1. Introduction
Ferroptosis was first reported in 2012 [
2,
3], as an iron-dependent mechanism of non-apoptotic cell death [
4], which has attracted the researchers’ interest due to its implications in the pathogenesis of various age-related disorders. For instance, it can play both a negative and a positive role, as it may be involved in cancer initiation and development, but also suppression [see for instance the review] [
5].
The iron-dependent regulated necrosis cell death is characterized by massive membrane damage mediated by uncontrolled lipid peroxidation [
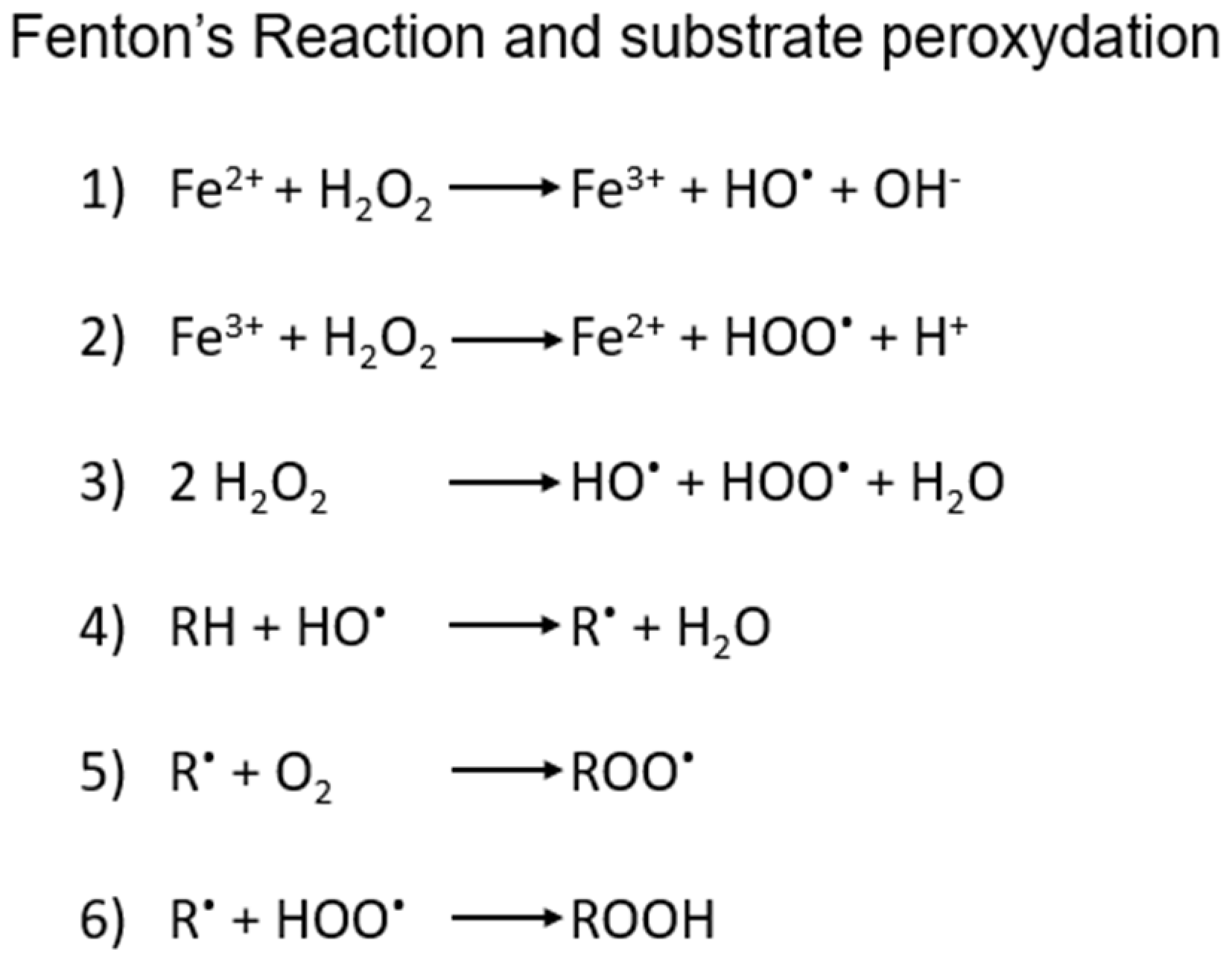
6], which is in turn triggered by redox mechanisms connected to the Fenton’s reaction. The latter is a rather common process in cells, involving the Fe(II/III)-catalyzed oxidation of organic substrates by H
2O
2 through a multi-step formation of highly reactive oxidizing species, such as the hydroxyl and hydroperoxyl radicals (shortly summarized in
Scheme 1), that in some cases may involve also lipids and phospholipids at their carbon-carbon double bonds, especially the polyunsaturated fatty acids (PUFAs) of the plasma membrane or membrane-enclosed organelles.
Ferroptosis demonstrated to play a key role in cellular homeostasis leading to a gradual and progressive functional decline that eventually causes inevitable death. It was first identified in the early 2000s, but the understanding of the mechanisms was completed between 2012 and 2014, and ferroptosis was included among the 12 different types of cell death pathways, mechanistically distinct from apoptosis and other forms of necrosis, including necroptosis and pyroptosis [
7,
8].
Dixon and Stockwell (2019) provided an overview of the hallmarks of ferroptosis. It was Dixon's research that demonstrated that ferroptosis can occur in cells without a functioning mitochondrial electron transport chain (ETC), as seen in HT1080 cells [
9].
The authors described the distinguishing features of ferroptosis as being related to three characteristics:
(a) the availability of redox-active iron
(b) the availability of chemically competent substrates to undergo peroxidation (e.g. PUFA phospholipids)
(c) the loss of the cellular lipid repair system that normally prevents the accumulation of these lethal species.
Ferroptosis, as described by the authors, varies among different cell types and even within the same cell type, linked to a unique expression pattern for proteins involved in iron, lipid, and antioxidant processes.
