Submitted:
05 January 2023
Posted:
12 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Susceptibility of Microorganisms towards Zinc(II) Ions
3. Antimicrobial Peptides
4. Nexus between Zn(II) and AMPs

4.1. Dermcidin Derived Peptides
4.2. Clavanin A
4.3. Histatins
4.4. Bacitracin
4.5. Kappacin
4.6.*ARVA Peptide
4.7. Calcitermin
4.8. Shepherin
4.9. Surfactant-Associated Anionic Peptides (SAAPs)
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Howell, M.; Wenc, A.; Donaghy, C.; Wasche, D.; Abissi, I.; Naing, M.; Pierce, S.; Angeles-Boza, A. M. Chapter Five - Exploring Synergy and Its Role in Antimicrobial Peptide Biology. In Methods in Enzymology; Academic Press, 2022; Vol. 663, pp 99–130.
- Maret, W. The Redox Biology of Redox-Inert Zinc Ions. Free Radical Biology and Medicine 2019, 134, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Kluska, K.; Adamczyk, J.; Krężel, A. Metal Binding Properties, Stability and Reactivity of Zinc Fingers. Coordination Chemistry Reviews 2018, 367, 18–64. [Google Scholar] [CrossRef]
- Ong, C. Y.; Berking, O.; Walker, M. J.; McEwan, A. G. New Insights into the Role of Zinc Acquisition and Zinc Tolerance in Group A Streptococcal Infection. Infect Immun 2018, 86(6), e00048–18. [Google Scholar] [CrossRef]
- Djoko, K. Y.; Ong, C. Y.; Walker, M. J.; McEwan, A. G. The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens. Journal of Biological Chemistry 2015, 290(31), 18954–18961. [Google Scholar] [CrossRef] [PubMed]
- Ibs, K.-H.; Rink, L. Zinc-Altered Immune Function. The Journal of Nutrition 2003, 133(5), 1452S–1456S. [Google Scholar] [CrossRef] [PubMed]
- R., Milanino; Marrella, M.; Gasperini, R.; Pasqualicchio, M.; Velo, G. Copper and Zinc Body Levels in Inflammation: An Overview of the Data Obtained from Animal and Human Studies. Agents Actions 1993, 39 (3–4), 195–209. [CrossRef]
- Wang, C.; Zhang, R.; Wei, X.; Lv, M.; Jiang, Z. Metalloimmunology: The Metal Ion-Controlled Immunity. In Advances in Immunology; Academic Press, 2020; Vol. 145, pp 187–241.
- Stafford, S. L.; Bokil, N. J.; Achard, M. E. S.; Kapetanovic, R.; Schembri, M. A.; McEwan, A. G.; Sweet, M. J. Metal Ions in Macrophage Antimicrobial Pathways: Emerging Roles for Zinc and Copper. Bioscience Reports 2013, 33(4), e00049. [Google Scholar] [CrossRef] [PubMed]
- Ong, C. Y.; Gillen, C. M.; Barnett, T. C.; Walker, M. J.; McEwan, A. G. An Antimicrobial Role for Zinc in Innate Immune Defense Against Group A Streptococcus. The Journal of Infectious Diseases 2014, 209(10), 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Maser, J.; Lai, B.; Cai, Z.; Barry, C. E.; Höner zu Bentrup, K.; Russell, D. G.; Bermudez, L. E. Elemental Analysis of Mycobacterium Avium -, Mycobacterium Tuberculosis -, and Mycobacterium Smegmatis -Containing Phagosomes Indicates Pathogen-Induced Microenvironments within the Host Cell’s Endosomal System. J Immunol 2005, 174(3), 1491–1500. [Google Scholar] [CrossRef] [PubMed]
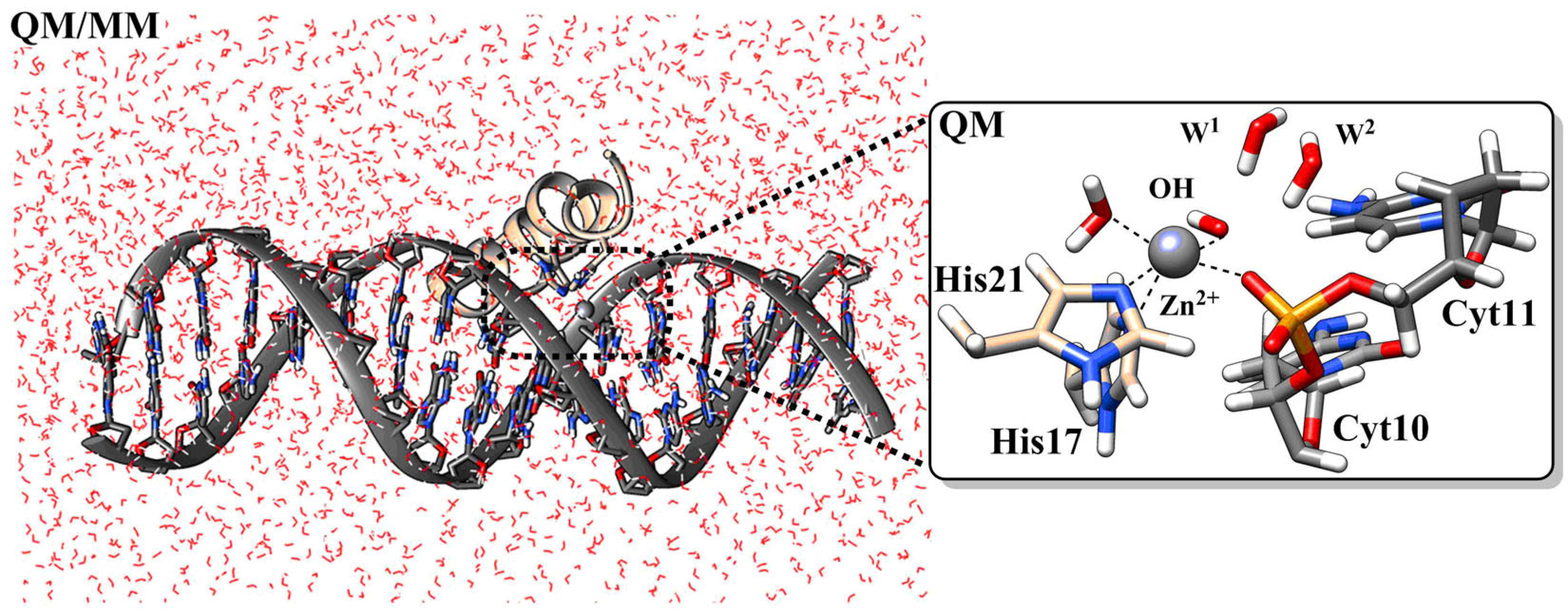
- Juliano, S. A.; Serafim, L. F.; Duay, S. S.; Heredia Chavez, M.; Sharma, G.; Rooney, M.; Comert, F.; Pierce, S.; Radulescu, A.; Cotten, M. L.; Mihailescu, M.; May, E. R.; Greenwood, A. I.; Prabhakar, R.; Angeles-Boza, A. M. A Potent Host Defense Peptide Triggers DNA Damage and Is Active against Multidrug-Resistant Gram-Negative Pathogens. ACS Infect. Dis. 2020, 6(5), 1250–1263. [Google Scholar] [CrossRef]
