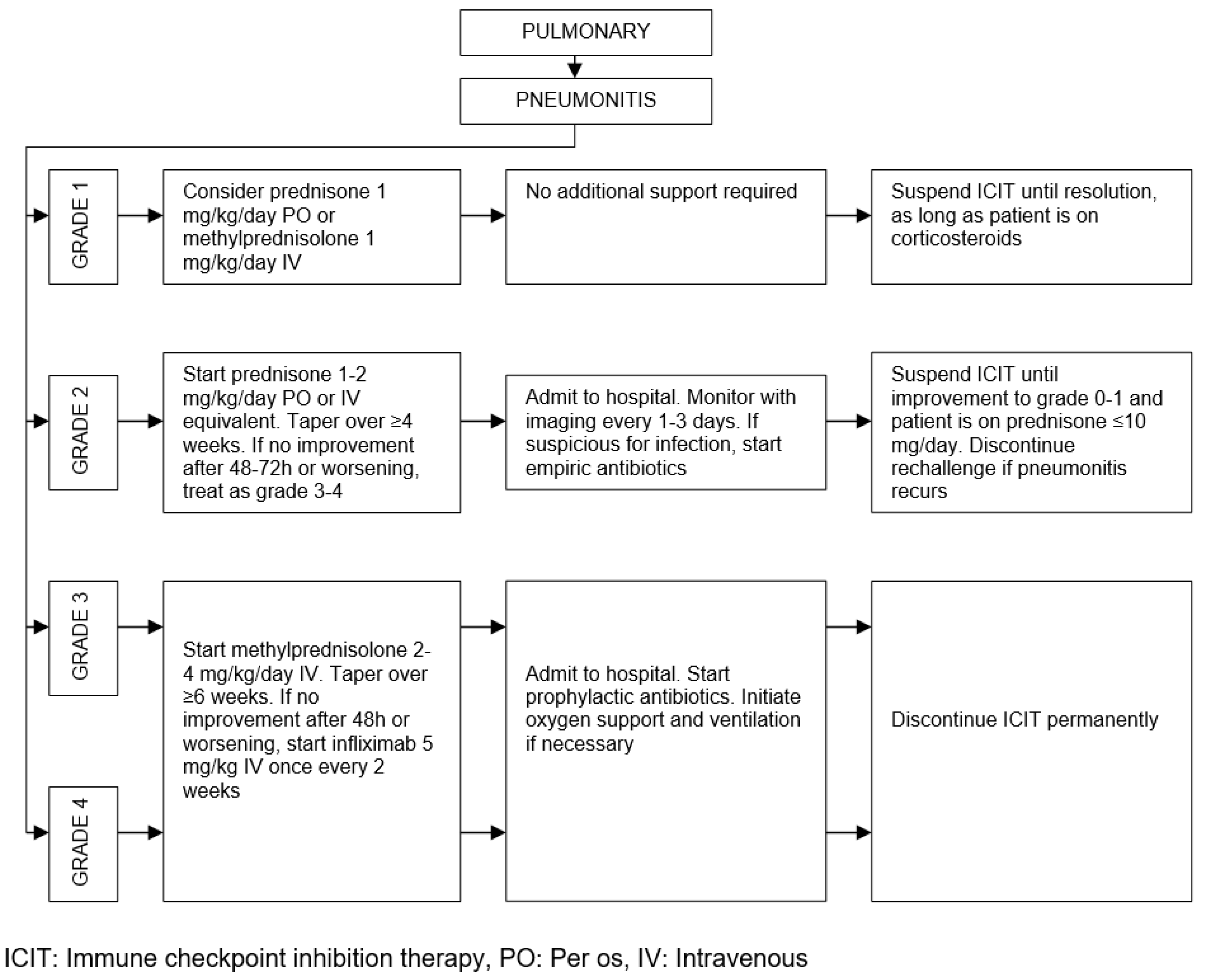
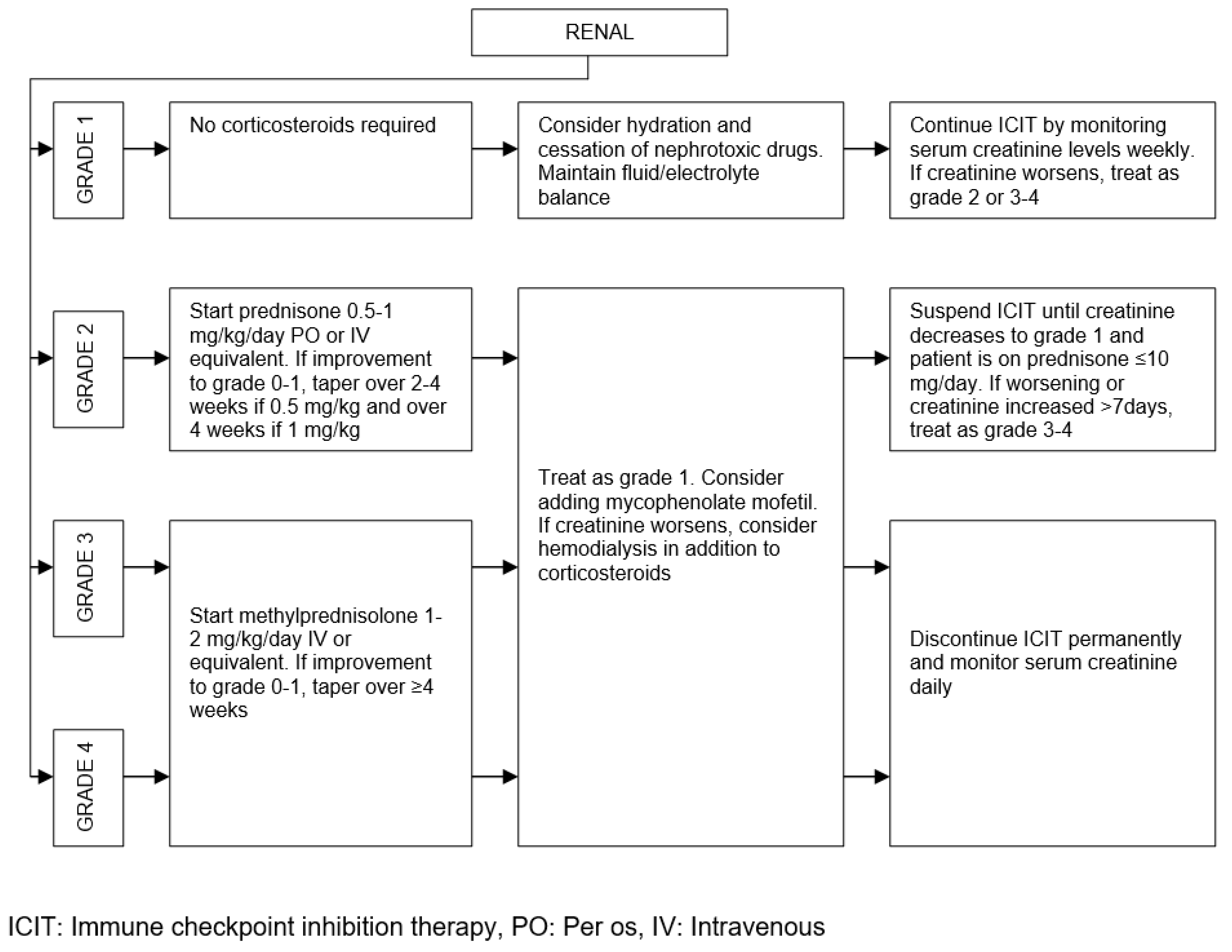
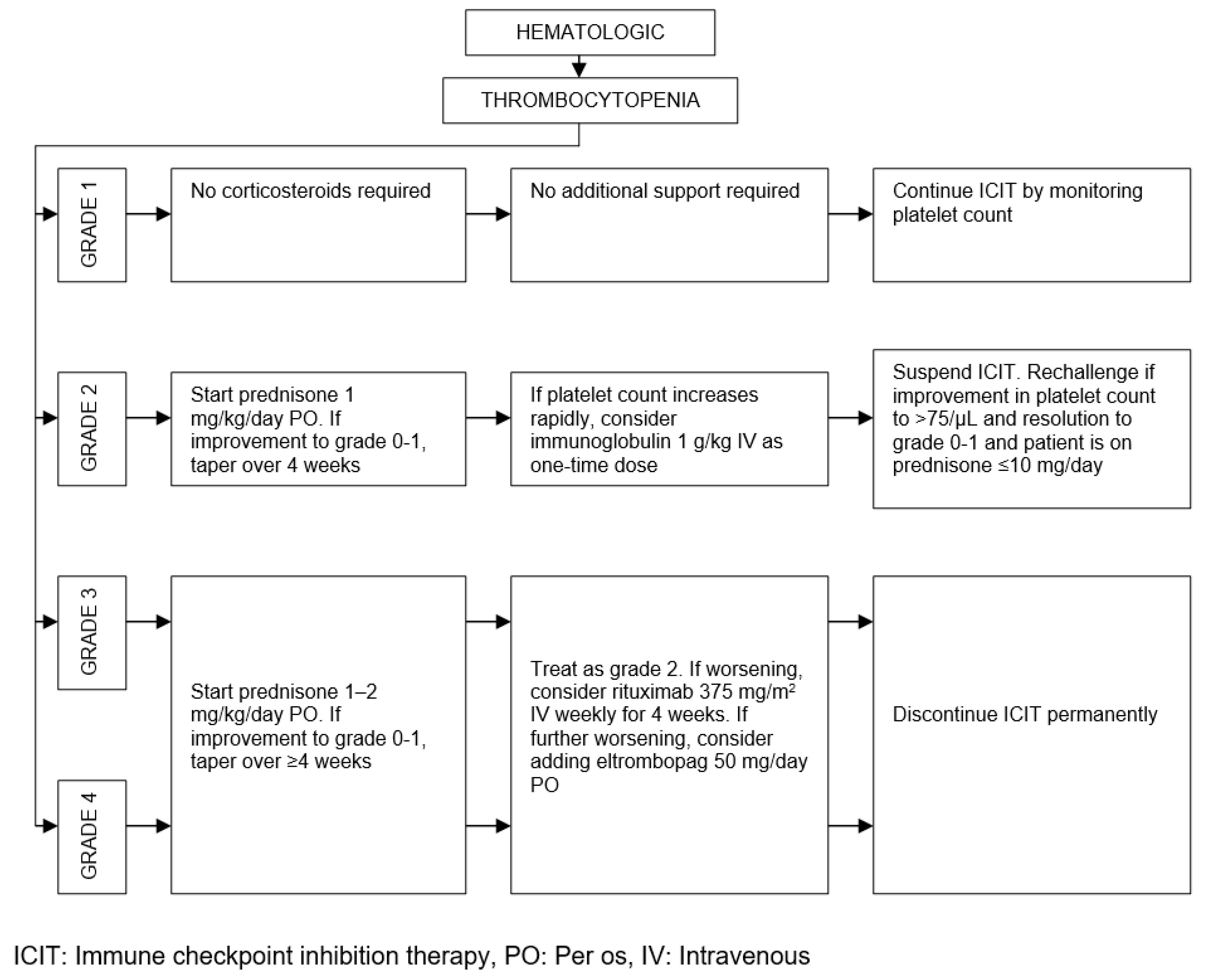
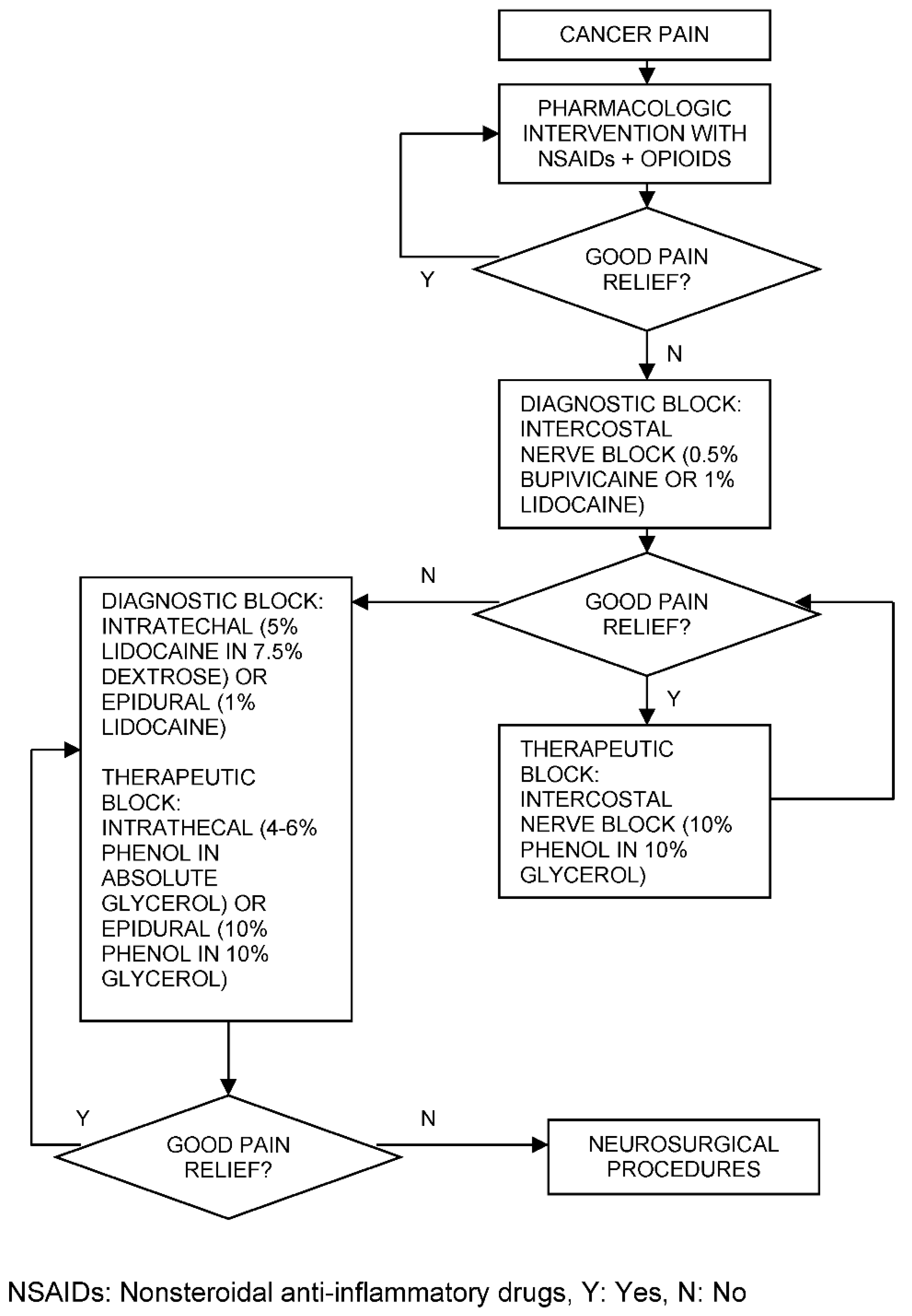
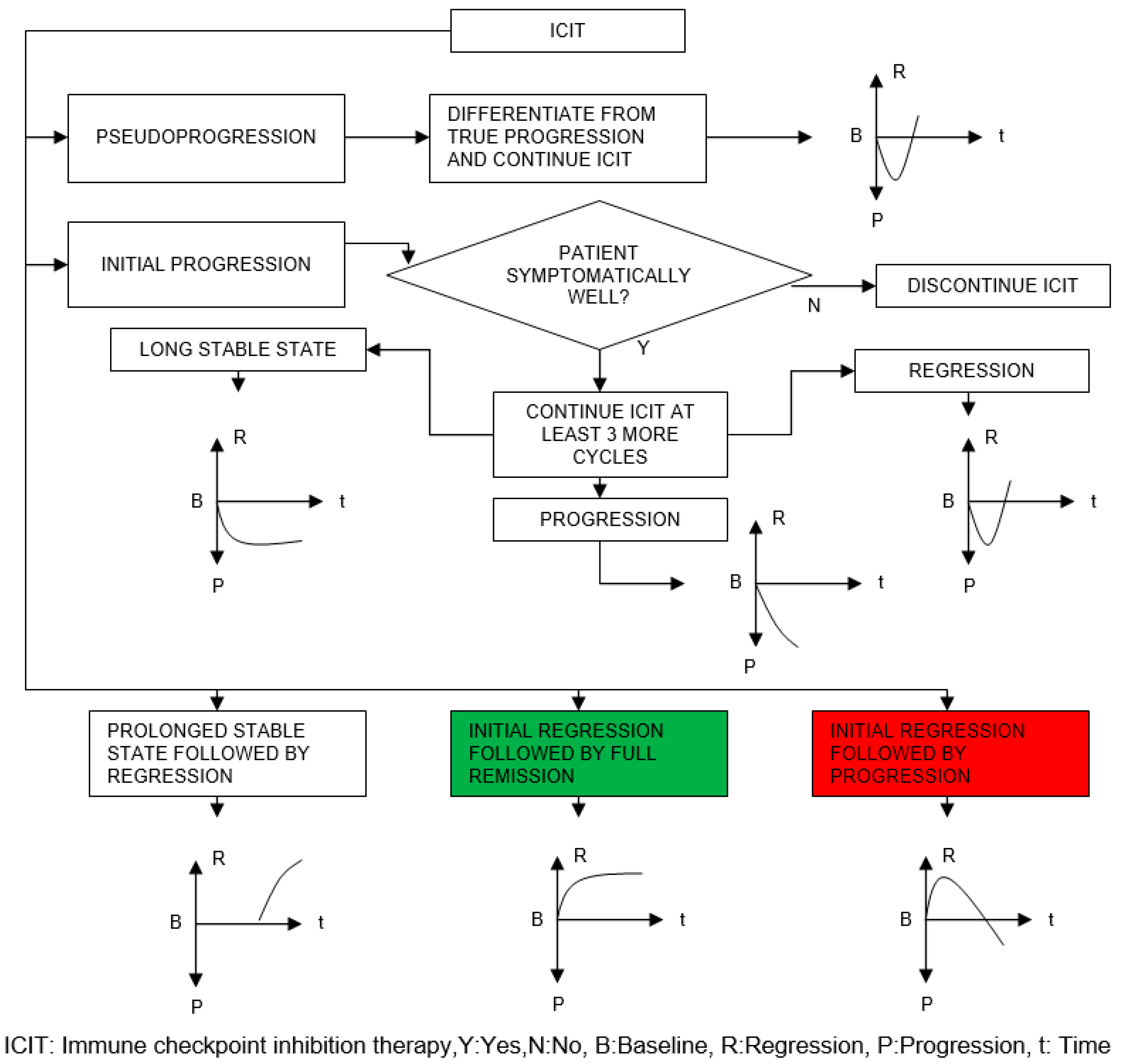
| Organ/Tissue System | irAEs | Approx. Onset Frequency (%) |
|---|---|---|
| Gastrointestinal | Oral mucositis | < 5 |
| Xerostomia | < 6 | |
| Gastritis | > 50 (PD-1), < 5 (PD-L1) | |
| Colitis | > 10 | |
| Ileitis | > 10 | |
| Hepatitis | 5 – 10 | |
| Pancreatitis | < 5 | |
| Dermatologic | Dermatitis | > 20 |
| Pruritus | 10 – 50 | |
| Stevens Johnson | < 5 | |
| Psoriasis | < 5 | |
| Vitiligo | 5 – 10 | |
| DRESS syndrome | < 4 | |
| Endocrine | Hypohysitis | 5 – 10 |
| Hyper-/hypothyroidism | < 10 | |
| Diabetes Mellitus | < 3 | |
| Addison's disease | 5 – 10 | |
| Pulmonary | Pleuritis | < 1 |
| Pneumonitis | < 5 | |
| Sarcoid-like granulomatosis | 5 – 7 (CTLA-4), < 0.5 (PD-L1) | |