Thus, the mechanisms governing ferroptosis are slowly being unveiled [
10], but they are mainly centered on cysteine and glutathione (GSH) metabolism and the ability of the phospholipid peroxidase GPX4 to prevent iron-dependent accumulation of peroxidized lipids. Basically, it is characterized by the failure of glutathione-dependent antioxidant defenses, resulting in iron-catalyzed peroxidation of polyunsaturated fatty acid phospholipids, which is countered by GSH activity as a ligand for cytosolic Fe
2+ [
11] and as a substrate for glutathione peroxidase-4, to eliminate membrane lipid peroxides that lead, when accumulated, to ferroptotic cell death [
9,
12,
13].
Many researchers have hypothesized that the increase in Fe
2+ levels catalyzes the Fenton’s reaction cascade to generate hydroxyl radicals from hydrogen peroxide, and the consequent depletion of GSH, which fuels the catalytic increase in lipid peroxidation, predisposing cells to ferroptosis and biological aging. Studies have shown the propagation of ferroptosis in a paracrine manner outward from the affected cells. Although the details of this mechanism are still unclear, the relatively stable and diffusible toxic lipid peroxidation end-products 4-hydroxynonenal and malondialdehyde may mediate this effect by contributing to cellular senescence [
14]. Directly or indirectly induced senescence is proposed to be a gradual deterioration of cellular, tissue, and living organism functional characteristics associated with redox imbalances with chronic induction and upregulation of pro-inflammatory mediators (e.g., TNF-α, IL-1β, IL-6, COX-2, iNOS) and signaling pathways such as NF-KB, and subsequent reduction in the activity of antioxidant pathways[
15].
During senescence, growth stops in a stable manner to limit the replication of old and damaged cells; cells undergo morphological changes, chromatin remodeling and metabolic reprogramming by secreting predominantly proinflammatory factors called senescence-associated secretory phenotype (SASP) [
16]. While aging, the amount of circulating iron is reduced and tissue and intracellular iron stores increase, leading to deleterious effects on cellular functions due to redox imbalances causing ferroptosis and/or contributing to aging, associated morbidity and increased mortality [
17]. It has been hypothesized that iron dyshomeostasis and ferroptosis are central to the mechanisms underlying the gradual dysfunction observed and underlying aging, certainly acting towards the end of life as “executioners” (
vide infra).
Authors as Stroustrup et al. [
18] demonstrated interventions in longevity by interfering in metabolic pathways of insulin-like growth factor 1 (IGF-1), hypoxia-inducible factor (hif-1) and heat shock factor (HSF-1) on
C. elegans, which alter lifespan with apparent “temporal scaling”. It was found that salicylaldehyde isonicotinoyl hydrazone (SIH), a lipophilic acyl-hydrazone that eliminates intracellular iron for extracellular clearance, and Liproxstatin-
1 (Lip-1), an inhibitor of ferroptosis through the inactivation of lipid peroxide radicals, can reshape life stages.
SIH reduces the risk of death in middle age through cellular downsizing, while Lip-1 acts in late post-reproductive age. While iron accumulation contributes to many processes that can modulate the rate of aging, inhibition of ferroptosis reduces frailty (late-life survival) rather than modulating the overall rate of aging. It is then possible that iron dyshomeostasis and ferroptosis are not central to the mechanisms underlying the observed gradual dysfunction, but act towards the end of life as “executioners” to rapidly damage and degrade functions.
2. Methods
This review work was undertaken under the guidance of Preferred Reporting Items for Systematic Review and Meta-analysis.
Search strategy
A comprehensive search of multiple databases was conducted in order to identify relevant studies. The databases searched were PubMed, Scopus, Google Scholar, Web of Science, and the Cochrane Library. The search included papers published until December 2022. The search terms used were “senescence”, “ferroptosis”, “aging” and “disease”. The search was limited to English language papers only.
Inclusion criteria
This review considered studies investigating senescence and/or ferroptosis in any tissue or organ as they satisfied the eligibility criteria. Inclusion criteria were directed towards studies focusing on both human or animal subjects.
The following information was extracted from each paper: study design, sample size, participant characteristics such as age, gender, disease status, interventions, outcomes, and results. The study characteristics were then tabulated.
Quality Assessment
The quality of the included studies was assessed using the Cochrane Risk of Bias tool. This tool comprises six domains: random sequence generation, allocation concealment, blinding participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting. Each domain was rated as “low risk”, “high risk”, or “unclear risk” based on the information provided in the study. Two independent reviewers performed the quality assessment, and any discrepancies were resolved through discussion and consensus. The overall quality of each study was rated as “low risk”, “high risk”, or “unclear risk” based on the ratings of the individual domains.
Analysis
This review aims to assess the current evidence on the potential mechanisms underlying the link connecting senescence, ferroptosis, aging, and disease, although the exact mechanisms underlying this connection are still not fully understood.