- Ning, C.; Wang, X.; Li, L.; Zhu, Y.; Li, M.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Tan, G.; Zhang, Y.; Wang, Y.; Mao, C. Concentration Ranges of Antibacterial Cations for Showing the Highest Antibacterial Efficacy but the Least Cytotoxicity against Mammalian Cells: Implications for a New Antibacterial Mechanism. Chem. Res. Toxicol. 2015, 28(9), 1815–1822. [Google Scholar] [CrossRef]
- Choudhury, R.; Srivastava, S. Zinc Resistance Mechanisms in Bacteria. 2022, 9.
- Braymer, J. J.; Giedroc, D. P. Recent Developments in Copper and Zinc Homeostasis in Bacterial Pathogens. Current Opinion in Chemical Biology 2014, 19, 59–66. [Google Scholar] [CrossRef]
- Steven, J. Beard, Rohani Hashim, Jorge Membrillo-Hernández, Martin N. Hughes, Robert K. Poole. Zinc(II) Tolerance in Escherichia Coli K-12: Evidence That the ZntA Gene (O732) Encodes a Cation Transport ATPase. Molecular Microbiology 2004, 25 (5), 883–891. [CrossRef]
- Ong, C. Y.; Walker, M. J.; McEwan, A. G. Zinc Disrupts Central Carbon Metabolism and Capsule Biosynthesis in Streptococcus Pyogenes. Sci Rep 2015, 5(1), 10799. [Google Scholar] [CrossRef] [PubMed]
- Soares, L. W.; Bailão, A. M.; Soares, C. M. de A.; Bailão, M. G. S. Zinc at the Host–Fungus Interface: How to Uptake the Metal? JoF 2020, 6(4), 305. [Google Scholar] [CrossRef]
- Jung, W. H. The Zinc Transport Systems and Their Regulation in Pathogenic Fungi. Mycobiology 2015, 43(3), 179–183. [Google Scholar] [CrossRef]
- Volkova, M.; Atamas, A.; Tsarenko, A.; Rogachev, A.; Guskov, A. Cation Transporters of Candida Albicans—New Targets to Fight Candidiasis? Biomolecules 2021, 11(4), 584. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, C. A.; Ogunniyi, A. D.; Valkov, E.; Lawrence, M. C.; Kobe, B.; McEwan, A. G.; Paton, J. C. A Molecular Mechanism for Bacterial Susceptibility to Zinc. PLoS Pathog 2011, 7(11), e1002357. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ren, X.; Fan, B.; Huang, Z.; Wang, W.; Zhou, H.; Lou, Z.; Ding, H.; Lyu, J.; Tan, G. Zinc Toxicity and Iron-Sulfur Cluster Biogenesis in Escherichia Coli. Appl Environ Microbiol 2019, 85(9), e01967–18. [Google Scholar] [CrossRef] [PubMed]
- Lewis, B. E.; Mason, Z.; Rodrigues, A. V.; Nuth, M.; Dizin, E.; Cowan, J. A.; Stemmler, T. L. Unique Roles of Iron and Zinc Binding to the Yeast Fe–S Cluster Scaffold Assembly Protein “Isu1.” Metallomics 2019, 11 (11), 1820–1835. [CrossRef]
- Bunnell, B. E.; Escobar, J. F.; Bair, K. L.; Sutton, M. D.; Crane, J. K. Zinc Blocks SOS-Induced Antibiotic Resistance via Inhibition of RecA in Escherichia Coli. PLoS ONE 2017, 12(5), e0178303. [Google Scholar] [CrossRef] [PubMed]
- Gutknecht, J. Cadmium and Thallous Ion Permeabilities through Lipid Bilayer Membranes. Biochimica et Biophysica Acta (BBA) - Biomembranes 1983, 735 (1), 185–188. [CrossRef]
- Duay, S. S.; Sharma, G.; Prabhakar, R.; Angeles-Boza, A. M.; May, E. R. Molecular Dynamics Investigation into the Effect of Zinc(II) on the Structure and Membrane Interactions of the Antimicrobial Peptide Clavanin A. J. Phys. Chem. B 2019, 123(15), 3163–3176. [Google Scholar] [CrossRef]
