Submitted:
03 March 2023
Posted:
06 March 2023
You are already at the latest version
Abstract
Keywords:
Introductıon
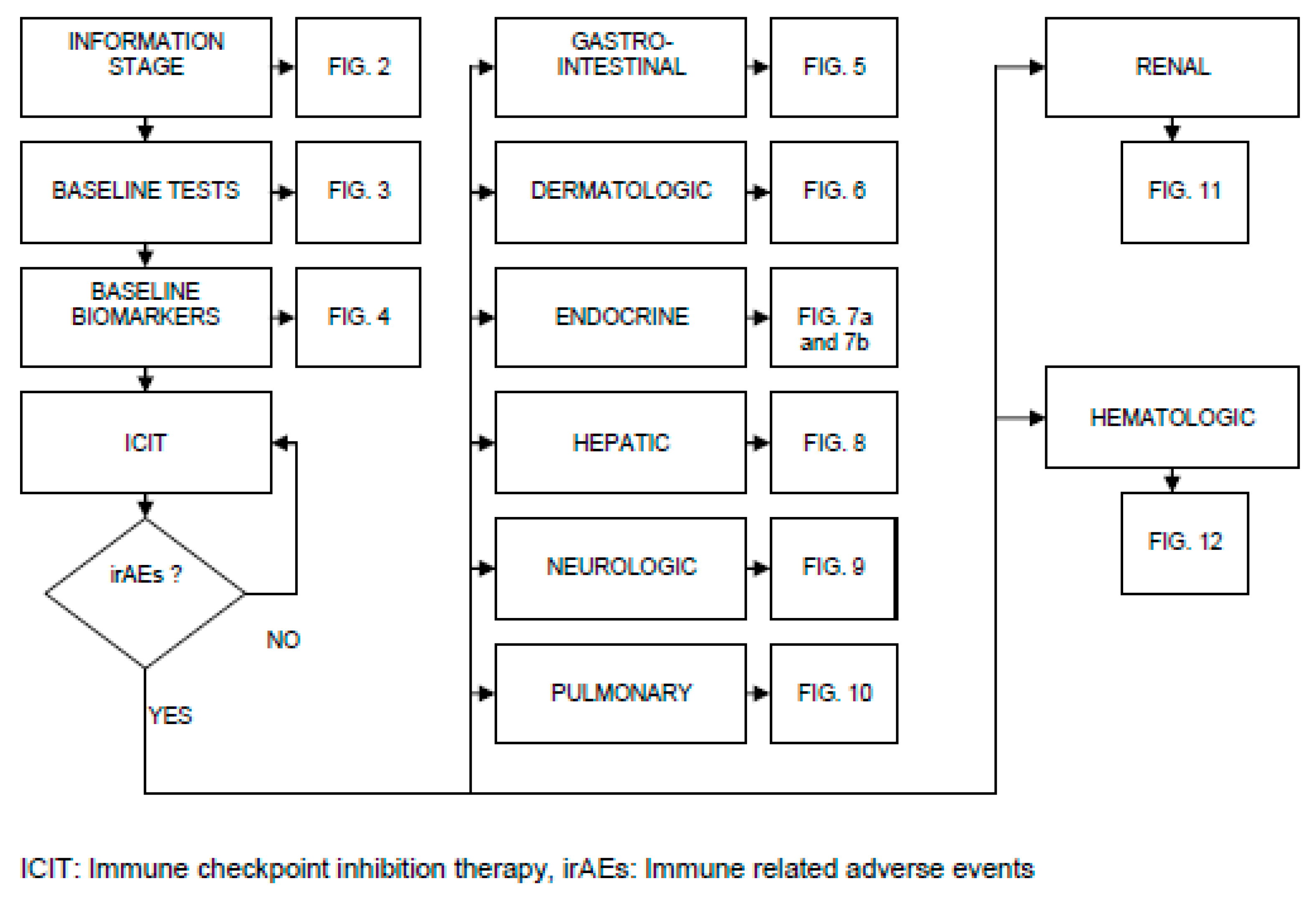
Applıcatıon and Mechanısms of Icıt
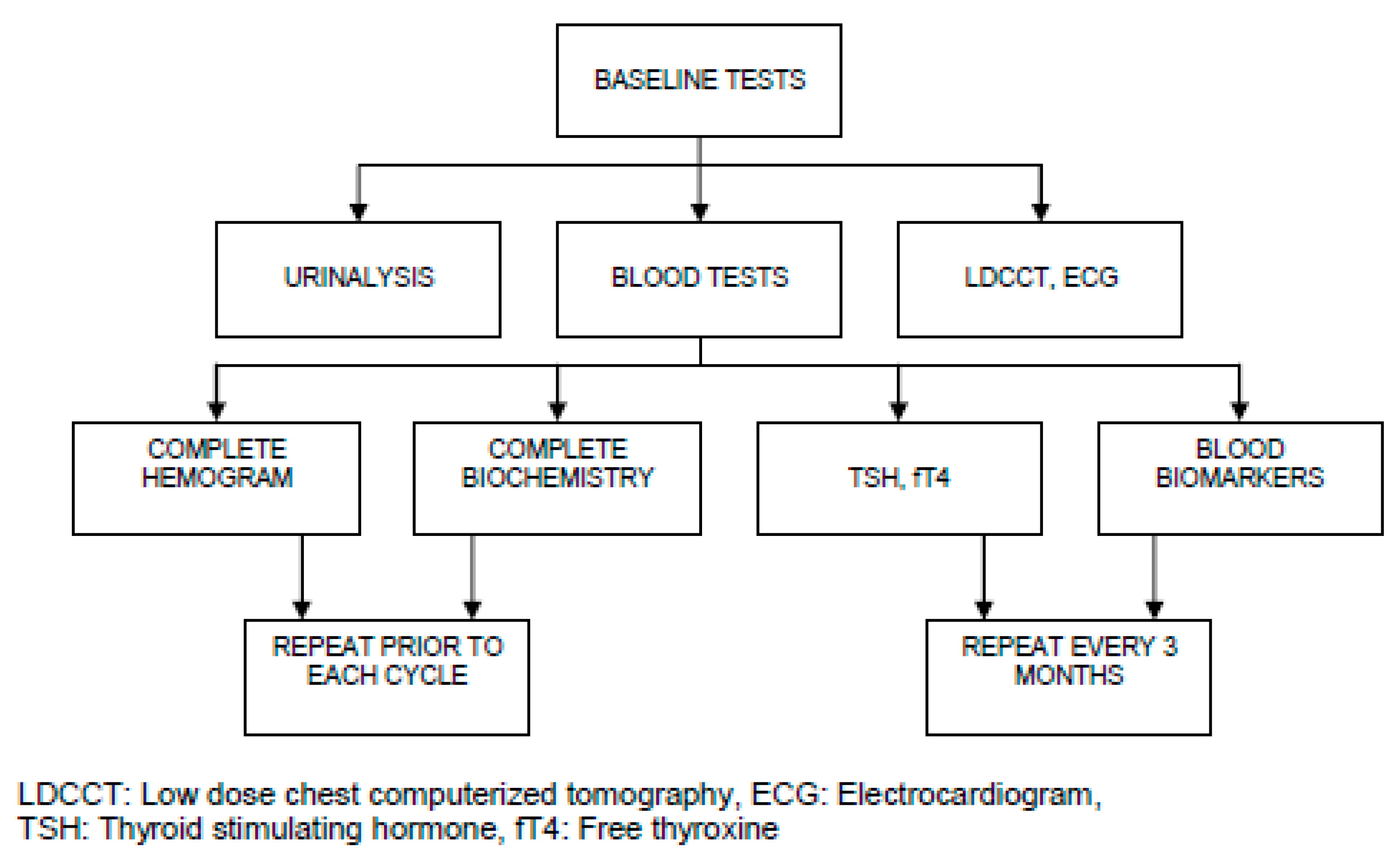
The Bottlenecks of Icıt, the Complexıty and Effectıve Management
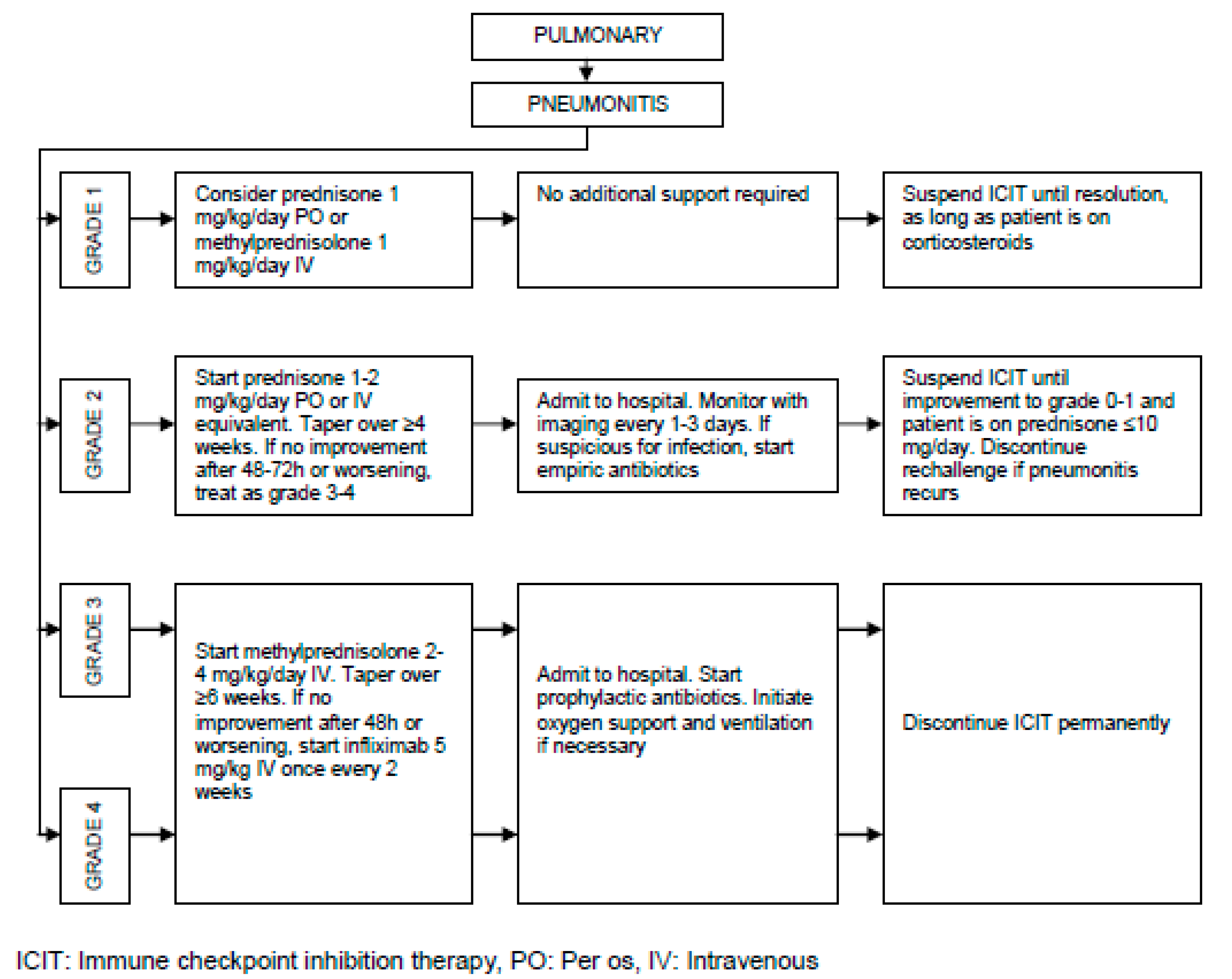
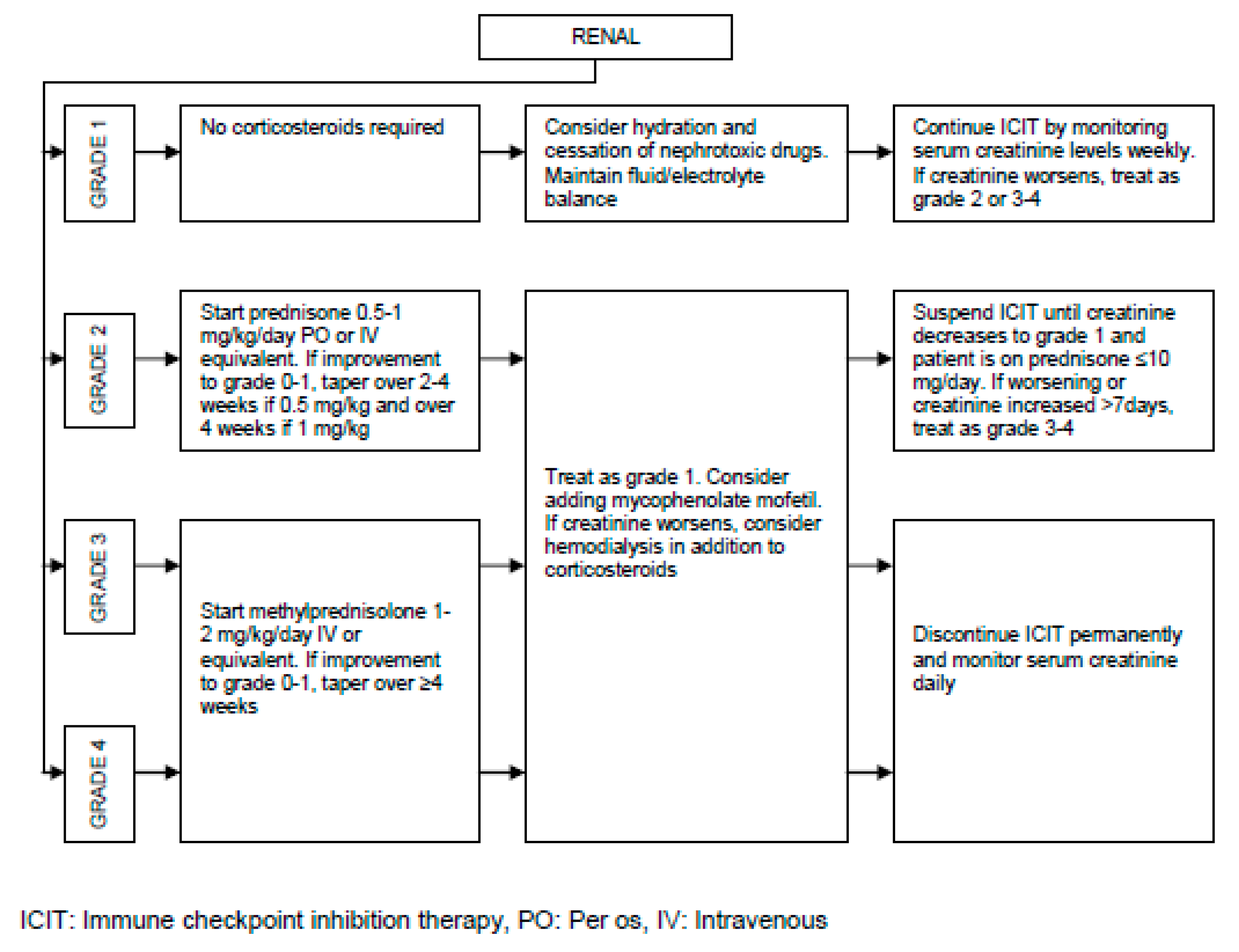
Pathogenesis of irAEs, Management Burden and Strategies:
Cancer Pain Management throughout ICIT:
Dıscussıon
- How soon the regression starts after the initial infusion
- What the rate of regression is
- How soon the remission is reached after the initial infusion
- The general personal immune strength
- The compatibility of baseline biomarkers with the ICIT
- Number of days without any irAE after the initial infusion
Decision about Optimum ICIT Duration:
Conclusıons
Funding
IRB approval
Clinical trials
Patient consent
Conflict of interest
References
- Mahoney, D.J.; Stojdl, D.F.; Laird, G. Virus Therapy for Cancer. Sci. Am. 2014, 311, 54–59. [Google Scholar] [CrossRef]
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer Immunoediting: Integrating Immunity’s Roles in Cancer Suppression and Promotion. Science 2011, 331, 1565–1570. [Google Scholar] [CrossRef]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef]
- Champiat, S.; Lambotte, O.; Barreau, E.; Belkhir, R.; Berdelou, A.; Carbonnel, F.; Cauquil, C.; Chanson, P.; Collins, M.; Durrbach, A.; et al. Management of immune checkpoint blockade dysimmune toxicities: a collaborative position paper. Ann. Oncol. 2015, 27, 559–574. [Google Scholar] [CrossRef] [PubMed]
- Chiou, V.L.; Burotto, M. Pseudoprogression and Immune-Related Response in Solid Tumors. J. Clin. Oncol. 2015, 33, 3541–3543. [Google Scholar] [CrossRef]
- Abril-Rodriguez, G.; Ribas, A. SnapShot: Immune Checkpoint Inhibitors. Cancer Cell 2017, 31, 848–848. [Google Scholar] [CrossRef]
- Linsley, P.S.; Wallace, P.M.; Johnson, J.; Gibson, M.G.; Greene, J.L.; Ledbetter, J.A.; Singh, C.; Tepper, M.A. Immunosuppression in Vivo by a Soluble Form of the CTLA-4 T Cell Activation Molecule. Science 1992, 257, 792–795. [Google Scholar] [CrossRef] [PubMed]
- Egen, J.G.; Allison, J.P. Cytotoxic T Lymphocyte Antigen-4 Accumulation in the Immunological Synapse Is Regulated by TCR Signal Strength. Immunity 2002, 16, 23–35. [Google Scholar] [CrossRef]
- Leach, D.R.; Krummel, M.F.; Allison, J.P. Enhancement of Antitumor Immunity by CTLA-4 Blockade. Science 1996, 271, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: current researches in cancer. Am J Cancer Res 2020, 10, 727–742. [Google Scholar]
- Dong, H.; Zhu, G.; Tamada, K.; Chen, L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999, 5, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Mohsenzadegan, M.; Bavandpour, P.; Nowroozi, M.R.; Amini, E.; Kourosh-Arami, M.; Momeni, S.A.; Bokaie, S.; Sharifi, L. The Potential of T Cell Immunoglobulin and Mucin-Domain Containing-3 (Tim-3) in Designing Novel Immunotherapy for Bladder Cancer. Endocrine, Metab. Immune Disord. - Drug Targets 2021, 21, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Sprangers, B. Pembrolizumab-related renal toxicities: diagnosis first, treatment later. Clin. Kidney J. 2018, 12, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, F.B.; Marrone, K.A.; Troxell, M.L.; Ralto, K.M.; Hoenig, M.P.; Brahmer, J.R.; Le, D.T.; Lipson, E.J.; Glezerman, I.G.; Wolchok, J.; et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int. 2016, 90, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Hanna, R.M.; Selamet, U.; Bui, P.; Sun, S.