1. Introduction
Intervertebral disc degeneration (IVDD) is a key recognizable fact for back and neck pain in adults leading to decrease quality life sometimes making disable (1-4). IVDD is a pathological and physiological process that can be chronic and progressive (1).The disease is of widespread prevalence. About 80% of the mature individuals would be subjected to back and neck pain at any stages in their life (2, 5, 6). Intervertebral disc (IVD),an important element which can tolerate various kinds of load depending on the intensity of load (7).Many studies have been conducted, where mentioned excessive external stress, hereditary disease, obesity and aging are most common enhancer of IVDD (1, 3, 6, 8). However, mechanical stimulus is another important key contributor for IVDD, when it exceeds tolerable margin of load baring elements (9). Reasonable mechanical signal creates defensive effect on IVD but, too much mechanical pressure often exacerbates IVD cells diminish. IVD is composed of three distinct but interdependent tissues: central gelatinous nucleus pulposus (NP), outer annulus fibrosus (AF) and cartilage end plates on the superior and inferior surfaces (1, 3, 10). NP cells play a vital role to maintain normal skeleton and physiological function of IVD by producing extracellular matrix (ECM), including type II collagen and proteoglycans (mainly aggrecans)(1, 10).Innermost NP cells are the basic part of IVD. The effect of pathological loading is usually harsher in NP cells than in other types of IVD cells to compression-mediated disc deterioration (11). Reducing NP cells number is the key element of IVDD which is strongly connected with programmed cell death (PCD) (11, 12).
In cell biology, there are two classic and morphologically unique modes of PCD: autophagy and apoptotic cell death (1). Stress can influence frequently both types of PCD. Under stress, autophagy provides energy by degrading with accumulating the miss-folded protein and damaged organelles (1, 13).Many studies have been conducted regarding compression mediated apoptosis; conversely, a few studies have been conducted regarding role of autophagy in IVD cells. But, it has not been well established, when IVD discs expose to compression. So, still there is a space to investigate the relationship of autophagy in IVD.So, it is thought that NP cells have strong connection with etiology of IVDD, and excessive compression advances the process of IVD degeneration (1). Whether autophagy or apoptosis which play major role in compression mediated NP cell injury is not clear yet.
Hence, the IVD cells would demonstrate different forms of cells death, besides apoptosis, autophagy in response to compression (13).So, we hypothesized that EF and SI may exert autophagy and apoptosis mediated IVDD through NP cells injury in rat tail discs (
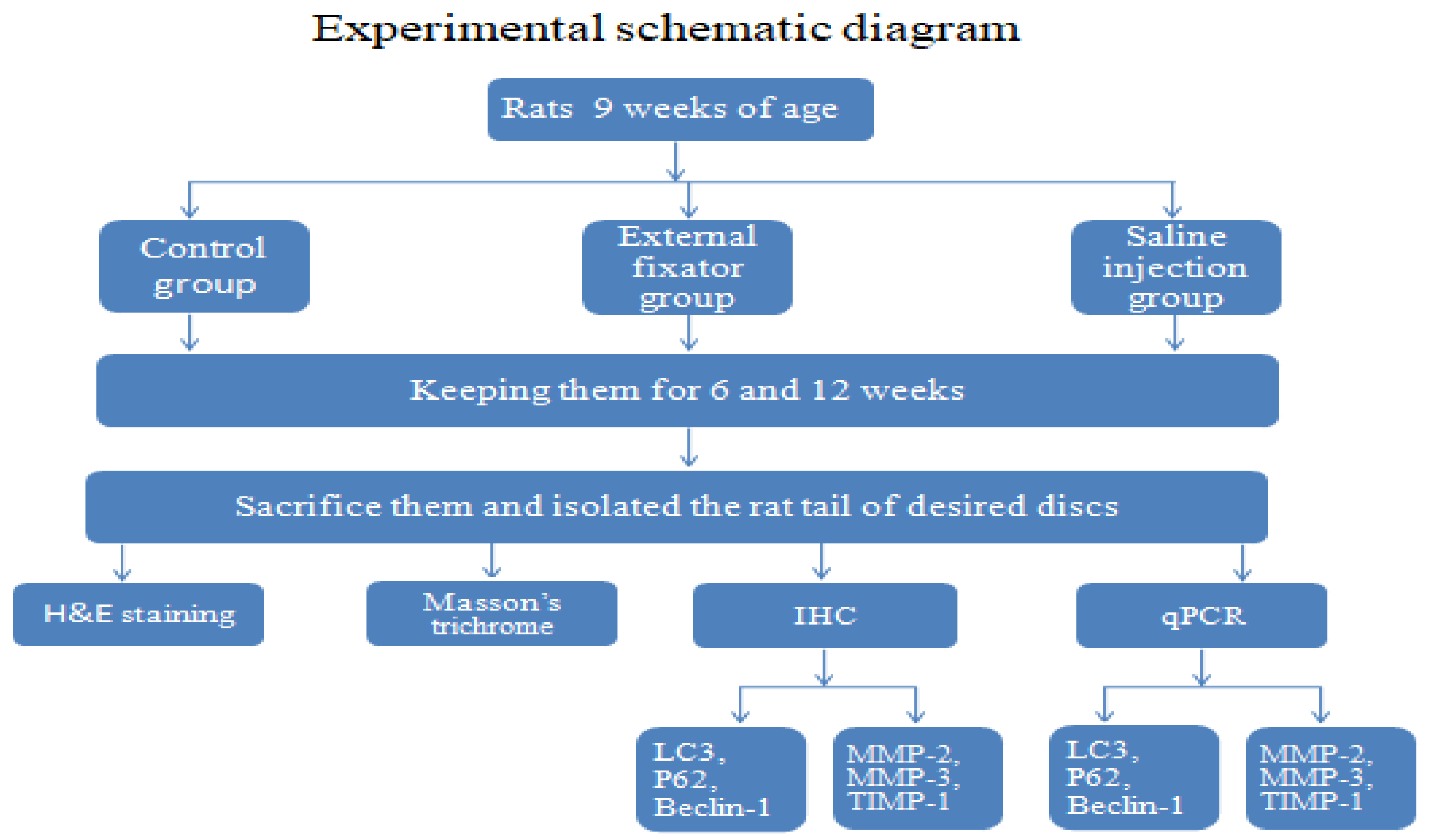
Figure 1).