- Kučerka, N.; Dushanov, E.; Kholmurodov, K. T.; Katsaras, J.; Uhríková, D. Calcium and Zinc Differentially Affect the Structure of Lipid Membranes. Langmuir 2017, 33(12), 3134–3141. [Google Scholar] [CrossRef]
- Portelinha, J.; Duay, S. S.; Yu, S. I.; Heilemann, K.; Libardo, M. D. J.; Juliano, S. A.; Klassen, J. L.; Angeles-Boza, A. M. Antimicrobial Peptides and Copper(II) Ions: Novel Therapeutic Opportunities. Chem. Rev. 2021, 121(4), 2648–2712. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J. L.; Thompson, Z.; Cowan, J. A. Antimicrobial Metallopeptides. ACS Chem. Biol. 2018, 13(4), 844–853. [Google Scholar] [CrossRef]
- Wimley, W. C. Describing the Mechanism of Antimicrobial Peptide Action with the Interfacial Activity Model. ACS Chem. Biol. 2010, 5(10), 905–917. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D. K.; Torres, M. D. T.; Duay, S. S.; Lovie, E.; Simpson, L.; von Köckritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D. A.; Angeles-Boza, A. M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell. Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef]
- Hancock, R. E. W.; Lehrer, R. Cationic Peptides: A New Source of Antibiotics. Trends in Biotechnology 1998, 16(2), 82–88. [Google Scholar] [CrossRef]
- Harris, F.; Dennison, S.; Phoenix, D. Anionic Antimicrobial Peptides from Eukaryotic Organisms and Their Mechanisms of Action. Curr. Chem. Biol. 2011, 5 (2), 142-153(12). [CrossRef]
- Lehrer, R.; Ganz, T. Antimicrobial Peptides in Mammalian and Insect Host Defence. Current Opinion in Immunology 1999, 11(1), 22–27. [Google Scholar] [CrossRef]
- Libardo, M. D.; Angeles-Boza, A. M. Bioinorganic Chemistry of Antimicrobial and Host-Defense Peptides. Crit Rev (N Y) 2014, 34 (1–2), 42–58. [CrossRef]
- Tonk, M.; Vilcinskas, A. The Medical Potential of Antimicrobial Peptides from Insects. Current Topics in Medicinal Chemistry 2017, 17(5), 554–575. [Google Scholar] [CrossRef]
- Walkenhorst, W. F. Using Adjuvants and Environmental Factors to Modulate the Activity of Antimicrobial Peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes 2016, 1858 (5), 926–935. [CrossRef]
- Guha, S.; Ghimire, J.; Wu, E.; Wimley, W. C. Mechanistic Landscape of Membrane-Permeabilizing Peptides. Chem. Rev. 2019, 119(9), 6040–6085. [Google Scholar] [CrossRef] [PubMed]
- Le, C.-F.; Fang, C.-M.; Sekaran, S. D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob Agents Chemother 2017, 61(4), e02340–16. [Google Scholar] [CrossRef]
- Madani, F.; Lindberg, S.; Langel, Ü.; Futaki, S.; Gräslund, A. Mechanisms of Cellular Uptake of Cell-Penetrating Peptides. Journal of Biophysics 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Subbalakshmi, C.; Sitaram, N. Mechanism of Antimicrobial Action of Indolicidin. FEMS Microbiology Letters 1998, 160(1), 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-H.; Shah, P.; Chen, Y.-W.; Chen, C.-S. Systematic Analysis of Intracellular-Targeting Antimicrobial Peptides, Bactenecin 7, Hybrid of Pleurocidin and Dermaseptin, Proline–Arginine- Rich Peptide, and Lactoferricin B, by Using Escherichia Coli Proteome Microarrays*□S. 11.