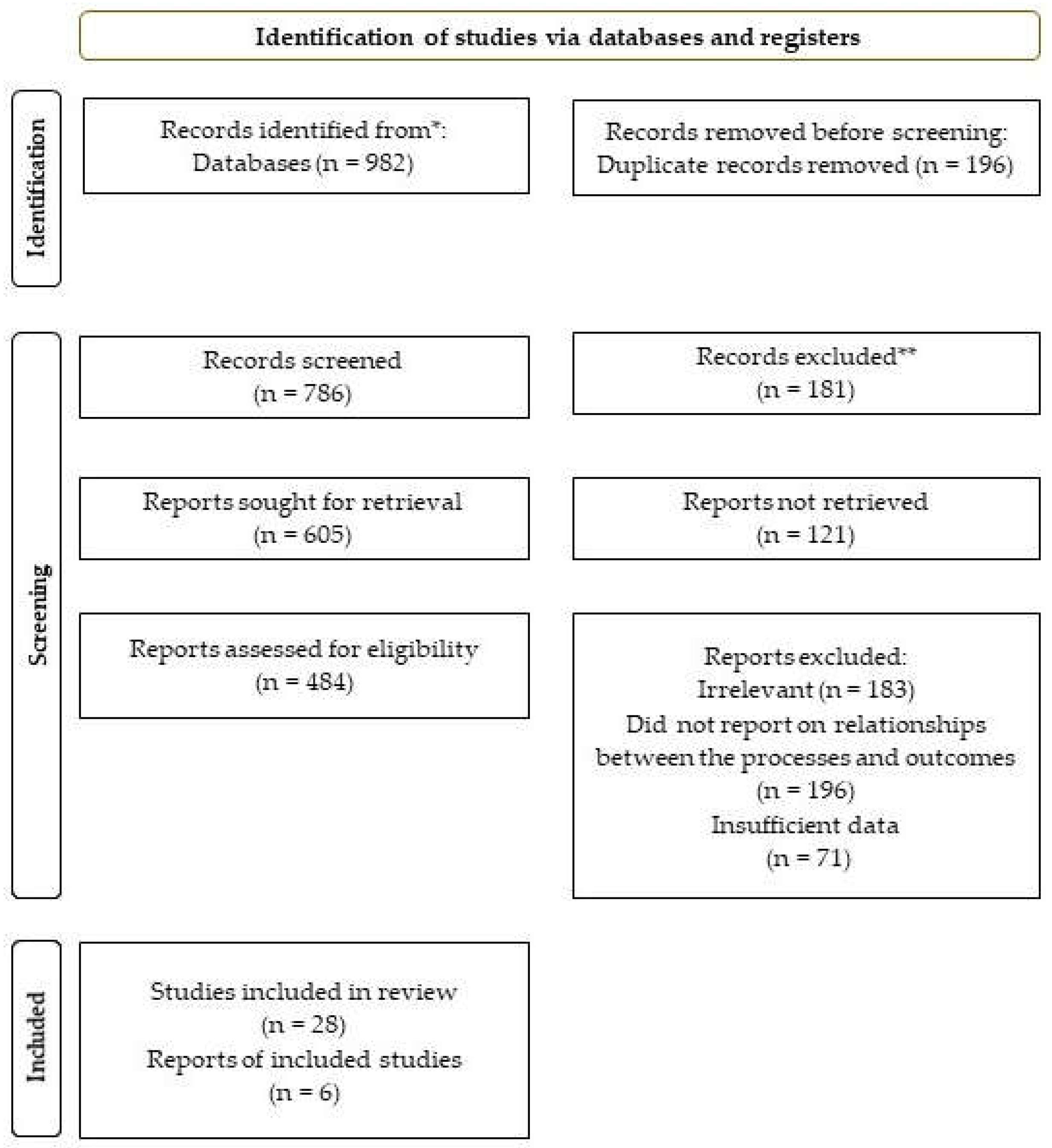
The search of study selection identified a total of 982 studies that met the inclusion criteria. They included a variety of designs, such as randomized controlled trials (RCTs), observational and in vitro studies. Most of the studies were conducted in animal models, although a few were in human subjects. Out of the 982 articles, 196 were excluded for being duplicates. Abstract screening resulted in the elimination of 181 studies. Of the remaining 605 articles, 121 could not be retrieved. The study design screening further excluded 183 articles since they were deemed unsuitable. A further exclusion decision eliminated 196 studies since they failed to report on the relationship linking senescence, ferroptosis, disease, aging, and cell death. Of the remaining studies, 71 were eliminated due to insufficient data, while some others were written in languages other than English. Therefore, 34 studies were deemed suitable for this review, as reported in
Scheme 2.
3. Results and Discussion
The implication of ferroptosis in senescence development and progress can be inferred by the interesting results reported in a number of studies focusing on the role of this regulated form of cell death (RCD) in ageing-related processes, such as cardiovascular conditions, neurodegeneration, and cancer.
Zhou et al. (2020) explored the mechanisms of ferroptosis and the implications of aging and age-related complications. This is a particularly burdensome problem for healthcare systems worldwide given the rapidly increasing aging population. Researchers have concluded that ferroptosis contributes significantly to age-related diseases, including cancer, cardiovascular diseases, and neurodegenerative conditions. This assertion is related to the mechanism by which cells undergoing ferroptosis secrete factors that strongly activate the innate immune system. The authors point out that to date there is still a lack of clear evidence of ferroptosis in human cells and tissues in human autopsy to understand the mechanism by which ferroptosis regulates degeneration, the main cause of tissue damage and organ failure [
19].
Zhao, T., (2021) [
20] discussed an overview of the mechanisms of iron accumulation and lipid peroxidation in the aging retina and the role of iron accumulation in the development of age-related macular degeneration (AMD), and the potential involvement of ferroptosis in this process. Intracellular iron concentration is finely regulated at three levels: control of iron uptake, modulation of the labile iron pool, and regulation of iron export[
21,
22,
23,
24]. Excess iron can be toxic by forming ROS through the Fenton’s mechanism, initiated by a reaction between ferrous iron and hydrogen peroxide [
25]. Among the proteins involved in the phototransduction cascade in the retina [
26], the RPE65 protein converts 11-cis-retinal to all-trans-retinyl as part of the retinoid cycle required for iron-dependent phototransduction [
27]. Furthermore, iron is essential for guanylate cyclase in the phototransduction pathway [
26]: if iron accumulates, it becomes toxic by forming ROS, through the Fenton’s reaction [
25], exceeding the cellular antioxidant capacity; the aberrant production of ROS damages DNA, and proteins and lipids within the retina[
28,
29]. This process leads to the production of two major products of lipid peroxidation, malondialdehyde (MDA) and 4-hydroxynonenal (4-HNE), which increase in the inner segments of photoreceptors, producing oxidative stress-induced cell death as the final event in the cell death cascade that underlies the pathogenesis of AMD. These findings have been observed both in AMD patients and animal models [
30,
31].
This, on the other hand, was already highlighted by Yun Sun et al in 2018 when examining cultured ARPE-19 cells and the effects of GSH depletion on stress-induced premature senescence (SIPS). Cell death was caused by GSH depletion which in turn induced ferroptosis, but also autophagy and SIPS, with autophagy being a negative regulator of SIPS. However, the study did not clarify whether ferroptosis is a process of autophagy activation or a prerequisite for it.