2. Materials and methods:
2.1. Animal preparation
In total, 16 of 9 weeks of age male Sprague-Dawley rats (KOATECH Korea Animal technology) were used of our experiment. It has been approved by the Institutional Review Board of the Animal Experimentation Committee (IRB number is CMCDJ-AP-2021-006). Rats were randomly divided into three groups. Control group (Ctrl), Saline injection group (SI) and External fixator group (EF) as well. Four (4) rats were used for Ctrl group, six (6)for SI group and remaining six (6) for EF group respectively. For EF group, kirschner wire was used for compression and divided into two subgroups like 6 week and 12 weeks. For SI group, 0.9% normal saline was used as a substitute of external fixator as well as two subgroups were made like EF group. Same subgroups were made for Ctrl group where four 4 rats were used and it was kept and housed without any treatment. After intraperitoneal anesthesia, two-cross 0.7 mm diameter kirschner wires were inserted percutaneously into each vertebral body perpendicular to the tail’s axis which attached with aluminum rings. Rings were connected longitudinally with three threaded rods. For Saline group, 0.9% normal saline was injected in the discs of rat tail using a syringe. All the surgery has been performed by registered orthopedic surgeon in a controlled room. After surgery, rats were housed at 12 h light/dark cycle with temperature (23 ± 2 ◦C) and humidity (55 ± 5%). Rats were kept separately in specific pathogen free housing case and giving free access to food and water.
2.2. Paraffin-Embedded Tissue Preparation
The experimental part of rat’s tail was taken out carefully and immediately immersed into 4% paraformaldehyde for keeping them at room temperature for 48 days. Afterwards, the excised tail was rinsed with water for immersing into decalcification reagents until fully decalcified. Then after, rat tail was cut out vertically with making appropriate size to place in a plastic block and immersed into 10% formaldehyde for overnight. Following day, dehydration steps were carried out. Each section was sliced at thickness about 5μm with microtome and mounted on slides according to a standard protocol. Eventually slides were allowed to dry for overnight and stored at room temperature until ready for use.
2.3. H&E staining
Paraffin-embedded sections were deparaffinized by following deparaffinization steps as per instruction of a standard protocol. Afterwards, hematoxylin and eosin staining was performed according to a standard procedure and photographed by using the OLYMPUS, BX53 U-CMAD3 microscope (T7 TOKYO, JAPAN).
2.4. Masson trichrome staining
Formalin-fixed paraffin-embedded sections were dehydrated by following dehydration steps. Afterwards, slides were placed in bouins for overnight at room temperature. Following day, slides were washed out by tap water until yellow was gone. Then, slides were stained in weigerts hematoxylin for 15 minutes and placed them in biebrich scarlet-acid fuchsin for 5-10 minutes after rinsing with water. Then after, slides were placed in the phosphomolybdic-phosphotungstic acid solution in a plastic coplin jar for 15 minutes after washing with water. Eventually, slides were stained with aniline blue solution for 10 minutes after rinsing with water. Finally, slides were mounted using appropriate cover slips after following dehydration steps and photographed using OLYMPUS, BX53 U-CMAD3 microscope (T7 TOKYO, JAPAN).
2.5. Immunohistochemistry (IHC)
Sections were deparaffinized in xylene, dehydrated in graded ethanol, and washed for 5 minutes in tap water. Sections were rinsed with distilled water and treated for antigen retrieval using protinase K enzyme. Sections were incubated with 1:200 rabbit monoclonal primary antibody LC3 (#3868; Cell Signaling Technology, USA), 1:400 diluted mouse monoclonal primary antibody Beclin 1 (#sc-48381; Santa Cruz Biotechnology, Inc. USA), 1:50 diluted mouse monoclonal primary antibody p62 (#ab56416; Abcam, UK)at 4°C overnight. 1:50 diluted mouse monoclonal antibody MMP2 (#sc53630; Santa Cruz Biotechnology, Inc. USA), 1:50 diluted mouse monoclonal antibody MMP3 (#sc21732; Santa Cruz Biotechnology, Inc. USA), and 1:100 diluted mouse monoclonal antibody TIMP1(#sc21732; Santa Cruz Biotechnology, Inc. USA) also used and kept them at 4°C overnight.Following day, slides were treated with peroxidase-labeled anti-mouse or anti-rabbit antibody (VECTASTIN Elite ABC Kit, Vector Laboratories, Inc, USA) at room temperature for 30 minutes. Subsequently HRP detection solutions (ImmPACTNovaRED Substrate kit peroxidase, Vector Laboratories, Inc, USA) were added and incubated them for 15 minutes. Eventually counterstaining was used with Mayer’s hematoxylin as well as dehydration steps were followed. Finally slides were mounted using appropriate cover slips and photographed using the OLYMPUS, BX53 U-CMAD3 microscope (T7 TOKYO, JAPAN).
2.6. RNA Extraction and qPCR:
Experimental discs were excised carefully (including AF and NP) and keep into liquid nitrogen to prevent RNA degradation, and finally stored at −80°C. Total RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany). Then, 2 μg RNA was reverse transcribed with a Revertra Ace qPCR RT Master Mix Kit (TOYOBO, Osaka, Japan).Relative mRNA expression encoding autophagy-related genes (LC3, Beclin-1, and P62) and apoptosis-related genes (MMP-2 and MMP-3 and TIMP-1) were checked out by real-time reverse transcription (RT)-polymerase chain reaction (PCR). Applied Biosystems 7500 Fast Real-Time PCR System(Thermo Fisher Scientific,USA) and thunderbird SYBR Green qPCR mix (TOYOBO, Osaka, Japan) was used for identifying them. Primer sequences are listed in
Table 1. Glyceraldehyde3-phosphate dehydrogenase (GAPDH) mRNA expression was measured as an endogenous control. mRNA expression of each protein in the experimental discs was transformed into a relevant number which represented the amount of mRNA compared with control group using the 2
−ΔΔCt method. We used the formula ΔCt = Cttarget gene – CtGAPDH to calculate the difference in threshold cycles for target gene and reference gene. Eventually, we have been able to figure out the mRNA expression fold change of the target gene as 2
−ΔΔCt in the treated discs.