- Nishikata, M.; Kanehira, T.; Oh, H.; Tani, H.; Tazaki, M.; Kuboki, Y. Salivary Histatin as an Inhibitor of a Protease Produced by the Oral Bacterium Bacteroides Gingivalis. Biochemical and Biophysical Research Communications 1991, 174(2), 625–630. [Google Scholar] [CrossRef]
- Campbell, J. X.; Gao, S.; Anand, K. S.; Franz, K. J. Zinc Binding Inhibits Cellular Uptake and Antifungal Activity of Histatin-5 in Candida Albicans. ACS Infect. Dis. 2022, 8(9), 1920–1934. [Google Scholar] [CrossRef] [PubMed]
- Juliano, S. A.; Pierce, S.; deMayo, J. A.; Balunas, M. J.; Angeles-Boza, A. M. Exploration of the Innate Immune System of Styela Clava : Zn 2+ Binding Enhances the Antimicrobial Activity of the Tunicate Peptide Clavanin A. Biochemistry 2017, 56(10), 1403–1414. [Google Scholar] [CrossRef]
- Eissa, A.; Amodeo, V.; Smith, C. R.; Diamandis, E. P. Kallikrein-Related Peptidase-8 (KLK8) Is an Active Serine Protease in Human Epidermis and Sweat and Is Involved in a Skin Barrier Proteolytic Cascade. Journal of Biological Chemistry 2011, 286(1), 687–706. [Google Scholar] [CrossRef] [PubMed]
- Schittek, B.; Hipfel, R.; Sauer, B.; Bauer, J.; Kalbacher, H.; Stevanovic, S.; Schirle, M.; Schroeder, K.; Blin, N.; Meier, F.; Rassner, G.; Garbe, C. Dermcidin: A Novel Human Antibiotic Peptide Secreted by Sweat Glands. Nat Immunol 2001, 2(12), 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Rieg, S.; Steffen, H.; Seeber, S.; Humeny, A.; Kalbacher, H.; Dietz, K.; Garbe, C.; Schittek, B. Deficiency of Dermcidin-Derived Antimicrobial Peptides in Sweat of Patients with Atopic Dermatitis Correlates with an Impaired Innate Defense of Human Skin In Vivo. The Journal of Immunology.
- Paulmann, M.; Arnold, T.; Linke, D.; Özdirekcan, S.; Kopp, A.; Gutsmann, T.; Kalbacher, H.; Wanke, I.; Schuenemann, V. J.; Habeck, M.; Bürck, J.; Ulrich, A. S.; Schittek, B. Structure-Activity Analysis of the Dermcidin-Derived Peptide DCD-1L, an Anionic Antimicrobial Peptide Present in Human Sweat. Journal of Biological Chemistry 2012, 287(11), 8434–8443. [Google Scholar] [CrossRef] [PubMed]
- Steffen, H.; Rieg, S.; Wiedemann, I.; Kalbacher, H.; Deeg, M.; Sahl, H.-G.; Peschel, A.; Götz, F.; Garbe, C.; Schittek, B. Naturally Processed Dermcidin-Derived Peptides Do Not Permeabilize Bacterial Membranes and Kill Microorganisms Irrespective of Their Charge. Antimicrob Agents Chemother 2006, 50(8), 2608–2620. [Google Scholar] [CrossRef] [PubMed]
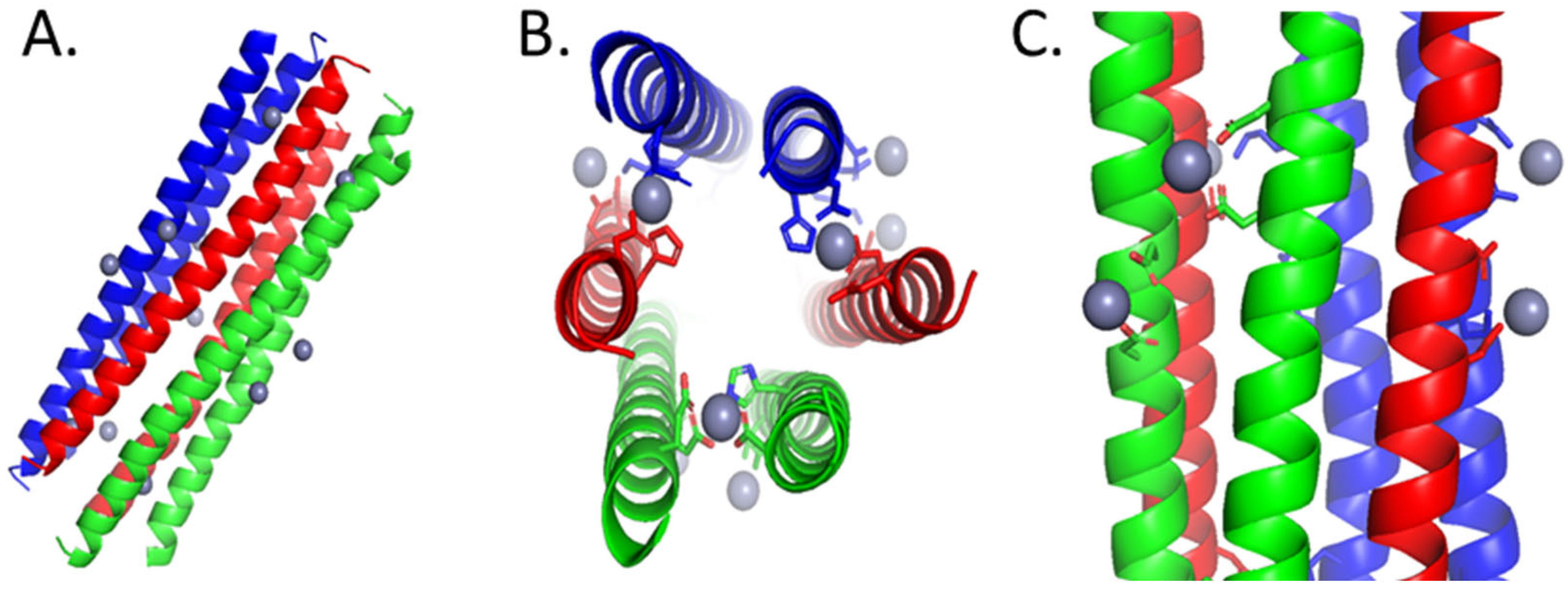
- Song, C.; Weichbrodt, C.; Salnikov, E. S.; Dynowski, M.; Forsberg, B. O.; Bechinger, B.; Steinem, C.; de Groot, B. L.; Zachariae, U.; Zeth, K. Crystal Structure and Functional Mechanism of a Human Antimicrobial Membrane Channel. Proc. Natl. Acad. Sci. U.S.A. 2013, 110(12), 4586–4591. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System, 2010.