| Neurologic | Encephalitis | < 5 |
| Meningitis | < 5 | |
| Guillain Barré | < 5 | |
| Neuropathy | < 5 | |
| Myasthenia gravis | < 5 | |
| Myelopathy | < 1 | |
| Optic neuritis | < 1 | |
| Cardiovascular | Myocarditis | < 5 |
| Pericarditis | < 3 | |
| Vasculitis | < 1 | |
| Hematologic | Thrombocytopenia | < 2 |
| Neutropenia | < 1 | |
| Hemolytic anemia | < 5 (CTLA-4), < 10 (PD-1) | |
| Pancytopenia | < 1 | |
| Renal | Acute interstitial nephritis | < 5 |
| Acute tubular injury | < 1 | |
| Glomerulonephritis | < 2 | |
| Musculoskeletal | Dermatomyositis | < 1 |
| Arthritis | < 4 | |
| Ocular | Retinitis | < 3 |
| Conjunctivitis | < 1 | |
| Uveitis | < 5 | |
| Scleritis | < 1 |
| ICIT | irAEs | Onset Time (Week) |
CTCAE Peak (Week) |
Damping Time (Week) |
|---|---|---|---|---|
| CTLA-4 | Colitis | 4 – 5 | 6 | 10 |
| Hepatitis | 6 – 7 | 8 – 13 | 15 | |