-F.; Shenouda, O.; Nobakht, N.; Barsoum, M.; Arman, F.; Rastogi, A. Acute Kidney Injury after Pembrolizumab-Induced Adrenalitis and Adrenal Insufficiency. Case Rep. Nephrol. Dial. 2018, 8, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Dumoulin, D.W.; Visser, S.; Cornelissen, R.; van Gelder, T.; Vansteenkiste, J.; von der Thusen, J.; Aerts, J.G.J.V. Renal Toxicity From Pemetrexed and Pembrolizumab in the Era of Combination Therapy in Patients With Metastatic Nonsquamous Cell NSCLC. J. Thorac. Oncol. 2020, 15, 1472–1483. [Google Scholar] [CrossRef]
- Izzedine, H.; Mathian, A.; Champiat, S.; Picard, C.; Mateus, C.; Routier, E.; Varga, A.; Malka, D.; Leary, A.; Michels, J.; et al. Renal toxicities associated with pembrolizumab. Clin. Kidney J. 2018, 12, 81–88. [Google Scholar] [CrossRef]
- Warner, B.M.; Baer, A.N.; Lipson, E.J.; Allen, C.; Hinrichs, C.; Rajan, A.; Pelayo, E.; Beach, M.; Gulley, J.L.; Madan, R.A.; et al. Sicca Syndrome Associated with Immune Checkpoint Inhibitor Therapy. Oncol. 2019, 24, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Som, A.; Mandaliya, R.; Alsaadi, D.; Farshidpour, M.; Charabaty, A.; Malhotra, N.; Mattar, M.C. Immune checkpoint inhibitor-induced colitis: A comprehensive review. World J. Clin. Cases 2019, 7, 405–418. [Google Scholar] [CrossRef]
- El Sabbagh, R.; Azar, N.S.; Eid, A.A.; Azar, S.T. Thyroid Dysfunctions Due to Immune Checkpoint Inhibitors: A Review. Int. J. Gen. Med. 2020, 13, 1003–1009. [Google Scholar] [CrossRef]
- Johnson, D.B.; McDonnell, W.J.; Gonzalez-Ericsson, P.I.; Al-Rohil, R.N.; Mobley, B.C.; Salem, J.-E.; Wang, D.Y.; Sanchez, V.; Wang, Y.; Chastain, C.A.; et al. A case report of clonal EBV-like memory CD4+ T cell activation in fatal checkpoint inhibitor-induced encephalitis. Nat. Med. 2019, 25, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liang, X.; Liang, J.; Li, Y.; Wang, J. Immune Thrombocytopenia Induced by Immune Checkpoint Inhibitors in Solid Cancer: Case Report and Literature Review. Front. Oncol. 2020, 10, 530478. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.; Nowak, E.; Etienne, M.; Legoupil, D.; Fouchard, M.; Brenaut, E.; Misery, L. Causes of Pruritus in Patients Treated With Immune Checkpoint Inhibitors for Melanomas or Skin Carcinomas. Front. Med. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Ghoraba, H.; Or, C.; Karaca, I.; Mishra, K.; Akhavanrezayat, A.; Park, S.; Than, N.; Leung, L.-S.; Sanislo, S.; Nguyen, Q.D. Immunotherapy-induced retinopathy mimicking cancer associated retinopathy. Am. J. Ophthalmol. Case Rep. 2022, 26, 101449. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, K.; Yanagihara, T.; Egashira, A.; Ogo, N.; Yoshizawa, S.; Sunami, S.; Asoh, T.; Maeyama, T. A Rare Case of Pembrolizumab-Induced Dermatomyositis in a Patient with Cancer of Unknown Primary Origin. Am. J. Case Rep. 2021, 22, e930286–1. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Go, M. Nivolumab Induced Adrenal Insufficiency: Rare Side-effect of a New Anti-cancer Therapy - Immune-checkpoint Inhibitors. Cureus 2020, 12, e7625. [Google Scholar] [CrossRef]