- Lee, I. H.; Cho, Y.; Lehrer, R. I. Effects of PH and Salinity on the Antimicrobial Properties of Clavanins. Infect Immun 1997, 65(7), 2898–2903. [Google Scholar] [CrossRef]
- Juliano, S. A. From Antimicrobial Activity to Zinc Binding: An In-Depth Analysis of the Tunicate Host Defense Peptide Clavanin A, 2020.
- van Kan, E. J. M.; van der Bent, A.; Demel, R. A.; de Kruijff, B. Membrane Activity of the Peptide Antibiotic Clavanin and the Importance of Its Glycine Residues. Biochemistry 2001, 40(21), 6398–6405. [Google Scholar] [CrossRef] [PubMed]
- van Kan, E. J. M.; Demel, R. A.; Breukink, E.; van der Bent, A.; de Kruijff, B. Clavanin Permeabilizes Target Membranes via Two Distinctly Different PH-Dependent Mechanisms. Biochemistry 2002, 41(24), 7529–7539. [Google Scholar] [CrossRef] [PubMed]
- Sabatini, L.; Azen, E. Histatins, a Family of Salivary Histidine-Rich Proteins, Are Encoded by at Least Two Loci (HIS1 and HIS2). Biochem. Biophys. Res. Commun. 1989, 160(2), 495–502. [Google Scholar] [CrossRef] [PubMed]
- Melino, S.; Santone, C.; Di Nardo, P.; Sarkar, B. Histatins: Salivary Peptides with Copper(II)- and Zinc(II)-binding Motifs. FEBS Lett. 281 (3), 657–672. [CrossRef]
- Oppenheim, F. G.; Xu, T.; McMillian, F. M.; Levitz, S. M.; Diamond, R. D.; Offner, G. D.; Troxler, R. F. Histatins, a Novel Family of Histidine-Rich Proteins in Human Parotid Secretion. Isolation, Characterization, Primary Structure, and Fungistatic Effects on Candida Albicans. Journal of Biological Chemistry 1988, 263 (16), 7472–7477. [CrossRef]
- Castagnola, M.; Inzitari, R.; Rossetti, D. V.; Olmi, C.; Cabras, T.; Piras, V.; Nicolussi, P.; Sanna, M. T.; Pellegrini, M.; Giardina, B.; Messana, I. A Cascade of 24 Histatins (Histatin 3 Fragments) in Human Saliva. Journal of Biological Chemistry 2004, 279(40), 41436–41443. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Edgerton, M. How Does It Kill?: Understanding the Candidacidal Mechanism of Salivary Histatin 5. Eukaryot Cell 2014, 13(8), 958–964. [Google Scholar] [CrossRef]
- Pan, L.; Zhang, X.; Gao, Q. Effects and Mechanisms of Histatins as Novel Skin Wound-Healing Agents. Journal of Tissue Viability 2021, 30(2), 190–195. [Google Scholar] [CrossRef]
- Du, H.; Puri, S.; McCall, A.; Norris, H. L.; Russo, T.; Edgerton, M. Human Salivary Protein Histatin 5 Has Potent Bactericidal Activity against ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Norris, H. L.; Kumar, R.; Ong, C. Y.; Xu, D.; Edgerton, M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. Albicans and C. Glabrata. JoF 2020, 6(3), 124. [Google Scholar] [CrossRef]