| Pancreatitis | 3 – 9 | N/A | N/A | |
| Pruritus | ≈ 4 | ≈ 5 | ≈ 6 | |
| Hypohysitis | 6 – 7 | 8 | ∞ | |
| Addison's disease | 6 – 7 | 8 | ∞ | |
| Myasthenia gravis | 2 – 6 | 1 – 4 | N/A | |
| Acute interstitial nephritis | 2 – 12 | N/A | N/A | |
| PD-1 or PD-L1 | Colitis | 4 – 5 | 6 | 10 |
| Hepatitis | 6 – 7 | 8 – 14 | 15 | |
| Pancreatitis | 3 – 30 | N/A | N/A | |
| Pruritus | ≈ 4 | ≈ 5 | ≈ 6 | |
| Hypohysitis | 6 – 7 | 8 | ∞ | |
| Addison's disease | 6 – 7 | 8 | ∞ | |
| Pneumonitis | 10 – 11 | 12 | 22 | |
| Myasthenia gravis | 4 – 5 | N/A | N/A | |
| Acute interstitial nephritis | 12 – 72 | N/A | N/A | |
| CTLA-4 and PD-1 | Colitis | 3 – 4 | 5 | 10 |
| Hepatitis | 5 – 6 | 7 – 13 | 15 | |
| Pruritus | ≈ 2 | ≈ 4 | ≈ 5 | |
| Hypohysitis | ≈ 2 | 4 | ∞ | |
| Addison's disease | ≈ 2 | 4 | ∞ | |
| Pneumonitis | ≈ 5 | ≈ 7 | ≈ 11 | |
| Myasthenia gravis | 2 | N/A | N/A |