Guo et al. (2022) as well explored the molecular mechanism of aging and age-related diseases and their respective treatments and interventions. The study of the mechanisms of aging included various areas such as epigenetic regulation, proteostasis, autophagy, cellular senescence, and stem cells. Among the many causes of aging, whose mechanisms are extremely complex, they highlighted how the levels of autophagy-related proteins directly influence the organismal aging. In other words, the study found that the concentration of autophagy-related proteins decreases in an individual with advancing age and that translocation to lysosomes is reduced, which promotes the aging of the organism. Furthermore, the researchers found much higher iron levels in senescent cells than in non-senescent or immortalized cells due to defective autophagic degradation of ferritin in lysosomes [
32].
Evidence from other studies suggests that several mechanisms may be involved in the association of senescence, ferroptosis, aging, and disease [
33]. Some of these mechanisms we already mentioned, such as lipid peroxidation, glutathione peroxidase 4 enzyme activity, and iron metabolism, are able to trigger the secretion of pro-inflammatory cytokines and other molecules, including interleukin-6 (IL-6) and tumour necrosis factor-alpha (TNF-alpha) by senescent cells. Their involvement is relevant in the pathophysiology of certain neurodegenerative conditions such as Alzheimer's, Parkinson's, and Huntington’s diseases [
34].
From a mechanistic point of view, ferroptosis is believed to be linked to the activation of these pro-inflammatory pathways, which may contribute to the death of damaged or sick cells and thus counteract senescence characterized by the evasion of programmed cell death mechanisms. It is worth noting that disease mechanisms are broad and vary depending on the type and changes involved at the organismal, cellular, or molecular level [
35]. Some notable disease mechanisms are genetic mutations, environmental exposures, and infections. Mutations in specific genes can trigger changes in molecular structures and the production of abnormal proteins, resulting in disease [
36,
37]. In addition, exposure to environmental polyforms and other harmful substances can cause incidences such as cancer. One limitation of the studies included in this review is that many were conducted in animal models, which may not fully reflect the processes occurring in humans. In addition, the studies included a variety of designs and methods, which may make it difficult to compare the results across studies. It is also worth noting that the mechanisms underlying the link among senescence, ferroptosis, aging, and disease are likely complex and multifaceted. They may involve other factors in addition to those discussed in this review. Further research is, however, needed to fully understand the role of these mechanisms and to identify potential interventions that could target such processes in order to prevent or treat these diseases.
From the observation of these results, it appears that senescence and ferroptosis may be directly related, but there are also many other studies that focused on specific aspects linked to the general decay of the organism during the ageing process that should be examined in detail, as follows.
3.1. Brain damage
Among age-related conditions, cerebrovascular diseases are some of the most frequent medical challenges that raise awareness on the limitations in clinical treatment strategies. These represent the second leading cause of death in the world population and the sixth most common cause of disability. Ferroptosis, caused by abnormal metabolism of lipids, in which the brain is particularly rich, accelerates acute injury to the central nervous system. Recent studies have gradually uncovered the pathological process of ferroptosis in the neurovascular unit of acute stroke. Altered homeostasis of iron [
38], the most abundant metal in the brain necessary for cerebral metabolism [
39], produces abnormal overload in specific regions of the brain, promoting disease progression [
37]. This process accompanies pathological aggravation and is considered to be an important pathophysiological factor involved in secondary damage after ischemic stroke [
40]. The study aimed at evaluating molecular targets for plant active ingredients of natural products that regulate ferroptosis after ischemic stroke. The molecules evaluated belonged to the macro-families of saponins, flavonoids, and polyphenols. These biomolecules were used in animal models while a follow-up of clinical studies in large-scale, long-term patients is lacking.
Yihang Pan [
41] put attention in describing the link between ferroptosis and ischemia-reperfusion (I/R) injury in various conditions, including ischemic stroke, myocardial infarction, heart attack, acute lung injury, liver transplantation, acute kidney injury, and hemorrhagic shock. Through the study of ferroptosis-regulated genes studied in the context of I/R, they suggest beneficial applications of ferroptosis regulators as a therapeutic target to alleviate I/R injury.
It was previously reminded that iron dyshomeostasis and lipid peroxidation are associated with aging. Brain iron homeostasis is finely regulated by a delicate balance of iron movement between blood and tissue across the blood-brain barrier; intracellular and extracellular environments; and between different iron pools. This process is the major risk factor for neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD) where intracellular iron accumulation was first observed. For this reason, a number of studies and reviews attempt to elucidate the role of ferroptosis in the brain and the understanding of the mechanism of neurological diseases to provide potential prevention and treatment interventions for neurological conditions including neurodegeneration, stroke, and neurotrauma [
1]. Masaldan et al. (2018), have studied senescence in mouse embryonic fibroblasts (MEFs) and measured intracellular iron by inductively coupled plasma mass spectrometry (ICP-MS). while examining the relationship between altered cellular iron acquisition and storage and also impedes iron-mediated cell death pathways, noted a link between impaired ferritinophagy, a lysosomal process that explains the accumulation of iron, and ferroptosis development [
42].
Thereafter, Masaldan et al. (2019), gathered evidence linking neurodegenerative disorders to the induction of senescence in brain cells following iron accumulation while examining iron dyshomeostasis in the context of Alzheimer’s disease [
43].
Derry et al., analyzed, among all the pathologies associated with senescence, the dysfunctions involved in the course of Alzheimer's disease, an insidious and multi-aetiological illness. The discovery of oligomeric tau and of the numerous mechanisms that lead to iron binding in various forms increases intracellular iron, which may contribute to sensitivity to ferroptosis. To this it should be added that in Alzheimer's disease certain aspects of the glutathione synthesis and utilization pathways are disrupted. Analysis of the mechanisms leads to the proposal of new pharmaceutical targets and therapies that address multiple pathways, in particular iron-mediated [
44].
Consistent with this study is that of Majerníková, den Dunnen and Dolga (2021), where the authors evaluated the potential of therapeutic interventions on ferroptosis as a treatment for Alzheimer’s disease. The authors found that ferroptosis contributes to Alzheimer’s disease development and progression. The study also explored other mechanisms, such as transcriptomic analysis, to help understand the impacts of ferroptosis on Alzheimer’s disease targeted therapies [
45].