- Helmerhorst, E. J.; Reijnders, I. M.; van ’t Hof, W.; Simoons-Smit, I.; Veerman, E. C. I.; Amerongen, A. V. N. Amphotericin B- and Fluconazole-Resistant Candida Spp., Aspergillus Fumigatus, and Other Newly Emerging Pathogenic Fungi Are Susceptible to Basic Antifungal Peptides. Antimicrob Agents Chemother 1999, 43 (3), 702–704. [CrossRef]
- 66) A Khan, S. Impaired Histatin-5 Levels and Salivary Antimicrobial Activity against C.Albicans in HIV Infected Individuals. J AIDS Clin Res 2013, 04 (02). [CrossRef]
- 67) McCaslin, T. G.; Pagba, C. V.; Yohannan, J.; Barry, B. A. Specific Metallo-Protein Interactions and Antimicrobial Activity in Histatin-5, an Intrinsically Disordered Salivary Peptide. Sci Rep 2019, 9(1), 17303. [Google Scholar] [CrossRef]
- Cragnell, C.; Staby, L.; Lenton, S.; Kragelund, B.; Skepö, M. Dynamical Oligomerisation of Histidine Rich Intrinsically Disordered Proteins Is Regulated through Zinc-Histidine Interactions. Biomolecules 2019, 9(5), 168. [Google Scholar] [CrossRef]
- Kurut, A.; Henriques, J.; Forsman, J.; Skepo, M.; Lund, M. Role of Histidine for Charge Regulation of Unstructured Peptides at Interfaces and in Bulk. Proteins 2013, 82(4), 657–667. [Google Scholar] [CrossRef] [PubMed]
- Stewart, L.; Hong, Y.; Holmes, I.; Firth, S.; Bolton, J.; Santos, Y.; Cobb, S.; Jakubovics, N.; Djoko, K. The Role of Metal Binding in the Function of the Human Salivary Antimicrobial Peptide Histatin-5; preprint; Microbiology, 2022. [CrossRef]
- Stewart, L.; Hong, Y.; Holmes, I.; Firth, S.; Ahmed, Y.; Quinn, J.; Santos, Y.; Cobb, S.; Jakubovics, N.; Djoko, K. Role of Zinc in the Activity of Histatin-5 against Streptococci. ACS Infectious Diseases.
- Norris, H.; Kumar, R.; Edgerton, M. A Novel Role for Histatin 5 in Combination with Zinc to Promote Commensalism in C. Albicans Survivor Cells. Pathogens 2021, 10(12), 1609. [Google Scholar] [CrossRef] [PubMed]
- Flora, B.; Heloisa, G.; Helmerhorst, E. J.; Troxler, R.; Oppenheim, F. G. A New Method for the Isolation of Histatins 1, 3, and 5 from Parotid Secretion Using Zinc Precipitation. Protein Expr. Purif. 2001, 23(1), 198–206. [Google Scholar] [CrossRef] [PubMed]
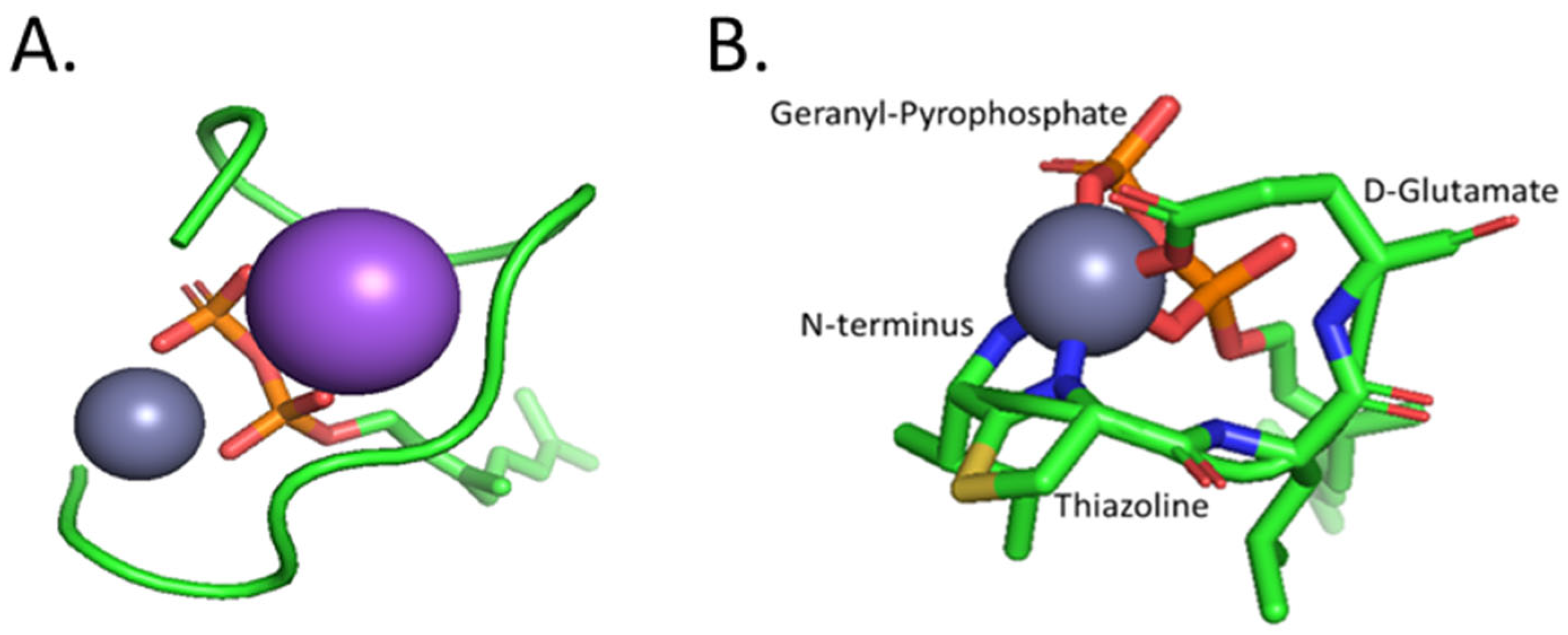
- Tay, W. M.; Epperson, J. D.; da Silva, G. F. Z.; Ming, L.-J. 1 H NMR, Mechanism, and Mononuclear Oxidative Activity of the Antibiotic Metallopeptide Bacitracin: The Role of d -Glu-4, Interaction with Pyrophosphate Moiety, DNA Binding and Cleavage, and Bioactivity. J. Am. Chem. Soc. 2010, 132(16), 5652–5661. [Google Scholar] [CrossRef] [PubMed]