- Hercun, J.; Vincent, C.; Bilodeau, M.; Lapierre, P. Immune-Mediated Hepatitis During Immune Checkpoint Inhibitor cancer Immunotherapy: Lessons From Autoimmune Hepatitis and Liver Immunology. Front. Immunol. 2022, 13, 907591. [Google Scholar] [CrossRef]
- Weber, J.S.; Kähler, K.C.; Hauschild, A. Management of Immune-Related Adverse Events and Kinetics of Response With Ipilimumab. J. Clin. Oncol. 2012, 30, 2691–2697. [Google Scholar] [CrossRef] [PubMed]
- Hassel, J.C.; Heinzerling, L.; Aberle, J.; Bähr, O.; Eigentler, T.K.; Grimm, M.-O.; Grünwald, V.; Leipe, J.; Reinmuth, N.; Tietze, J.K.; et al. Combined immune checkpoint blockade (anti-PD-1/anti-CTLA-4): Evaluation and management of adverse drug reactions. Cancer Treat. Rev. 2017, 57, 36–49. [Google Scholar] [CrossRef]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef]
- Wang, D.Y.; Salem, J.-E.; Cohen, J.V.; Chandra, S.; Menzer, C.; Ye, F.; Zhao, S.; Das, S.; Beckermann, K.E.; Ha, L.; et al. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors A Systematic Review and Meta-analysis. JAMA Oncol. 2018, 4, 1721–1728. [Google Scholar] [CrossRef]
- Oble, D.A.; Mino-Kenudson, M.; Goldsmith, J.; Hodi, F.S.; Seliem, R.M.; Dranoff, G.; Mihm, M.; Hasserjian, R.; Lauwers, G.Y. α-CTLA-4 mAb-associated Panenteritis. Am. J. Surg. Pathol. 2008, 32, 1130–1137. [Google Scholar] [CrossRef]
- Read, S.; Malmström, V.; Powrie, F. Cytotoxic T Lymphocyte–Associated Antigen 4 Plays an Essential Role in the Function of Cd25+Cd4+ Regulatory Cells That Control Intestinal Inflammation. J. Exp. Med. 2000, 192, 295–302. [Google Scholar] [CrossRef]
- Troxell, M.L.; Higgins, J.P.; Kambham, N. Antineoplastic Treatment and Renal Injury: An Update on Renal Pathology Due to Cytotoxic and Targeted Therapies. Adv. Anat. Pathol. 2016, 23, 310–329. [Google Scholar] [CrossRef] [PubMed]
- Shirali, A.C.; Perazella, M.A.; Gettinger, S. Association of Acute Interstitial Nephritis With Programmed Cell Death 1 Inhibitor Therapy in Lung Cancer Patients. Am. J. Kidney Dis. 2016, 68, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef]
- Matson, D.R.; Accola, M.A.; Rehrauer, W.M.; Corliss, R.F. Fatal Myocarditis Following Treatment with the PD-1 Inhibitor Nivolumab. J. Forensic Sci. 2017, 63, 954–957. [Google Scholar] [CrossRef] [PubMed]
- Zha, H.; Han, X.; Zhu, Y.; Yang, F.; Li, Y.; Li, Q.; Guo, B.; Zhu, B. Blocking C5aR signaling promotes the anti-tumor efficacy of PD-1/PD-L1 blockade. OncoImmunology 2017, 6, e1349587. [Google Scholar] [CrossRef]
- Moller, D.R. Cells and cytokines involved in the pathogenesis of sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 1999, 16, 24–31. [Google Scholar]