2.7. Statistical analysis:
Data were expressed as mean ± standard deviation;t test was used to investigate the changes for effecting of EF and SIafter 6 weeks and 12 weeks. We analyzed 12 discs for EF of 6 six rats and same number of discs were also used for SI group but for control group total 8 discs of 4 four rats were used. Statistical significance was assessed with P< 0.05< 0.01, and < 0.005(Level of significance is * P< 0.05, **P< 0.01, and ***P< 0.005) by using GraphPad Prism (GraphPad Software, Inc., USA).
3. Results
3.1. External fixator exerted insidious degradation of NP cells
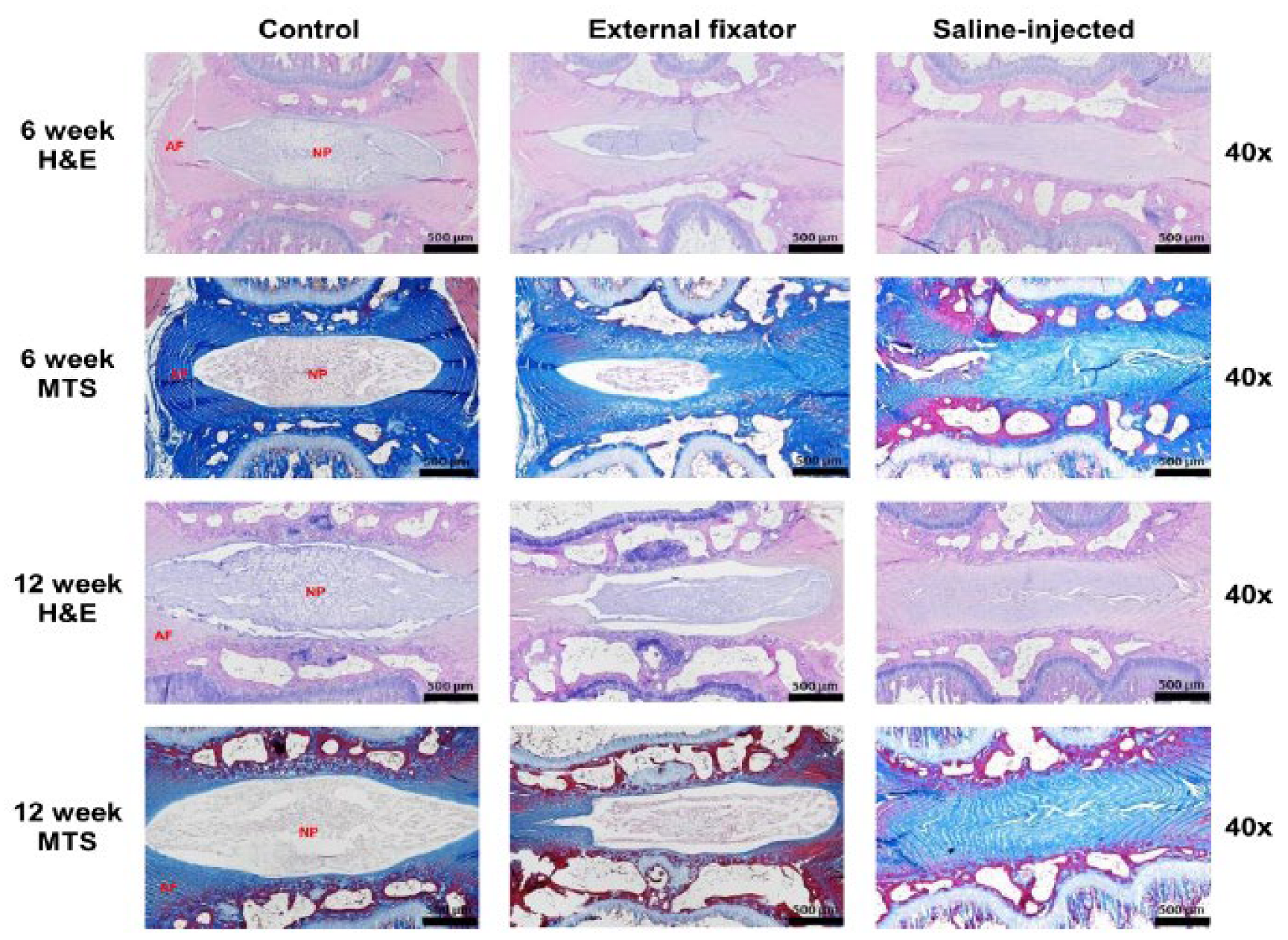
To identify NP cells degradation, we performed H&E staining where EF group and SI group showed more insidious NP cell degeneration than Ctrl group even if SI group showed there was no margin between AF and NP. But rate of degradation was elevated with increasing duration of compression (
Figure 2). To understand the status of NP cells morphology, we performed Masson’s trichrome staining. Where, EF and group SI group showed more subtle NP cells degeneration than control group. And rate of degradation was inclined with increasing duration of compression. Whereas SI group showed there was no boundary between AF and NP cells (
Figure 2).