- Economou, N. J.; Cocklin, S.; Loll, P. J. High-Resolution Crystal Structure Reveals Molecular Details of Target Recognition by Bacitracin. Proc. Natl. Acad. Sci. U.S.A. 2013, 110(35), 14207–14212. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, K.; Robertson, J.; Bach, J. Quantitative High-Pressure Liquid Chromatographic Analysis of Bacitracin, a Polypeptide Antibiotic. J. Chromatogr. A 1974, 99, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Ming, L.-J.; Epperson, J. D. Metal Binding and Structure–Activity Relationship of the Metalloantibiotic Peptide Bacitracin. J. Inorg. Biochem. 2002, 91(1), 46–58. [Google Scholar] [CrossRef]
- Adler, R. H.; Snoke, J. E. REQUIREMENT OF DIVALENT METAL IONS FOR BACITRACIN ACTIVITY. J Bacteriol 1962, 83(6), 1315–1317. [Google Scholar] [CrossRef] [PubMed]
- Storm, D. R. Mechanism of Bacitractin Action: A Specific Lipid-Peptide Interaction. Ann. N. Y. Acad. Sci. 1974, 235(1), 387–398. [Google Scholar] [CrossRef]
- Storm, D. R.; Strominger, J. L. Complex Formation between Bacitracin Peptides and Isoprenyl Pyrophosphates. Journal of Biological Chemistry 1973, 248(11), 3940–3945. [Google Scholar] [CrossRef]
- Malkoski, M.; Dashper, S. G.; O’Brien-Simpson, N. M.; Talbo, G. H.; Macris, M.; Cross, K. J.; Reynolds, E. C. Kappacin, a Novel Antibacterial Peptide from Bovine Milk. Antimicrob Agents Chemother 2001, 45(8), 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Dashper, S. G.; O’Brien-Simpson, N. M.; Cross, K. J.; Paolini, R. A.; Hoffmann, B.; Catmull, D. V.; Malkoski, M.; Reynolds, E. C. Divalent Metal Cations Increase the Activity of the Antimicrobial Peptide Kappacin. Antimicrob Agents Chemother 2005, 49(6), 2322–2328. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Edwards, P.; Palmano, K.; Creamer, L. Structural Features of Bovine Caseinomacropeptide A and B by 1H Nuclear Magnetic Resonance Spectroscopy. J. Dairy Res. 69 (1), 85–94. [CrossRef]
- Rathinakumar, R.; Walkenhorst, W. F.; Wimley, W. C. Broad-Spectrum Antimicrobial Peptides by Rational Combinatorial Design and High-Throughput Screening: The Importance of Interfacial Activity. J. Am. Chem. Soc. 2009, 131(22), 7609–7617. [Google Scholar] [CrossRef] [PubMed]
- Walkenhorst, W. F.; Sundrud, J. N.; Laviolette, J. M. Additivity and Synergy between an Antimicrobial Peptide and Inhibitory Ions. Biochimica et Biophysica Acta (BBA) - Biomembranes 2014, 1838 (9), 2234–2242. [CrossRef]
- Aoki, S.; Iwaida, K.; Hanamoto, N.; Shiro, M.; Kimura, E. Guanidine Is a Zn 2+ -Binding Ligand at Neutral PH in Aqueous Solution. J. Am. Chem. Soc. 2002, 124(19), 5256–5257. [Google Scholar] [CrossRef] [PubMed]
- Cole, A. M.; Kim, Y.-H.; Tahk, S.; Hong, T.; Weis, P.; Waring, A. J.; Ganz, T. Calcitermin, a Novel Antimicrobial Peptide Isolated from Human Airway Secretions. FEBS Letters 2001, 504 (1–2), 5–10. [CrossRef]