- Facco, M.; Cabrelle, A.; Teramo, A.; Olivieri, V.; Gnoato, M.; Teolato, S.; Ave, E.; Gattazzo, C.; Fadini, G.P.; Calabrese, F.; et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2010, 66, 144–150. [Google Scholar] [CrossRef]
- Lomax, A.J.; McGuire, H.M.; McNeil, C.; Choi, C.J.; Hersey, P.; Karikios, D.; Shannon, K.; van Hal, S.; Carr, U.; Crotty, A.; et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Int. J. Rheum. Dis. 2017, 20, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017, 27, 894–901. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.K.; Shure, A.K.; Agrawal, D.K. Immune-mediated adverse events of anticytotoxic T lymphocyte–associated antigen 4 antibody therapy in metastatic melanoma. Transl. Res. 2015, 166, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Polakos, N.K.; Cornejo, J.C.; Murray, D.A.; Wright, K.O.; Treanor, J.J.; Crispe, I.N.; Topham, D.J.; Pierce, R.H. Kupffer Cell-Dependent Hepatitis Occurs during Influenza Infection. Am. J. Pathol. 2006, 168, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Teulings, H.-E.; Limpens, J.; Jansen, S.N.; Zwinderman, A.H.; Reitsma, J.B.; Spuls, P.I.; Luiten, R.M. Vitiligo-Like Depigmentation in Patients With Stage III-IV Melanoma Receiving Immunotherapy and Its Association With Survival: A Systematic Review and Meta-Analysis. J. Clin. Oncol. 2015, 33, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Freeman-Keller, M.; Kim, Y.; Cronin, H.; Richards, A.; Gibney, G.; Weber, J.S. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin. Cancer Res. 2016, 22, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abu-Sbeih, H.; Mao, E.; Ali, N.; Qiao, W.; Trinh, V.A.; Zobniw, C.; Johnson, D.H.; Samdani, R.; Lum, P.; et al. Endoscopic and Histologic Features of Immune Checkpoint Inhibitor-Related Colitis. Inflamm. Bowel Dis. 2018, 24, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- González-Rodríguez, E.; Rodríguez-Abreu, D.; on behalf of the Spanish Group for Cancer Immuno-Biotherapy (GETICA). Immune Checkpoint Inhibitors: Review and Management of Endocrine Adverse Events. Oncologist 2016, 21, 804–816. [Google Scholar] [CrossRef]
- Kumar, V.; Chaudhary, N.; Garg, M.; Floudas, C.S.; Soni, P.; Chandra, A.B. Current Diagnosis and Management of Immune Related Adverse Events (irAEs) Induced by Immune Checkpoint Inhibitor Therapy. Front. Pharmacol. 2017, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Friedman, C.F.; Proverbs-Singh, T.A.; Postow, M.A. Treatment of the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors. JAMA Oncol. 2016, 2, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Torino, F.; Barnabei, A.; De Vecchis, L.; Salvatori, R.; Corsello, S.M. Hypophysitis Induced by Monoclonal Antibodies to Cytotoxic T Lymphocyte Antigen 4: Challenges from a New Cause of a Rare Disease. Oncol. 2012, 17, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Linardou, H.; Gogas, H. Toxicity management of immunotherapy for patients with metastatic melanoma. Ann. Transl. Med. 2016, 4, 272–272. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Yang, J.C.; Atkins, M.B.; Disis, M.L. Toxicities of Immunotherapy for the Practitioner. J. Clin. Oncol. 2015, 33, 2092–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.C.; Luo, W.; Wang, Y. Acute liver injury in the context of immune checkpoint inhibitor-related colitis treated with infliximab. J. Immunother. Cancer 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Calvo, R. Hematological Side Effects of Immune Checkpoint Inhibitors: The Example of Immune-Related Thrombocytopenia. Front. Pharmacol. 2019, 10, 454. [Google Scholar] [CrossRef]