3.2. External fixator induced autophagy mediated NP cells degradation:
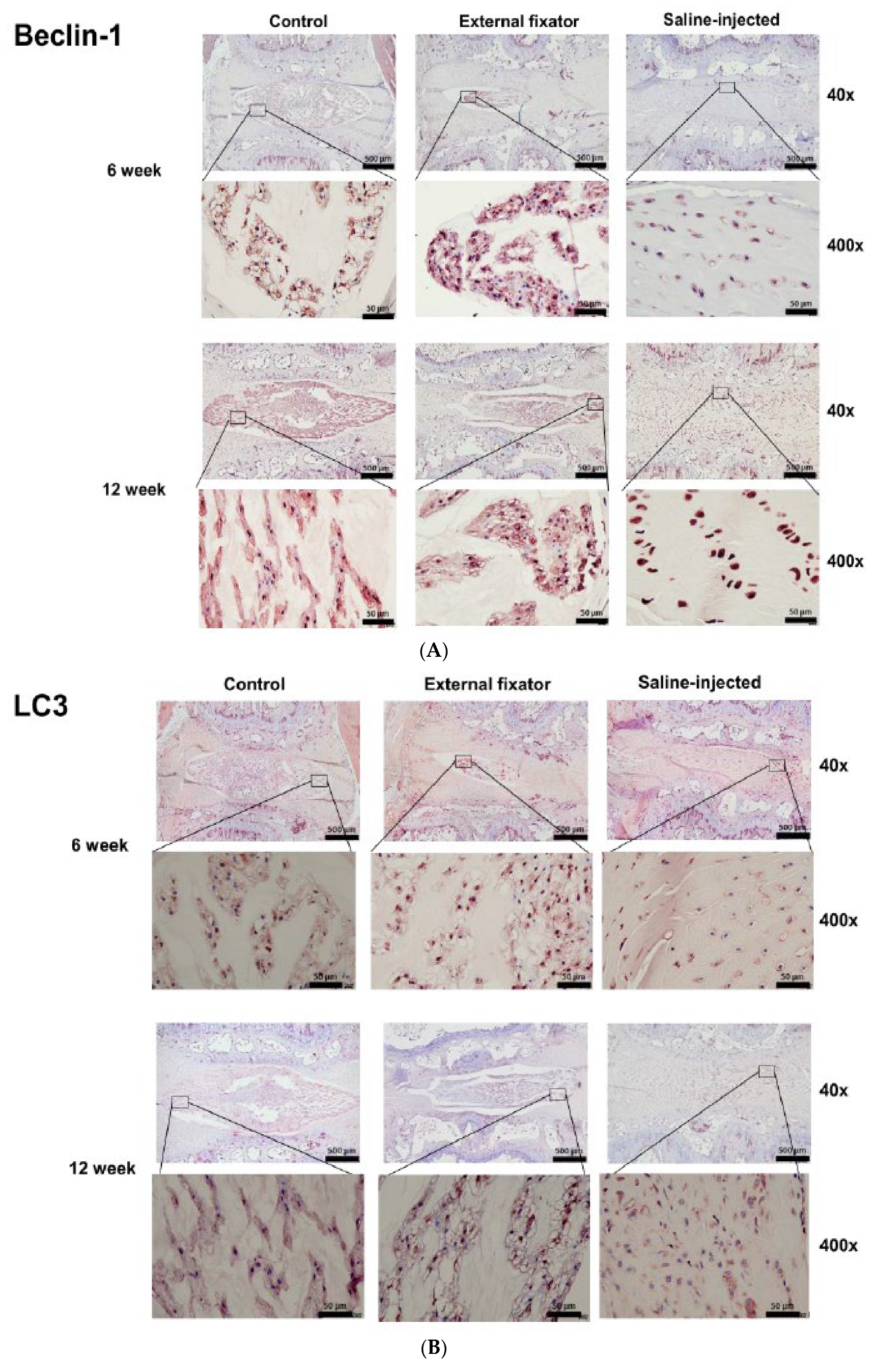
To observe the autophagy mediated NP cells degradation, we performed immunohistochemistry for LC3, Beclin-1and P62. Highest and laterally beclin-1 protein expression was indentified in EF group after 6 weeks and it down-regulated after 12 weeks. SI group showed also up-regulated and lateral expression, but it showed significantly up-regulated expression after 12 weeks than 6 weeks. Even though, SI group cannot distinguish the margin between NP cells and AF cells. However, SI group showed less up-regulated expression than EF group (Figure-3A).Up-regulated and lateral LC3 expression was found both EF and SI groups after 6 weeks and 12 weeks than Ctrl. EF group showed elevated expression than SI group although SI group cannot differentiate the boundary of AF and NP cells (
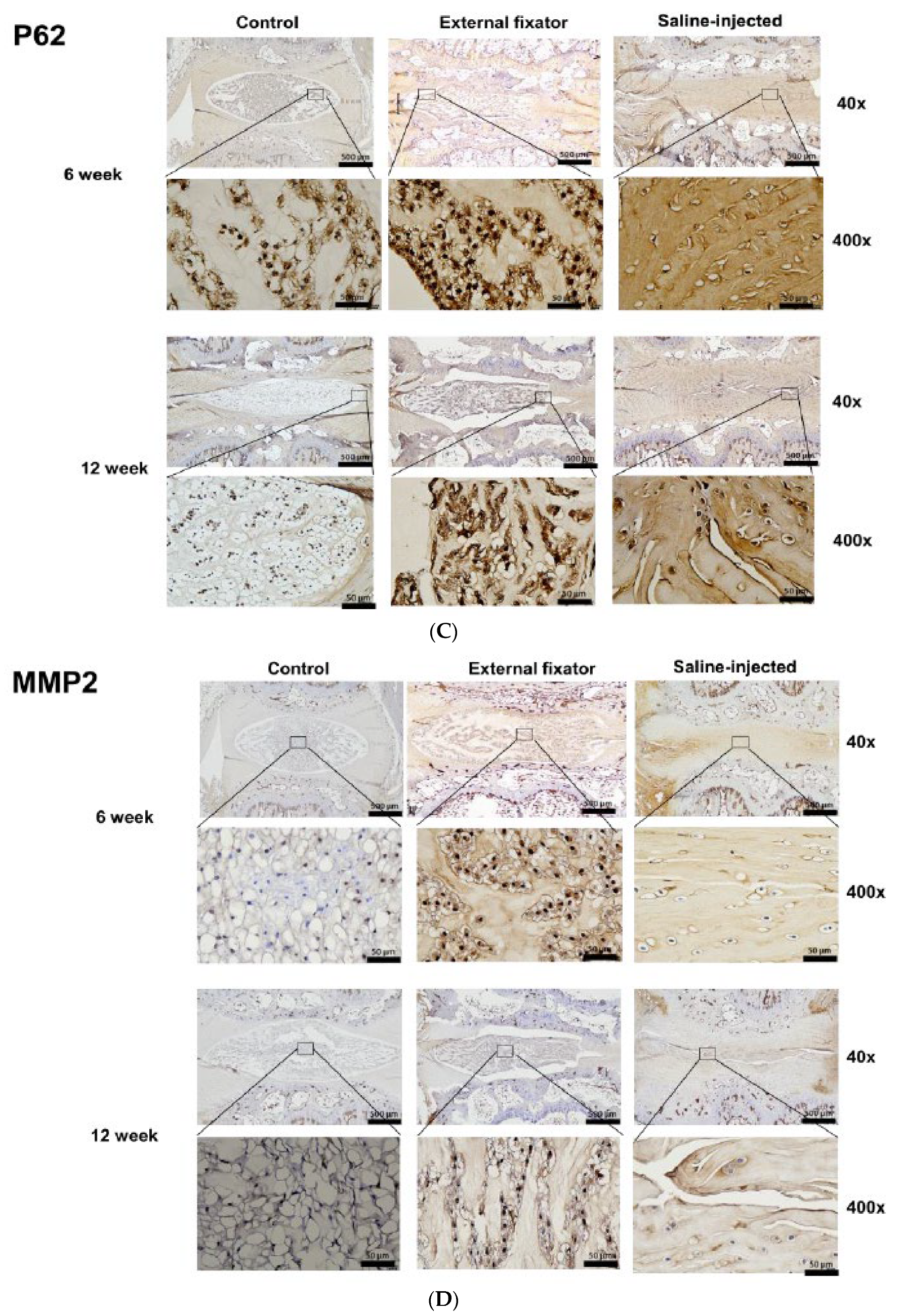
Figure 3B). Up-regulated and lateral P62 expression was found in both EF and SI groups after 6 weeks but after 12 weeks they showed down-regulated expression than 6 weeks. But EF group showed more upregulated expression than SI group (
Figure 3A-C). It dictates that EF induced more and lateral autophagy mediated NP cells degradation but not central.
3.3. External fixator induced apoptosis mediated NP cells death:
To identify the apoptotic mediated NP cells degradation, we performed immunohistochemistry for MMP-2, MMP-3 and TIMP-1. Highest and central MMP-2 expression was identified in EF group but SI group cannot differentiate the border between AF cells and NP cells. And after 12 weeks, somewhat and central down-regulation expression was observed in EF group and in SI group although SI group showed less expression than EF group (
Figure 3D). Up-regulated and central MMP-3 expression was found in both EF and SI group. But MMP-3 expression was upregulated, and it amplified with increasing the duration compression (
Figure 3E). Highest TIMP-1 was recognized which expressed centrally in EF group and SI group than in Ctrl group after 6 weeks and 12 weeks. But EF group showed more central expression after 6 weeks than 12 weeks. On the other hand, SI group showed more upregulated expression than 6 weeks (
Figure 3F), although; SI group cannot recognize the margin between AF and NP cells. It indicates that there may have apoptosis mediated degradation in NP cells.
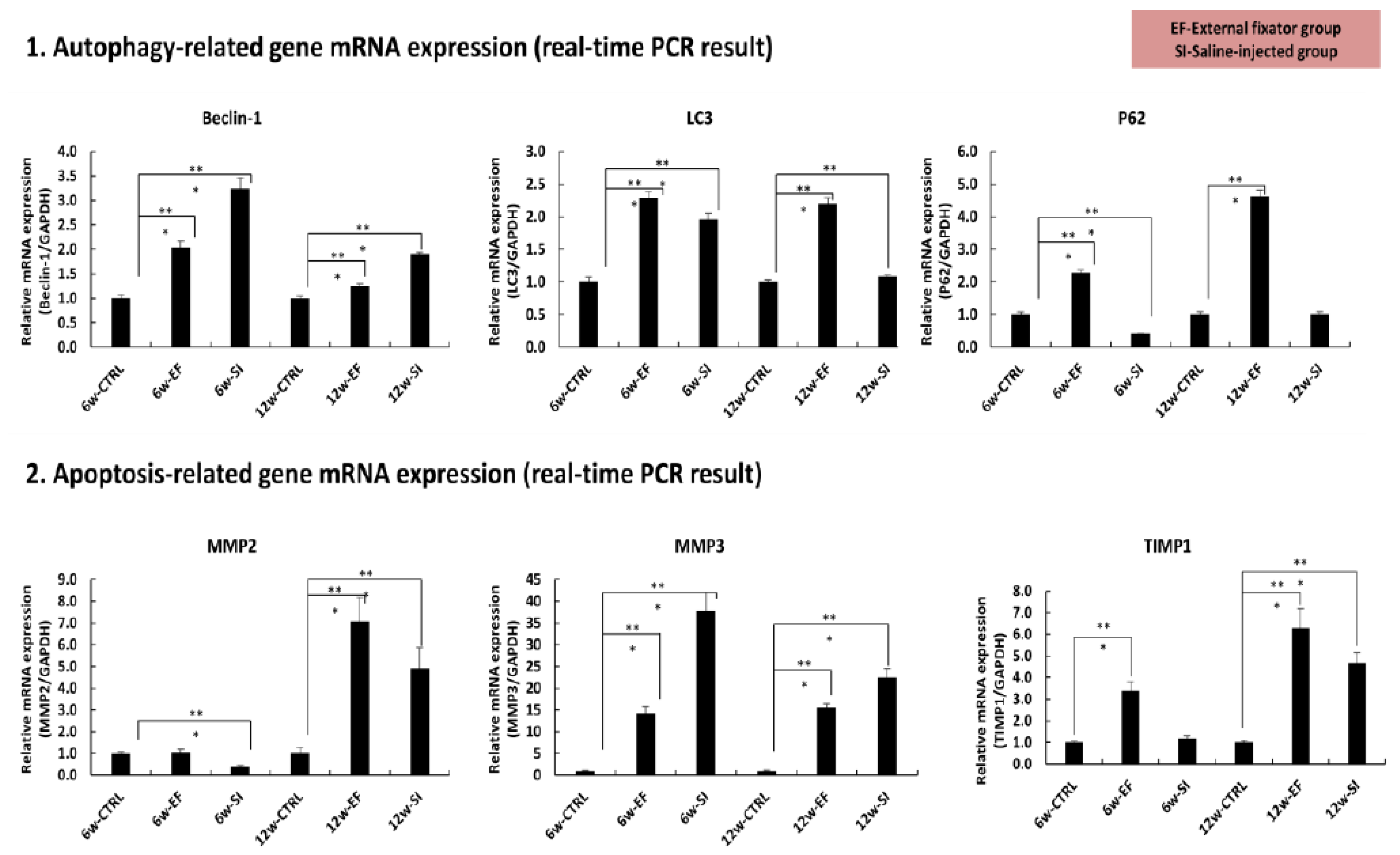
3.4. qPCR results
3.4.1. External fixator up-regulated autophagy related gene expression:
To observe the catabolic gene expression, we checked the mRNA expression for beclin-1, LC3 and P62.Up-regulated beclin-1mRNA expression was found in both groups after 6 and 12 weeks as well but more expression was identified after 6 weeks than 12 weeks. But, after 12 weeks, the entire groups were showed down-regulated expression compared to 6 weeks except SI group which showed up-regulated expression (
Figure 4). Up- regulated LC3 mRNA expression was observed in both groups after 6 weeks compared to Ctrl group. After 12 weeks, EF group showed similar expression compared to 6 weeks. But SI group showed no significant up-regulated expression than Ctrl group (
Figure 4). After 6 weeks, less p62 mRNA expression was observed in SI group than in Ctrl group whereas EF group showed significant up-regulation than Ctrl group. On contrary, after 12 weeks, the utmost expression was found in EF group than in Ctrl group whereas no significant changes found in SI group compared in Ctrl group. It indicates that highest autophagy degradation material was accumulated in EF group after 6 and 12 weeks also and after 12 weeks it showed utmost expression (
Figure 4).
From above these findings, it can be mentioned that EF group induced more expression of autophagy related gene than SI group, although the autophagy initiation gene expression was high in both EF and SI group, but it reduced after 12 weeks. And highest autophagy accumulating materials was found in EF group than SI group and it elevated along with increasing the duration of compression.
3.4.2. Saline injection upregulated the apoptosis related gene expression:
After 6 weeks, down-regulated mRNA expression of MMP-2 was identified in SI group than in Ctrl group, but no significant change was found in EF group compared to Ctrl group. On contrary, after 12 weeks, highest mRNA expression was observed in both groups compared in Ctrl group, but maximum mRNA expression was recognized in EF group than SI group (
Figure 4). Elevated of mRNA expression of MMP-3 was identified in both groups after 6 weeks but SI group showed utmost expression than EF group. After 12 weeks, more of mRNA expression was found in both groups than in ctrl group but SI group expressed more mRNA than EF group. It dictates that SI group expressed more apoptosis related gene and it reached peak position after 6 weeks than 12 weeks (
Figure 4).After 6 weeks, no significant changes of TIMP1 were observed in SI group than in Ctrl group but EF group showed significant up-regulation than Ctrl group. After 12 weeks, remarkable uppermost mRNA expressions were recognized in both groups but EF group was showed highest mRNA expression than SI group. It indicates that TIMP-1 showed highest protective approach against EF group of MMP3 (
Figure 4).