| ICIT | irAEs | Onset Time (Week) |
Frequency Peak (Week and %) |
Damping Time (Week) |
|
|---|---|---|---|---|---|
| CTLA-4 and PD-1 | Gastrointestinal | 0 – 1 | ≈ 5 | ≈ 46 | 6 – 8 |
| Dermatologic | 0 | ≈ 2 | ≈ 52 | 4 – 5 | |
| Endocrine | 2 – 3 | ≈ 10 | ≈ 9 | ≈ 18 | |
| Pulmonary | ≈ 1 | 5 – 6 | 25 | ≈ 10 | |
| Hepatic | 0 | 4 – 5 | 39 | ≈ 8 | |
| Renal | 1 | 5 – 6 | ≈ 5 | 10 | |
| Other | 2 | 10 | ≈ 2 | 18 | |
| Diagnosis | TSH | fT4 |
|---|---|---|
| Primary hypothyroidism | High | Low |
| Subclinical hypothyroidism | High | Normal |
| Secondary hypothyroidism | Normal | Low |
| Primary hyperthyroidism | Low | High |
| Subclinical hyperthyroidism | Low | Normal |
| Class | Type | Examples | Notes |
|---|---|---|---|
| Nonopioids | NSAIDs | Ibuprofen, aspirin, diflunisal, piroxicam, naproxen | Risk of renal failure in chronic use, not recommended in hemostatic disorders, risk of gastrointestinal bleeding |