Altered interaction in the metabolic and nutritional coupling between glial cells (astroglial cells, oligodendrocytes, and microglia) and neurons can lead to neuronal death due to excessive iron accumulation, especially in ferroptosis [
46].
In the central nervous system, iron, as an important cofactor [
47], is mainly combined with ferritin and neuromelanin and participates in several important processes, including oxygen transport, oxidative phosphorylation, myelin production and neurotransmitter synthesis and metabolism [
48].
Ren et al., compared the differences and relationships among the various cell death mechanisms involved in neurological diseases to elucidate the role of ferroptosis. This could improve the understanding of the mechanism to provide potential treatments for acute and chronic neurological diseases.
Other authors described the pathological process of I/R to explore the molecular pathogenesis of ferroptosis and evaluate its role. They also analysed the role of tested iron-chelating agents that have shown efficacy in improving outcomes in a variety of I/R-associated symptoms, while not overlooking the potentially negative impact on the bloodstream and little organ specificity, suggesting that iron alone may not be an optimal target [
49].
Among diseases that threaten human health and quality of life, Wang et al. (2022), focused their attention on the emerging role of ferroptosis in cardiovascular conditions as a potential therapeutic target. In recent years, the pathogenic role of iron overload in cardiotoxicity has been recognized, mainly on animal models and cellular levels. Gu et al. found that protein p53 participates in the nonclassical pathway of ferroptosis regulation; this pathway if better understood could be a valuable tool in the prevention and treatment of cardiovascular disease, but experimental verification is still lacking
in vivo. [
50].
3.2. Cancer
Ferroptosis is implicated in several pathological pathways, as suggested by Yang et al. [
39] in a review where they summarize the current knowledge on the mechanism of iron regulation, and lipid and cysteine metabolism. They further discussed the contribution of ferroptosis to the pathogenesis of several diseases. It emerged that ferroptosis may be useful in circumventing therapy resistance of cancer cells especially after they have acquired drug resistance. Neutrophils can promote, at the tumour site, myeloperoxidase (MPO), which in turn catalyses iron-dependent phospholipid peroxidation and promotes ferroptosis in neighbouring tumour cells, showing a strong anti-tumour function [
51]. In addition, ferroptosis may participate in cancer immunity through adaptive mechanisms. The role of ferroptosis in cancer is therefore ambiguous, as it may be either an immunogenic or immunosuppressive form of cell death [
52]. Therefore, it may represent an exploitable feature critical for the treatment of cancer and certain types of diseases. In fact, understanding the mechanisms of a form of regulated cell death driven by phospholipid peroxidation could prove to be a promising approach in the treatment of a range of conditions, including neoplasms. The growing body of research has allowed describing at least three pathways controlling the sensitivity of cells to ferroptosis: glutathione-GPX4, NADPH-FSP1-CoQ 10 and GCH1-BH4 pathways. To date, it is known that ferroptosis is the most conserved form of cell death in all the diversity of life on earth than any other, and cells have evolved a complex control system to regulate when and how to activate ferroptosis and prevent tumorigenesis. Identification of targets to induce and inhibit ferroptosis is a promising strategy for future therapies [
2] since, for instance, it activates the NRF2 pathway, altering the associated molecular makeup [
53]. Dixon and Stockwell (2019) linked ferroptosis to the function of key tumour suppressor pathways, underlining how this is an interesting checkpoint in stress responses, and in tumour suppressor mechanisms. Indeed, protein p53 is known to affect cell cycle arrest, senescence, and apoptosis. However, its interaction with ferroptosis is of great interest [
9]. Through its metabolic targets, p53 modulates the response to ferroptosis through the canonical (GPX4-dependent) and non-canonical (GPX4-independent) ferroptosis pathways [
54].
Xuhui Tong et al., described in a review the mechanisms of necroptosis, pyroptosis, ferroptosis, and cuproptosis and the effects on tumor cell proliferation and cancer metastasis. The authors draw attention to the potential agents and nanoparticles for cancer treatment or the association of these with already existing therapies inducing RCD compared to conventional treatments [
55].
Cell death on cancer development and potential interventions and treatments. The authors examined how ferroptosis contributes to the development and progression of cancer in the ageing population through the reduction of mitochondria via parkin-mediated mitophagy which significantly reduces the susceptibility of cells to ferroptosis induced by cysteine deprivation, as already observed in the literature.
Another paper by Gao et al. also established the crucial role of mitochondria in ferroptosis. Cysteine deprivation leads to hyperpolarisation of the mitochondrial membrane and accumulation of lipid peroxide. The complex mechanism discussed by the authors implies the relevance of ferroptosis in tumour suppression [
56].
These findings led Bano and colleagues to define ferroptosis as a new avenue for cancer management after analysing the factors and molecular mechanisms that play a role in the initiation and susceptibility of ferroptosis in various malignancies [
57].
3.3. Further findings
Most of the studies showed that ferroptosis plays a pathological role in various contexts. A very interesting finding in this area of research is microbial virulence factors’ alleged ability to exploit or dampen ferroptosis regulatory pathways to their own benefit and the immune consequences. During an infection, reactive oxygen species are rapidly increased to facilitate pathogen elimination and implement inflammation and immune responses [
58].
There is indirect evidence linking lipid peroxidation, iron, and ferroptosis to host-pathogen interactions whether they are bacteria, viruses, fungi, or parasites. Attention is predominantly focused on the involvement of lipid peroxidation-driven ferroptosis in infectious diseases through its ability to spread a ferroptosis signal to neighbouring cells following a “wave” pattern [
59].This paradigm will probably help translate ferroptosis to various clinical contexts.
One limitation of the studies included in this review is that many were conducted in animal models, which may not fully reflect the processes occuring in humans. In addition, the studies included a variety of designs and methods, which may make it difficult to compare the results across studies. It is also worth noting that the mechanisms underlying the link connecting senescence, ferroptosis, aging, and disease is likely complex and multifaceted. They may involve other factors in addition to those discussed in this review. Further research is, however, needed to fully understand the role of these mechanisms to identify potential interventions that could target these mechanisms to prevent or treat age-related diseases.