From above this evidence, it expressed that highest amount of apoptotic related gene expressed in SI group where as EF group showed lowest expression. When mRNA expression of TIMP-1 expression increased where as the MMP3 expression was decreased that is inversely correlated and on the other hand mRNA expression of MMP2 was proportionally correlated with TIMP1.
4. Discussion
There are several reasons which may play role for the pathogenesis of IVDD, like nutrition, age, mechanical force and others (1). To underlying the disease of IVDD, a lot of research has been conducted, however it remains indistinct. From the contributing factors, it has been well documented that mechanical pressure is one of the key enhancer for IVDD (3, 9). (9, 14) It has been documented that diminishing NP cell is the key reason to IVDD which can up-regulate through compression. And also (12, 15) it has mentioned that increasing compression may induce autophagy and apoptosis mediated NP cell diminish. It has been well established that NP cell dysfunction is closely linked with IVDD (2).Some studies have also been mentioned that apoptosis and autophagy mediated disc degeneration is another cause for low back pain (1, 16). Our research group explains a comprehensive contribution of autophagy and apoptosis in intervertebral disc degeneration after exposing SI and EF. From our study, we identified more insidious NP cell destruction in EF group. Whereas, in SI group could not differentiate the margin between NP cells and AF cells because of destructing the boundary of cells.
Beclin 1, the mammalian orthologue of yeast Atg6, has a key function in autophagy, a pathway of programmed cell survival, which is increased during periods of cell stress and extinguished during the cell cycle (17). In some studies, it has mentioned compression induces the generation of morphological and biochemical markers of autophagy in rat NP cells, and triggers autophagosome formation by LC3B expression(13, 18). From our study, we identified higher and more laterally expressed Beclin-1 protein in EF. But SI cannot differentiate the boundary between NP cells and AF cells. Highest and laterally LC3 expression was identified after 6 weeks and 12 weeks in EF. It dictates that, there was no autophagy mediated degradation in central NP cells. P62 expression was higher and more laterally in EF group after 6 weeks and after 12 weeks and its expression was inversely related with duration of compression. It means that more autophagy material was accumulated laterally but did not degrade initially but after increasing duration of compression, autophagy accumulation materials destroyed. It proves that there may have lateral nucleus pulposus cells degradation but not central.