| Acetaminophen | Paracetamol | Risk of hepatotoxicity in overdosage | |
| Weak opioids | Codeine | Oxycodone, hydrocodone, propoxyphene | Mostly in combination with NSAIDs or acetaminophen |
| Potent opioids with short half-life | MorphineFentanyl | Oxymorphone, hydromorphone, meperidine, pentazocine, butorphanol | Meperidine not recommended in patients with renal disease |
| Potent opioids with long half-life | MethadoneLevorphanol | Risk of withdrawal symptoms in physically dependent patients |
| Parameter | Definition of Scoring |
|---|---|
| Score with 100, if regression starts after the first initial infusion | |
| Score with 100 – 5x, if regression starts after the xth infusion where x=2,...,6 | |
| Score with 100 – 7y, if regression starts after the yth infusion where y=7,...,11 | |
| Score with 10, if regression starts beyond the 12th infusion | |
| Score with 0, if no regression is observed at the assessment stage | |
| Score with x, where x denotes tumor volume shrinkage in % | |
| Score with the average for multiple tumors, i.e. number of tumors | |
| Score with 100, if remission starts latest after the 3rd infusion | |
| Score with 100 – 10(x – 3), if remission starts after the xth infusion where x=4,...,11 | |
| Score with 10, if remission starts beyond the 12th infusion | |
| Score with 0, if no remission is observed at the assessment stage | |
| Score between 100 – 75, if there is no preexisting comorbidity and autoimmune disorder | |
| Score between 74 – 50, if there is only one preexisting comorbidity or autoimmune disorder | |
| Score between 49 – 0, if there is more than one preexisting comorbidity or autoimmune disorder | |
| Score with 100, if all of the baseline biomarkers in Figure 4 present compatibility with ICIT | |
| Score with 100 – 14(7 – x), if x of the baseline biomarkers in Figure 4 present compatibility with ICIT where x=1,...,6 | |
| Score with 0, if none of the baseline biomarkers in Figure 4 present compatibility with ICIT | |
| Score with 5, if there is no irAE after the first initial infusion | |
| Score with 5x, if there is no irAE after the xth infusion where x=2,...,20 | |
| Score with 0 when any irAE is observed at the assessment stage | |
| Increment each w with whose corresponding parameter scored between 100 – 60 in the previous assessment stage where denotes number of weightings to be incremented | |
| Decrement each w with whose corresponding parameter scored between 40 – 0 in the previous assessment stage where denotes number of weightings to be decremented |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