Table 1.
This table summarized the article’s data and characteristics.
Table 1.
This table summarized the article’s data and characteristics.
| Article |
Study design |
Sample size |
Population |
Outcome measures |
| Masaldan et al. (2018) |
In vitro study |
N/A |
Senescent cells |
Ferroptosis, ferritinophagy, and iron accumulation |
| Guo et al. (2022) |
Review |
N/A |
N/A |
Age-related diseases, ageism |
| Masaldan et al. (2019) |
Review |
N/A |
N/A |
Cellular senescence, Alzheimer’s disease, and iron dyshomeostasis |
| Majerníková et al. (2021) |
Review |
N/A |
N/A |
Alzheimer's disease and Ferroptosis |
| Gong et al. (2022) |
Review |
N/A |
N/A |
Cancer, regulated cell death |
| Elmore |
Review |
N/A |
N/A |
Apoptosis |
| Diwan and Sharma (2022) |
Review |
N/A |
N/A |
Cellular ageing, cellular senescence. |
| Dixon & Stockwall (2019) |
Review |
N/A |
N/A |
Ferroptosis |
| Zhou et al. (2020) |
Review |
N/A |
N/A |
Age-related illnesses, cellular senescence. |
| Zhao et al. (2021) |
Review |
N/A |
N/A |
iron accumulation, lipid peroxidation and ferroptosis in AMD |
| Yun et al. (2018) |
In vitro study |
N/A |
ARPE-19 cells |
Effects of GSH depletion on stress-induced premature senescence (SIPS); ferroptosis; autophagy |
| Yihang et al. (2022) |
Review |
N/A |
N/A |
Ferroptosis and ischemia-reperfusion (I/R) |
| Yang et al. (2022) |
Review |
N/A |
N/A |
mechanism of regulation of iron, lipid, and cysteine metabolism; role of ferroptosis in several disease |
| Ratan, et al. (2020) |
In vitro study |
N/A |
N/A |
Ferroptosis, iron accumulation in brain, neurons death |
| Ren et al. (2020) |
|
N/A |
N/A |
Ferroptosis in neurological desease |
| Wang et al. (2022) |
Review |
N/A |
N/A |
Ferroptosis in cardiovascular disease |
| Tong et al. (2022) |
Review |
N/A |
N/A |
Mechanisms of necroptosis, pyroptosis, ferroptosis, and cuproptosis; the effects on tumor cell proliferation and cancer metastasis |
| Gao et al. (2020) |
In vitro study |
N/A |
Mammalian cells |
Role of mitochondria in ferroptosis |
| Derry et al. (2020) |
Review |
N/A |
N/A |
Alzheimer and ferroptosis |
| Riegman et al. (2020) |
In vitro study |
N/A |
N/A |
Involvement of lipid peroxidation-driven ferroptosis in infectious diseases |
| Verzola et al. (2008) |
In vivo study |
17 |
patients |
senescence in kidneys of patients with type 2 diabetic nephropathy |
| Reichert et al. (2020) |
Review |
N/A |
N/A |
ferroptosis and Alzheimer’s, Parkinson’s, and Huntington’s diseases |
| Campisi et al. (2016) |
Review |
N/A |
N/A |
senescence, ferroptosis, aging, and disease. |
| Paez-Ribes et al. (2019) |
Review |
N/A |
N/A |
Targeting senescent cells in age-related disease |
4. Conclusions
The data reviewed suggests several mechanisms may be interrelated and involved in the link connecting senescence, ferroptosis, aging, and disease. These mechanisms include the production of pro-inflammatory cytokines and other molecules by senescent cells, the accumulation of ROS and other oxidative stress-related molecules, and the activation of cell death pathways.
Ferroptosis is mediated by accumulated iron and the body's subsequent production of reactive oxygen species. This process may play a role in various age-related diseases, including diabetic nephropathy, cardiovascular diseases, ischemia/reperfusion-related injury, and age-related macular degeneration. Some studies suggest that ferroptosis may be a potential therapeutic target to counteract senescence, while others provide an overview of the mechanisms and links between ferroptosis and these diseases. Overall, these studies suggest that ferroptosis may be a promising area of research for understanding and potentially treating age-related conditions. They also reveal many research gaps in understanding the role of ferroptosis and its potential mechanisms connected with senescence. While most of them provide an overview of the mechanisms by which ferroptosis may contribute to the development of age-related diseases, there is a need for more research to fully understand these mechanisms and how they may differ between different conditions or whether targeting ferroptosis would be most effective at certain stages of the disease development as a potential therapeutic target. There is also a need for large-scale clinical studies as most of the available literature is relatively small, with only a few dozen participants, to confirm these studies' findings and determine the generalizability of the results for future research in these areas.
Author Contributions
“Conceptualization, D.C., and S.M.; methodology, D.C.; validation, S.M., C.C., investigation, D.C.; resources, C.C.; data curation, S.M.; writing—original draft preparation, D.C., A.C.; supervision, C.C.; project administration, S.M.; All authors have read and agreed to the published version of the manuscript. ” Authorship must be limited to those who have contributed substantially to the work reported.