Matrix metalloproteinase (MMP)-3 have function in the ER stress-induced apoptosis. Catalytic activity of MMP-3 enzyme can be upregulated through down-regulated of its endogenous inhibitor protein TIMP-1. TIMP-1 is declined in reply to ER stress, and TIMP-1 higher expression leads to cell protection and a decline in MMP-3 function (19).It has been documented that NP cells apoptosis, senescence, and imbalance between matrix anabolism and catabolism are widely recognized as main contributors to IVDD(16, 20). From our study, we identified, MMP-2 expression was higher which expressed centrally in EF group. On contrary, no significant changes were found for MMP-2 expression in SI group and it cannot distinguish the margin between NP and AF cells. Higher and centrally expression of MMP-3 was observed in EF group for both 6 and 12 weeks but after 12 weeks, it showed more expression than 6 weeks. It denotes that the apoptotic marker highly expressed in central NP cells. And it had upregulated after increasing the duration of compression. Expression of TIMP-1 was higher and expressed more centrally in 6 weeks. But after 12 weeks, the expression was decreased and it also expressed centrally. It dictates that apoptosis mediated degradation of extracellular matrix elevated after increasing the duration of compression. Whereas, IS group cannot distinguish the margin between NP cells and AF cells. It proves that more apoptosis mediated degradation was present in central NP cells in EF group.
We identified, mRNA expression of Beclin-1 was upregulated in both groups but SI group showed sharp expression than EF group after 6 weeks. But after 12 weeks, mRNA expression was declined in both groups, therefore, over expressed than Ctrl. It means that mRNA of beclin-1 highly expressed after immediately using compression, but it decreased after increasing the duration of compression. Highest LC3mRNA expression was identified in EF group than in SI group. But surprisingly, up-regulation mRNA expression of P62 was indentified in EF group than in SI group and Ctrl group all times. This means that maximum autophagy material was accumulated but not destroyed. This result shows that an inconsistent relationship may present between LC3 and p62/SQSTM1 unlike in vitro. The inconsistency between in vitro and in vivo expression patterns of LC3 and p62/SQSTM1 should be distinguished. Unlike in vitro autophagy stimulates with LC3 inclines and p62/SQSTM1 declines under serum deprivation, in vivo decreases in LC3 and p62/SQSTM1 under static compression do not necessarily indicate autophagy deficiency. Decreased LC3 indicates reductions in autophagosome formation and maturation (3), resulting in the loss of autophagic potential. Meanwhile, decreased p62/SQSTM1, an adaptor protein in autophagosome degradation (3, 21), may reflect severely degenerated conditions after impaired autophagy by unphysiological mechanical pressure (21).
Apoptosis is a key ways to cell diminish which linked with characteristic morphological changes including formation of membrane blebs, apoptotic bodies, chromatin and nuclear condensation, and DNA fragmentation (22).It is well known that tissue inhibitor of metalloproteinases-1 (TIMP-1) has the anti-apoptotic activity to enhance the cell growth (23). And also some data has published that MMP members have a key role in cell apoptosis. During apoptosis, MMP member is needed for remodeling of the cell matrix and cell-to-cell contact (24).From our study, we indentified, down regulated mRNA expression for MMP-2 after 6 weeks in SI group and no significant changes were found in EF group. But after 12 weeks, EF and SI groups showed vast up-regulatedMMP-2 expression, but EF group showed highest expression than SI group. Up-regulated mRNA expression was observed for MMP-3 in both 6 and 12 weeks, but the expression was decreased after 12 weeks compared to 6 weeks. In both cases, SI group showed over expression than EF group. It indicates that apoptotic promoting mRNA expression was elevated in SI group than in EF group. mRNA expression of TIMP-1 was up-regulated in EF group but no significant change was identified in SI group after 6 weeks. And after 12 weeks, TIMP-1 expression was amplified in both EF and in SI group but EF group showed more expression than SI group. From this evidence it can be mentioned that anti-apoptotic gene was induced more in EF group than in SI group. It proves that compression induced apoptotic gene expression and it elevates the expression along with increasing duration of compression. Although MMP-2 showed some ant-apoptotic activity, however, that was not unusual because some documents have mentioned that MMP-2 has anti-apoptotic activity (25). So, this result was consistent compared to previous mentioned results.
Limited number of rats were used in this study which was the major limitation of our study, for more extensive investigation has to be performed for identification the root cause of increasing the label of P62 after compression in vivo but not in vitro. There may another halting mechanism that has to be investigated.SI cannot give us true data to prove that whether there was central nucleus pulposus cells death or annulus fibrosus cells death.
5. Conclusions
External fixator method can be a best method for studying autophagy and apoptosis, because autophagy exerted lateral not central and apoptosis exerted central not lateral NP cells degradation. Although, saline injection cannot distinguish the boundary between NP cells and AF cells, so it cannot be a good method to study autophagy and apoptosis.