- Menzies, A.M.; Johnson, D.B.; Ramanujam, S.; Atkinson, V.G.; Wong, A.N.M.; Park, J.J.; McQuade, J.L.; Shoushtari, A.N.; Tsai, K.K.; Eroglu, Z.; et al. Anti-PD-1 therapy in patients with advanced melanoma and preexisting autoimmune disorders or major toxicity with ipilimumab. Ann. Oncol. 2017, 28, 368–376. [Google Scholar] [CrossRef]
- Johnson, D.B.; Sullivan, R.J.; Ott, P.A.; Carlino, M.S.; Khushalani, N.I.; Ye, F.; Guminski, A.; Puzanov, I.; Lawrence, D.P.; Buchbinder, E.I.; et al. Ipilimumab Therapy in Patients With Advanced Melanoma and Preexisting Autoimmune Disorders. JAMA Oncol. 2016, 2, 234–40. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Shah, M.; Lopez-Olivo, M.A.; Suarez-Almazor, M.E. Use of Immune Checkpoint Inhibitors in the Treatment of Patients With Cancer and Preexisting Autoimmune Disease. Ann. Intern. Med. 2018, 168, 121. [Google Scholar] [CrossRef]
- Horvat, T.Z.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D'Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients With Melanoma Treated With Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [PubMed]
- Bessede, A.; Marabelle, A.; Guégan, J.; Danlos, F.; Cousin, S.; Peyraud, F.; Chaput, N.; Spalato, M.; Roubaud, G.; Cabart, M.; et al. Impact of acetaminophen on the efficacy of immunotherapy in cancer patients. Ann. Oncol. 2022, 33, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Lissoni, P.; MandalÀ, M.; Brivio, F. Abrogation of the Negative Influence of Opioids on IL–2 Immunotherapy of Renal Cell Cancer by Melatonin. Eur. Urol. 2000, 38, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, R.A.; Metselaar-Albers, M.; Engels, F. Concomitant use of analgesics and immune checkpoint inhibitors in non-small cell lung cancer: A pharmacodynamics perspective. Eur. J. Pharmacol. 2021, 906, 174284. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Saâda-Bouzid, E.; Defaucheux, C.; Karabajakian, A.; Coloma, V.P.; Servois, V.; Paoletti, X.; Even, C.; Fayette, J.; Guigay, J.; Loirat, D.; et al. Hyperprogression during anti-PD-1/PD-L1 therapy in patients with recurrent and/or metastatic head and neck squamous cell carcinoma. Ann. Oncol. 2017, 28, 1605–1611. [Google Scholar] [CrossRef] [PubMed]
- Freixinos, V.R.; Garcia, A.; Fasani, R.; Castellvi, J.; Ruiz-Pace, F.; Viaplana, C.; Fariñas-Madrid, L.; Casal, C.; Matos, I.; Martin-Liberal, J.; et al. Immune profile and outcomes of patients (pts) with gynecological malignancies (GYN) enrolled in early phases immunotherapy (IO) trials. J. Clin. Oncol. 2018, 36, 5595–5595. [Google Scholar] [CrossRef]