- Gottsch, J. D.; Eisinger, S. W.; Liu, S. H.; Scott, A. L. Calgranulin C Has Filariacidal and Filariastatic Activity. Infect Immun 1999, 67(12), 6631–6636. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Toniolo, M.; Dudek, D.; Mikołajczyk, A.; Guerrini, R.; Matera-Witkiewicz, A.; Remelli, M.; Rowińska-Żyrek, M. Bioinorganic Chemistry of Calcitermin – the Picklock of Its Antimicrobial Activity. Dalton Trans. 2019, 48(36), 13740–13752. [Google Scholar] [CrossRef] [PubMed]
- Park, C. J.; Park, C. B.; Hong, S.-S.; Lee, H.-S.; Lee, S. Y.; Kim, S. C. Characterization and CDNA Cloning of Two Glycine- and Histidine-Rich Antimicrobial Peptides from the Roots of Shepherd’s Purse, Capsella Bursa-Pastoris. Plant Mol. Biol. 2000, 44(2), 187–197. [Google Scholar] [CrossRef] [PubMed]
- Remuzgo, C.; Oewel, T. S.; Daffre, S.; Lopes, T. R. S.; Dyszy, F. H.; Schreier, S.; Machado-Santelli, G. M.; Teresa Machini, M. Chemical Synthesis, Structure–Activity Relationship, and Properties of Shepherin I: A Fungicidal Peptide Enriched in Glycine-Glycine-Histidine Motifs. Amino Acids 2014, 46(11), 2573–2586. [Google Scholar] [CrossRef]
- Reichert, T. Análogos sintéticos da Cheferina I: interação com íons metálicos divalentes e o seu efeito na internalização celular e nas atividades anticandida e candidacida. Dissertation, University of São Paulo, 2018.
- Libardo, M. D. J.; De La Fuente-Nuñez, C.; Anand, K.; Krishnamoorthy, G.; Kaiser, P.; Pringle, S. C.; Dietz, C.; Pierce, S.; Smith, M. B.; Barczak, A.; Kaufmann, S. H. E.; Singh, A.; Angeles-Boza, A. M. Phagosomal Copper-Promoted Oxidative Attack on Intracellular Mycobacterium Tuberculosis. ACS Infectious Diseases 2018, 4(11), 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K. A.; De Lucca, A. J.; Bland, J.; Elliott, S. Isolation of an Ovine Pulmonary Surfactant-Associated Anionic Peptide Bactericidal for Pasteurella Haemolytica. Proc. Natl. Acad. Sci. U.S.A. 1996, 93(1), 412–416. [Google Scholar] [CrossRef]
- Brogden, K. A. Ovine Pulmonary Surfactant Induces Killing of Pasteurella Haemolytica, Escherichia Coli, and Klebsiella Pneumoniae by Normal Serum. Infect Immun 1992, 60(12), 5182–5189. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.; Matera-Witkiewicz, A.; Mikołajczyk, A.; Wątły, J.; Wilcox, D.; Witkowska, D.; Rowińska-Żyrek, M. Zn-Enhanced Asp-Rich Antimicrobial Peptides: N-Terminal Coordination by Zn(II) and Cu(II), Which Distinguishes Cu(II) Binding to Different Peptides. IJMS 2021, 22(13), 6971. [Google Scholar] [CrossRef] [PubMed]
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