Acknowledgments
This work has been supported by The Catholic University of Korea Daejeon St. Mary's Hospital, Clinical research institute (No.: CMCDJ-A-2022-016) and funded by The Catholic University of Korea Daejeon St. Mary's Hospital.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Quan M, Hong MW, Ko MS, Kim YY. Relationships Between Disc Degeneration and Autophagy Expression in Human Nucleus Pulposus. Orthop Surg. 2020;12(1):312-20. [CrossRef]
- Zhang S, Liang W, Abulizi Y, Xu T, Cao R, Xun C, et al. Quercetin Alleviates Intervertebral Disc Degeneration by Modulating p38 MAPK-Mediated Autophagy. Biomed Res Int. 2021;2021:6631562. [CrossRef]
- Yurube T, Hirata H, Ito M, Terashima Y, Kakiuchi Y, Kuroda R, et al. Involvement of Autophagy in Rat Tail Static Compression-Induced Intervertebral Disc Degeneration and Notochordal Cell Disappearance. Int J Mol Sci. 2021;22(11). [CrossRef]
- Banala RR, Vemuri SK, Ev S, Av GR, Gpv S. The Anti-Inflammatory and Cytoprotective Efficiency of Curvularin, a Fungal Macrolactone against Lipopolysaccharide-Induced Inflammatory Response in Nucleus Pulposus Cells: An In Vitro Study. Asian Spine J. 2021;15(2):143-54. [CrossRef]
- Rajasekaran S, Soundararajan DCR, Tangavel C, K SS, Nayagam SM, Matchado MS, et al. Proteomic Signature of Nucleus Pulposus in Fetal Intervertebral Disc. Asian Spine J. 2020;14(4):409-20. [CrossRef]
- Baldia M, Mani S, Walter N, Kumar S, Srivastava A, Prabhu K. Development of a Unique Mouse Intervertebral Disc Degeneration Model Using a Simple Novel Tool. Asian Spine J. 2021;15(4):415-23. [CrossRef]
- Zhao L, Lin H, Chen S, Chen S, Cui M, Shi D, et al. Hydrogen peroxide induces programmed necrosis in rat nucleus pulposus cells through the RIP1/RIP3-PARP-AIF pathway. J Orthop Res. 2018;36(4):1269-82. [CrossRef]
- Che H, Li J, Li Y, Ma C, Liu H, Qin J, et al. p16 deficiency attenuates intervertebral disc degeneration by adjusting oxidative stress and nucleus pulposus cell cycle. Elife. 2020;9. [CrossRef]
- Yurube T, Hirata H, Kakutani K, Maeno K, Takada T, Zhang Z, et al. Notochordal cell disappearance and modes of apoptotic cell death in a rat tail static compression-induced disc degeneration model. Arthritis Res Ther. 2014;16(1):R31. [CrossRef]
- Yurube T, Ito M, Kakiuchi Y, Kuroda R, Kakutani K. Autophagy and mTOR signaling during intervertebral disc aging and degeneration. JOR Spine. 2020;3(1):e1082. [CrossRef]
- Chen S, Lv X, Hu B, Shao Z, Wang B, Ma K, et al. RIPK1/RIPK3/MLKL-mediated necroptosis contributes to compression-induced rat nucleus pulposus cells death. Apoptosis. 2017;22(5):626-38. [CrossRef]
- Chen S, Lv X, Hu B, Zhao L, Li S, Li Z, et al. Critical contribution of RIPK1 mediated mitochondrial dysfunction and oxidative stress to compression-induced rat nucleus pulposus cells necroptosis and apoptosis. Apoptosis. 2018;23(5-6):299-313. [CrossRef]
- Tsujimoto R, Yurube T, Takeoka Y, Kanda Y, Miyazaki K, Ohnishi H, et al. Involvement of autophagy in the maintenance of rat intervertebral disc homeostasis: an in-vitro and in-vivo RNA interference study of Atg5. Osteoarthritis Cartilage. 2022;30(3):481-93. [CrossRef]
- Hirata H, Yurube T, Kakutani K, Maeno K, Takada T, Yamamoto J, et al. A rat tail temporary static compression model reproduces different stages of intervertebral disc degeneration with decreased notochordal cell phenotype. J Orthop Res. 2014;32(3):455-63. [CrossRef]
- Ma KG, Shao ZW, Yang SH, Wang J, Wang BC, Xiong LM, et al. Autophagy is activated in compression-induced cell degeneration and is mediated by reactive oxygen species in nucleus pulposus cells exposed to compression. Osteoarthritis Cartilage. 2013;21(12):2030-8. [CrossRef]
- Kang L, Xiang Q, Zhan S, Song Y, Wang K, Zhao K, et al. Restoration of Autophagic Flux Rescues Oxidative Damage and Mitochondrial Dysfunction to Protect against Intervertebral Disc Degeneration. Oxid Med Cell Longev. 2019;2019:7810320. [CrossRef]
- Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18(4):571-80. [CrossRef]
- Kakiuchi Y, Yurube T, Kakutani K, Takada T, Ito M, Takeoka Y, et al. Pharmacological inhibition of mTORC1 but not mTORC2 protects against human disc cellular apoptosis, senescence, and extracellular matrix catabolism through Akt and autophagy induction. Osteoarthritis Cartilage. 2019;27(6):965-76. [CrossRef]
- Kim EM, Shin EJ, Choi JH, Son HJ, Park IS, Joh TH, et al. Matrix metalloproteinase-3 is increased and participates in neuronal apoptotic signaling downstream of caspase-12 during endoplasmic reticulum stress. J Biol Chem. 2010;285(22):16444-52. [CrossRef]
- Li YX, Ma XX, Zhao CL, Wei JH, Mei AH, Liu Y. Nucleus pulposus cells degeneration model: a necessary way to study intervertebral disc degeneration. Folia Morphol (Warsz). 2022. [CrossRef]
- Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN, Jr. Autophagy in the Degenerating Human Intervertebral Disc: In Vivo Molecular and Morphological Evidence, and Induction of Autophagy in Cultured Annulus Cells Exposed to Proinflammatory Cytokines-Implications for Disc Degeneration. Spine (Phila Pa 1976). 2015;40(11):773-82. [CrossRef]
- Zhang F, Zhao X, Shen H, Zhang C. Molecular mechanisms of cell death in intervertebral disc degeneration (Review). Int J Mol Med. 2016;37(6):1439-48. [CrossRef]
- Kandasamy AD, Chow AK, Ali MA, Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: beyond the matrix. Cardiovasc Res. 2010;85(3):413-23. [CrossRef]
- Lao G, Ren M, Wang X, Zhang J, Huang Y, Liu D, et al. Human tissue inhibitor of metalloproteinases-1 improved wound healing in diabetes through its anti-apoptotic effect. Exp Dermatol. 2019;28(5):528-35. [CrossRef]
- Shapiro S, Khodalev O, Bitterman H, Auslender R, Lahat N. Different activation forms of MMP-2 oppositely affect the fate of endothelial cells. Am J Physiol Cell Physiol. 2010;298(4):C942-51. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).