- Matos, I.; Martin-Liberal, J.; Hierro, C.; De Olza, M.O.; Viaplana, C.; Costa, M.; Felip-Falg’s, E.; Mur-Bonet, G.; Vieito, M.; Brana, I.; et al. Incidence and clinical implications of a new definition of hyperprogression (HPD) with immune checkpoint inhibitors (ICIs) in patients treated in phase 1 (Ph1) trials. J. Clin. Oncol. 2018, 36, 3032–3032. [Google Scholar] [CrossRef]
- Arnold, C.E.; Rajnicek, A.M.; Hoare, J.I.; Pokharel, S.M.; Mccaig, C.D.; Barker, R.N.; Wilson, H.M. Physiological strength electric fields modulate human T cell activation and polarisation. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Salerno, S.; La Mendola, C.; La Manna, M.P.; Casto, A.L.; Caccamo, N.; Salerno, A. Reversible Effect of Magnetic Fields on Human Lymphocyte Activation Patterns: Different Sensitivity of Naive and Memory Lymphocyte Subsets. Radiat. Res. 2009, 172, 444–450. [Google Scholar] [CrossRef]










| Organ/Tissue System | irAEs | Approx. Onset Frequency (%) |
|---|---|---|
| Gastrointestinal | Oral mucositis | < 5 |
| Xerostomia | < 6 | |
| Gastritis | > 50 (PD-1), < 5 (PD-L1) | |
| Colitis | > 10 | |
| Ileitis | > 10 | |
| Hepatitis | 5 – 10 | |
| Pancreatitis | < 5 | |
| Dermatologic | Dermatitis | > 20 |
| Pruritus | 10 – 50 | |
| Stevens Johnson | < 5 | |
| Psoriasis | < 5 | |
| Vitiligo | 5 – 10 | |
| DRESS syndrome | < 4 | |
| Endocrine | Hypohysitis | 5 – 10 |
| Hyper-/hypothyroidism | < 10 | |
| Diabetes Mellitus | < 3 | |
| Addison’s disease | 5 – 10 | |
| Pulmonary | Pleuritis | < 1 |
| Pneumonitis | < 5 | |
| Sarcoid-like granulomatosis | 5 – 7 (CTLA-4), < 0.5 (PD-L1) | |
| Neurologic | Encephalitis | < 5 |
| Meningitis | < 5 | |
| Guillain Barré | < 5 | |
| Neuropathy | < 5 | |
| Myasthenia gravis | < 5 | |
| Myelopathy | < 1 | |
| Optic neuritis | < 1 | |
| Cardiovascular | Myocarditis | < 5 |
| Pericarditis | < 3 | |
| Vasculitis | < 1 | |
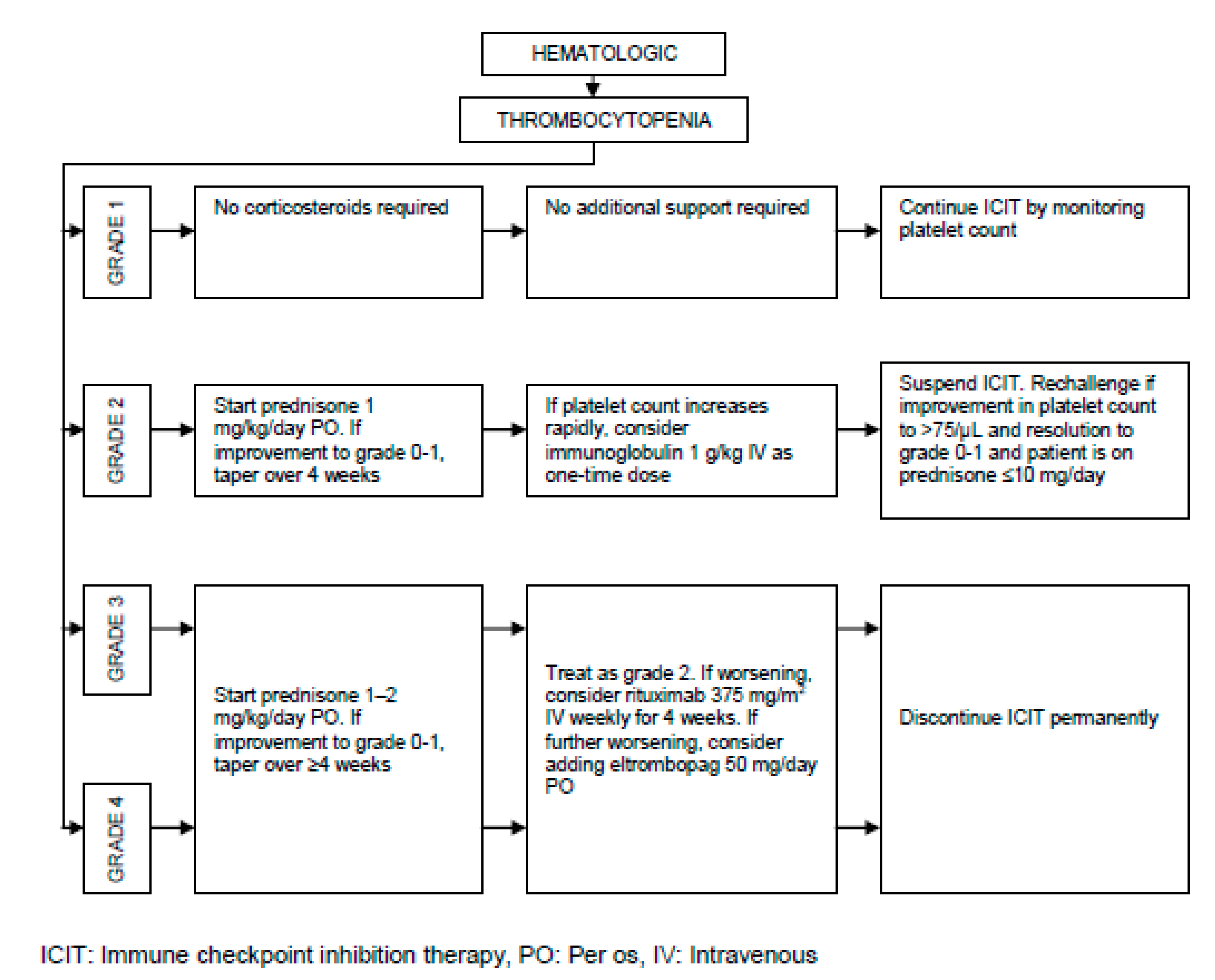
| Hematologic | Thrombocytopenia | < 2 |
| Neutropenia | < 1 | |
| Hemolytic anemia | < 5 (CTLA-4), < 10 (PD-1) | |
| Pancytopenia | < 1 | |
| Renal | Acute interstitial nephritis | < 5 |
| Acute tubular injury | < 1 | |
| Glomerulonephritis | < 2 | |
| Musculoskeletal | Dermatomyositis | < 1 |
| Arthritis | < 4 | |
| Ocular | Retinitis | < 3 |
| Conjunctivitis | < 1 | |
| Uveitis | < 5 | |
| Scleritis | < 1 | |
| irAEs: Immune related adverse events CTLA-4: Cytotoxic T lymphocyte associated protein 4 PD-1: Programmed cell death protein 1 PD-L1: Programmed cell death ligand 1 DRESS: Drug reaction with eosinophilia and systemic symptoms | ||
| ICIT | irAEs | Onset Time (week) |
CTCAE Peak (week) |
Damping Time (week) |
|---|---|---|---|---|
| CTLA-4 | Colitis | 4 – 5 | 6 | 10 |
| Hepatitis | 6 – 7 | 8 – 13 | 15 | |
| Pancreatitis | 3 – 9 | N/A | N/A | |
| Pruritus | ≈ 4 | ≈ 5 | ≈ 6 | |
| Hypohysitis | 6 – 7 | 8 | ∞ | |
| Addison’s disease | 6 – 7 | 8 | ∞ | |
| Myasthenia gravis | 2 – 6 | 1 – 4 | N/A | |
| Acute interstitial nephritis | 2 – 12 | N/A | N/A | |
| PD-1 or PD-L1 | Colitis | 4 – 5 | 6 | 10 |
| Hepatitis | 6 – 7 | 8 – 14 | 15 | |
| Pancreatitis | 3 – 30 | N/A | N/A | |
| Pruritus | ≈ 4 | ≈ 5 | ≈ 6 | |
| Hypohysitis | 6 – 7 | 8 | ∞ | |
| Addison’s disease | 6 – 7 | 8 | ∞ | |