Funding
This research received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ren, J.X.; Sun, X.; Yan, X.L.; Guo, Z.N.; Yang, Y. Ferroptosis in Neurological Diseases. Front Cell Neurosci 2020, 14, 218. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Jiang, X.; Gu, W. Emerging Mechanisms and Disease Relevance of Ferroptosis. Trends Cell Biol 2020, 30, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [PubMed]
- Tait, S.W.G.; Ichim, G.; Green, D.R. Die another way - non-apoptotic mechanisms of cell death. J Cell Sci 2014, 127, 2135–2144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, Z.; Pan, K.; Li, J.; Chen, Q. The function and mechanism of ferroptosis in cancer. Apoptosis 2020, 25, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Stockwell, B.R.; Friedmann Angeli, J.P.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascon, S.; Hatzios, S.K.; Kagan, V.E.; et al. Ferroptosis: A Regulated Cell Death Nexus Linking Metabolism, Redox Biology, and Disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Torresano, L.; Nuevo-Tapioles, C.; Santacatterina, F.; Cuezva, J.M. Metabolic reprogramming and disease progression in cancer patients. Biochim Biophys Acta Mol Basis Dis 2020, 1866, 165721. [Google Scholar] [CrossRef] [PubMed]
- Mou, Y.; Wang, J.; Wu, J.; He, D.; Zhang, C.; Duan, C.; Li, B. Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 2019, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Stockwell, B.R. The role of iron and reactive oxygen species in cell death. Nat Chem Biol 2014, 10, 9–17. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Organelle-specific regulation of ferroptosis. Cell Death Differ 2021, 28, 2843–2856. [Google Scholar] [CrossRef]
- Hider, R.C.; Kong, X.L. Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 2011, 24, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Friedmann Angeli, J.P.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E.; et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Jenkins, N.L.; James, S.A.; Salim, A.; Sumardy, F.; Speed, T.P.; Conrad, M.; Richardson, D.R.; Bush, A.I.; McColl, G. Changes in ferrous iron and glutathione promote ferroptosis and frailty in aging Caenorhabditis elegans. Elife 2020, 9, e56580. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Stockwell, B.R.J.P.b. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? 2018, 16, e2006203. [CrossRef]
- Mazhar, M.; Din, A.U.; Ali, H.; Yang, G.; Ren, W.; Wang, L.; Fan, X.; Yang, S. Implication of ferroptosis in aging. Cell Death Discov 2021, 7, 149. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J Clin Invest 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Toyokuni, S.; Yanatori, I.; Kong, Y.Y.; Zheng, H.; Motooka, Y.; Jiang, L. Ferroptosis at the crossroads of infection, aging and cancer. Cancer Sci 2020, 111, 2665–2671. [Google Scholar] [CrossRef] [PubMed]
- Stroustrup, N.; Anthony, W.E.; Nash, Z.M.; Gowda, V.; Gomez, A.; Lopez-Moyado, I.F.; Apfeld, J.; Fontana, W. The temporal scaling of Caenorhabditis elegans ageing. Nature 2016, 530, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.-P.; Chen, Y.; Wei, X.; Yu, B.; Xiong, Z.-G.; Lu, C.; Hu, W.J.T. Novel insights into ferroptosis: Implications for age-related diseases. 2020, 10, 11976. [CrossRef]
- Zhao, T.; Guo, X.; Sun, Y. Iron Accumulation and Lipid Peroxidation in the Aging Retina: Implication of Ferroptosis in Age-Related Macular Degeneration. Aging Dis 2021, 12, 529–551. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, N.; Pantopoulos, K.J.B., The Journal of the American Society of Hematology. IRP1 regulates erythropoiesis and systemic iron homeostasis by controlling HIF2α mRNA translation. 2013, 122, 1658-1668. [CrossRef]
- Kautz, L.; Jung, G.; Valore, E.V.; Rivella, S.; Nemeth, E.; Ganz, T.J.N.g. Identification of erythroferrone as an erythroid regulator of iron metabolism. 2014, 46, 678-684. [CrossRef]
- Shah, Y.M.; Xie, L. Hypoxia-inducible factors link iron homeostasis and erythropoiesis. Gastroenterology 2014, 146, 630–642. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Ferguson, D.J.; Nicholls, L.G.; Iredale, J.P.; Pugh, C.W.; Johnson, M.H.; Ratcliffe, P.J. Sites of erythropoietin production. Kidney Int 1997, 51, 393–401. [Google Scholar] [CrossRef]
- Enami, S.; Sakamoto, Y.; Colussi, A.J.J.P.o.t.N.A.o.S. Fenton chemistry at aqueous interfaces. 2014, 111, 623-628. [CrossRef]
- Gnana-Prakasam, J.P.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Expression and function of iron-regulatory proteins in retina. IUBMB Life 2010, 62, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Moiseyev, G.; Chen, Y.; Takahashi, Y.; Wu, B.X.; Ma, J.-x.J.P.o.t.N.A.o.S. RPE65 is the isomerohydrolase in the retinoid visual cycle. 2005, 102, 12413-12418. [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to tango: regulation of Mammalian iron metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.V.; Torti, F.M.J.N.R.C. Iron and cancer: more ore to be mined. 2013, 13, 342-355. [CrossRef]
- Chew, E.Y.; Clemons, T.E.; Agrón, E.; Sperduto, R.D.; SanGiovanni, J.P.; Kurinij, N.; Davis, M.D.; Ophthalmology, A.-R.E.D.S.R.G.J. Long-term effects of vitamins C and E, β-carotene, and zinc on age-related macular degeneration: AREDS report no. 35. 2013, 120, 1604-1611. e1604. [CrossRef]
- van Leeuwen, E.M.; Emri, E.; Merle, B.M.J.; Colijn, J.M.; Kersten, E.; Cougnard-Gregoire, A.; Dammeier, S.; Meester-Smoor, M.; Pool, F.M.; de Jong, E.K.; et al. A new perspective on lipid research in age-related macular degeneration. Prog Retin Eye Res 2018, 67, 56–86. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: from molecular mechanisms to interventions and treatments. Signal Transduct Target Ther 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Verzola, D.; Gandolfo, M.T.; Gaetani, G.; Ferraris, A.; Mangerini, R.; Ferrario, F.; Villaggio, B.; Gianiorio, F.; Tosetti, F.; Weiss, U.; et al. Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy. Am J Physiol Renal Physiol 2008, 295, F1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Reichert, C.O.; de Freitas, F.A.; Sampaio-Silva, J.; Rokita-Rosa, L.; Barros, P.L.; Levy, D.; Bydlowski, S.P. Ferroptosis Mechanisms Involved in Neurodegenerative Diseases. Int J Mol Sci 2020, 21, 8765. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Cellular Senescence and Lung Function during Aging. Yin and Yang. Ann Am Thorac Soc 2016, 13 Suppl 5, S402–S406. [Google Scholar] [CrossRef]
- Sumi, M.P.; Ghosh, A. Hsp90 in Human Diseases: Molecular Mechanisms to Therapeutic Approaches. Cells 2022, 11, 976. [Google Scholar] [CrossRef] [PubMed]
- Paez-Ribes, M.; Gonzalez-Gualda, E.; Doherty, G.J.; Munoz-Espin, D. Targeting senescent cells in translational medicine. EMBO Mol Med 2019, 11, e10234. [Google Scholar] [CrossRef]
- Wei, Z.; Xie, Y.; Wei, M.; Zhao, H.; Ren, K.; Feng, Q.; Xu, Y. New insights in ferroptosis: Potential therapeutic targets for the treatment of ischemic stroke. Front Pharmacol 2022, 13, 1020918. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Yuan, X.; Wang, S.; Ge, A.; Xu, H.; Zeng, J.; Ge, J. The mechanism of ferroptosis regulating oxidative stress in ischemic stroke and the regulation mechanism of natural pharmacological active components. Biomed Pharmacother 2022, 154, 113611. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Gao, P.; Chen, H.; Zhou, X.; Ou, Y.; He, Y. The Role of Iron, Its Metabolism and Ferroptosis in Traumatic Brain Injury. Front Cell Neurosci 2020, 14, 590789. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, X.; Liu, X.; Shen, L.; Chen, Q.; Shu, Q.J.A. Targeting Ferroptosis as a Promising Therapeutic Strategy for Ischemia-Reperfusion Injury. 2022, 11, 2196. [CrossRef]
- Masaldan, S.; Clatworthy, S.A.; Gamell, C.; Meggyesy, P.M.; Rigopoulos, A.-T.; Haupt, S.; Haupt, Y.; Denoyer, D.; Adlard, P.A.; Bush, A.I.J.R.b. Iron accumulation in senescent cells is coupled with impaired ferritinophagy and inhibition of ferroptosis. 2018, 14, 100-115. [CrossRef]
- Masaldan, S.; Belaidi, A.A.; Ayton, S.; Bush, A.I.J.P. Cellular senescence and iron dyshomeostasis in Alzheimer’s disease. 2019, 12, 93. [CrossRef]
- Derry, P.J.; Hegde, M.L.; Jackson, G.R.; Kayed, R.; Tour, J.M.; Tsai, A.-L.; Kent, T.A.J.P.i.n. Revisiting the intersection of amyloid, pathologically modified tau and iron in Alzheimer’s disease from a ferroptosis perspective. 2020, 184, 101716. [CrossRef]
- Majerníková, N.a.; den Dunnen, W.F.; Dolga, A.M.J.F.i.a.n. The potential of ferroptosis-targeting therapies for Alzheimer’s disease: from mechanism to transcriptomic analysis. 2021, 13. [CrossRef]
- Ratan, R.R. The Chemical Biology of Ferroptosis in the Central Nervous System. Cell Chem Biol 2020, 27, 479–498. [Google Scholar] [CrossRef] [PubMed]
- Moreau, C.; Danel, V.; Devedjian, J.C.; Grolez, G.; Timmerman, K.; Laloux, C.; Petrault, M.; Gouel, F.; Jonneaux, A.; Dutheil, M. Could conservative iron chelation lead to neuroprotection in amyotrophic lateral sclerosis? 2018. [CrossRef]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol 2014, 13, 1045–1060. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.F.; Tuo, Q.Z.; Yin, Q.Z.; Lei, P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res 2020, 41, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov 2022, 8, 394. [Google Scholar] [CrossRef]
- Yee, P.P.; Wei, Y.; Kim, S.-Y.; Lu, T.; Chih, S.Y.; Lawson, C.; Tang, M.; Liu, Z.; Anderson, B.; Thamburaj, K.J.N.c. Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. 2020, 11, 1-22. [CrossRef]
- Demuynck, R.; Efimova, I.; Naessens, F.; Krysko, D.V.J.J.f.I.o.C. Immunogenic ferroptosis and where to find it? 2021, 9. [CrossRef]
- Stockwell, B.R.; Jiang, X. A Physiological Function for Ferroptosis in Tumor Suppression by the Immune System. Cell Metab 2019, 30, 14-15. [CrossRef]
- Liu, Y.; Gu, W. p53 in ferroptosis regulation: the new weapon for the old guardian. Cell Death Differ 2022, 29, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Tang, R.; Xiao, M.; Xu, J.; Wang, W.; Zhang, B.; Liu, J.; Yu, X.; Shi, S. Targeting cell death pathways for cancer therapy: recent developments in necroptosis, pyroptosis, ferroptosis, and cuproptosis research. J Hematol Oncol 2022, 15, 174. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yi, J.; Zhu, J.; Minikes, A.M.; Monian, P.; Thompson, C.B.; Jiang, X. Role of Mitochondria in Ferroptosis. Mol Cell 2019, 73, 354–363 e353. [Google Scholar] [CrossRef]
- Bano, I.; Horky, P.; Abbas, S.Q.; Majid, M.; Bilal, A.H.M.; Ali, F.; Behl, T.; Hassan, S.S.U.; Bungau, S. Ferroptosis: A New Road towards Cancer Management. Molecules 2022, 27, 2129. [Google Scholar] [CrossRef]
- Sareila, O.; Kelkka, T.; Pizzolla, A.; Hultqvist, M.; Holmdahl, R.J.A.; signaling, r. Nox2 complex–derived ROS as immune regulators. 2011, 15, 2197-2208. [CrossRef]
- Riegman, M.; Sagie, L.; Galed, C.; Levin, T.; Steinberg, N.; Dixon, S.J.; Wiesner, U.; Bradbury, M.S.; Niethammer, P.; Zaritsky, A.J.N.c.b. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. 2020, 22, 1042-1048. [CrossRef]
Scheme 1.
1) and 2): schematic steps in iron-mediated generation of ROS by Fenton’s process and 3) overall reaction. 4), 5) and 6) possible steps in the radical peroxidation of organic substrates, including lipids.
Scheme 1.
1) and 2): schematic steps in iron-mediated generation of ROS by Fenton’s process and 3) overall reaction. 4), 5) and 6) possible steps in the radical peroxidation of organic substrates, including lipids.
Scheme 2.
PRISMA flow diagram.
Scheme 2.
PRISMA flow diagram.
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).