| Pneumonitis | 10 – 11 | 12 | 22 | |
| Myasthenia gravis | 4 – 5 | N/A | N/A | |
| Acute interstitial nephritis | 12 – 72 | N/A | N/A | |
| CTLA-4 and PD-1 | Colitis | 3 – 4 | 5 | 10 |
| Hepatitis | 5 – 6 | 7 – 13 | 15 | |
| Pruritus | ≈ 2 | ≈ 4 | ≈ 5 | |
| Hypohysitis | ≈ 2 | 4 | ∞ | |
| Addison’s disease | ≈ 2 | 4 | ∞ | |
| Pneumonitis | ≈ 5 | ≈ 7 | ≈ 11 | |
| Myasthenia gravis | 2 | N/A | N/A | |
| ICIT: Immune checkpoint inhibition therapy irAEs: Immune related adverse events CTCAE: Common terminology criteria for adverse events CTLA-4: Cytotoxic T lymphocyte associated protein 4 PD-1: Programmed cell death protein 1 PD-L1: Programmed cell death ligand 1 N/A: Not available | ||||
| ICIT | irAEs | Onset Time (week) |
Frequency Peak (week and %) |
Damping Time (week) |
|
|---|---|---|---|---|---|
| CTLA-4 and PD-1 | Gastrointestinal | 0 – 1 | ≈ 5 | ≈ 46 | 6 – 8 |
| Dermatologic | 0 | ≈ 2 | ≈ 52 | 4 – 5 | |
| Endocrine | 2 – 3 | ≈ 10 | ≈ 9 | ≈ 18 | |
| Pulmonary | ≈ 1 | 5 – 6 | 25 | ≈ 10 | |
| Hepatic | 0 | 4 – 5 | 39 | ≈ 8 | |
| Renal | 1 | 5 – 6 | ≈ 5 | 10 | |
| Other | 2 | 10 | ≈ 2 | 18 | |
| ICIT: Immune checkpoint inhibition therapy irAEs: Immune related adverse events CTLA-4: Cytotoxic T lymphocyte associated protein 4 PD-1: Programmed cell death protein 1 | |||||
| Diagnosis | TSH | fT4 |
|---|---|---|
| Primary hypothyroidism | High | Low |
| Subclinical hypothyroidism | High | Normal |
| Secondary hypothyroidism | Normal | Low |
| Primary hyperthyroidism | Low | High |
| Subclinical hyperthyroidism | Low | Normal |
| TSH: Thyroid stimulating hormone, fT4: Free thyroxine | ||
| Class | Type | Examples | Notes |
|---|---|---|---|
| Nonopioids | NSAIDs | Ibuprofen, aspirin, diflunisal, piroxicam, naproxen | Risk of renal failure in chronic use, not recommended in hemostatic disorders, risk of gastrointestinal bleeding |
| Acetaminophen | Paracetamol | Risk of hepatotoxicity in overdosage | |
| Weak opioids | Codeine | Oxycodone, hydrocodone, propoxyphene | Mostly in combination with NSAIDs or acetaminophen |
| Potent opioids with short half-life | Morphine Fentanyl |
Oxymorphone, hydromorphone, meperidine, pentazocine, butorphanol | Meperidine not recommended in patients with renal disease |
| Potent opioids with long half-life | Methadone Levorphanol |
Risk of withdrawal symptoms in physically dependent patients |
| Parameter | Definition of scoring |
|---|---|
| Score with 100, if regression starts after the first initial infusion | |
| Score with 100 – 5x, if regression starts after the xth infusion where x=2,...,6 | |
| Score with 100 – 7y, if regression starts after the yth infusion where y=7,...,11 | |
| Score with 10, if regression starts beyond the 12th infusion | |
| Score with 0, if no regression is observed at the assessment stage | |
| Score with x, where x denotes tumor volume shrinkage in % at the assessment stage | |
| Score with the average for multiple tumors, i.e. number of tumors | |
| Score with 100, if remission starts latest after the 3rd infusion | |
| Score with 100 – 10(x – 3), if remission starts after the xth infusion where x=4,...,11 | |
| Score with 10, if remission starts beyond the 12th infusion | |
| Score with 0, if no remission is observed at the assessment stage | |
| Score between 75 – 100, if there is no preexisting comorbidity and autoimmune disorder | |
| Score between 50 – 74, if there is only one preexisting comorbidity or autoimmune disorder | |
| Score between 0 – 49, if there is more than one preexisting comorbidity or autoimmune disorder | |
| Score with 100, if all of the baseline biomarkers in Figure 4 present compatibility with ICIT | |
| Score with 100 – 14(7 – x), if x of the baseline biomarkers in Figure 4 present compatibility with ICIT where x=1,...,6 | |
| Score with 0, if none of the baseline biomarkers in Figure 4 present compatibility with ICIT | |
| Score with 5, if there is no irAE after the first initial infusion | |
| Score with 5x, if there is no irAE after the xth infusion where x=2,...,20 | |
| Score with 0 when any irAE is observed at the assessment stage | |
| Increment each w with whose corresponding parameter scored between 60 – 100 in the previous assessment stage where denotes number of weightings to be incremented | |
| Decrement each w with whose corresponding parameter scored between 0 – 40 in the previous assessment stage where denotes number of weightings to be decremented | |
| ICIT: Immune checkpoint inhibition therapy : Timing of regression start : Regression rate : Timing of remission start : Personal immune strength score : Baseline biomarker compatibility : Time without toxicity or irAEs : Person-specific dynamic weightings | |
| score range | Optimum ICIT duration |
|---|---|
| 90 – 100 | Exit after 9th infusion |
| 75 – 89 | Exit after 12th infusion |
| 60 – 74 | Exit after 16th infusion |
| 50 – 59 | Exit after 22th infusion |
| 25 – 49 | Exit after 30th infusion |
| 0 – 24 | Exit after 35th infusion |
| ICIT: Immune checkpoint inhibition therapy : ICIT success rate | |
| Step# | Description of step |
|---|---|
| 1 | ICIT continues |
| 2 | Check whether the patient is psychologically doing well and GO TO step 3 if YES, otherwise WITHHOLD ICIT until improvement |
| 3 | Check whether the patient is symptomatically doing well and GO TO step 4 if YES, otherwise WITHHOLD ICIT until improvement |
| 4 | Check whether the patient has developed any irAE or toxicity and GO TO step 5 if NO, otherwise REFER to management algorithms presented in the previous section |
| 5 | Check whether MRNn+1 > MRNn (n є ℕ) and GO TO step 6 if NO, otherwise DISCONTINUE ICIT permanently |
| 6 | Check whether NTn+1 > NTn+2 (n є ℕ) and GO TO step 7 if NO, otherwise DISCONTINUE ICIT permanently |
| 7 | Check whether VTn+1 > 1.15xVTn (n є ℕ) and GO TO step 8 if NO, otherwise DISCONTINUE ICIT permanently |
| 8 | CONTINUE ICIT |
| 9 | GO TO step 1 and MAKE n=n+1 |
| ICIT: Immune checkpoint inhibition therapy irAE: Immune related adverse event MRNn: Number of metastatic regions at analysis stage n NTn: Number of existing tumors at analysis stage n VTn: Existing tumor volumes at analysis stage n ℕ: Natural numbers | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




