Submitted:
07 January 2023
Posted:
09 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Riverine Particulate Material
- 1)
- Mississippi (MS) bedload material collected in autumn 2015 in New Orleans near to the Department of Earth and Environmental Science of Tulane University. Due to the low water level in autumn 2015, the mud sample was collected from the usually flooded river bed. The sample consists of roughly 80 % SiO2 and is mainly composed of quartz, and feldspars with minor quantities of sheet silicates. It was chosen as a representative of continental riverine material.
- 2)
- Iceland (ICE) suspended particulates collected from Jökulsá á Dal at Brú, a glacial river in Eastern Iceland. The ICE riverine particulate material mainly consists of basaltic glass and crystalline basalt fragments. This sample is representative of the high relief, volcanic and tectonic active islands that contribute over 45 % of river suspended material to the oceans [78,79]. Details on sampling and filtration methods can be found in ref. [78], where the chemical composition is provided (sample ID 01A034 therein).
| Name | ICE | MS |
| Type | suspended | bedload |
| BET (m2/g) | 36.83 | 16.27 |
| SiO2 (%) | 51.87 | 77.65 |
| Na2O (%) | 2.22 | 1.20 |
| MgO (%) | 6.00 | 0.91 |
| Al2O3 (%) | 13.87 | 9.55 |
| P2O5 (%) | 0.24 | 0.14 |
| K2O (%) | 0.40 | 1.70 |
| CaO (%) | 10.15 | 1.67 |
| TiO2 (%) | 2.42 | 0.58 |
| MnO (%) | 0.21 | 0.09 |
| FeO (%) | 12.49 | |
| Fe2O3 (%) | 2.60 | |
| LOI | 3.78 |
2.2. Datoms
| IO | SW | ||
| Major cations (mmol/kg) | |||
| Na | 462 | 470 | |
| K | 9.4 | 10.2 | |
| Mg | 52 | 53 | |
| Ca | 9.4 | 10.3 | |
| Sr | 0.19 | 0.09 | |
| Major anions (mmol/kg) | |||
| Cl | 550 | 550 | |
| SO4 | 23 | 28 | |
| Trace elements (µmol/kg) | |||
| Li | 54 | 20 | |
| Si | 16 | 5 | |
| Mo | 1.8 | 0.1 | |
| Ba | 0.85 | 0.04 | |
| V | 2.95 | 0.04 | |
| Ni | 1.7 | 0.004 | |
| Cr | 7.5 | 0.003 | |
| Al | 240 | 0.002 | |
| Cu | 1.8 | 0.001 | |
| Zn | 0.5 | 0.001 | |
| Mn | 1.2 | 0.0004 | |
| Fe | 0.24 | 0.0001 | |
| Cd | 0.24 | 0.0001 | |
| Pb | 2.1 | 0.00006 | |
| Co | 1.3 | 0.00005 | |
| Ag | 2.3 | 0.00001 | |
| Ti | 0.67 | 0.00001 | |
| pure f/2 | 5 % f/2 | ||
| (mmol/kg) | |||
| 10-01 | 10-02 | ||
| 10-02 | 10-03 | ||
| 10-02 | 10-04 | ||
| 10-02 | 10-03 | ||
| 10-02 | 10-03 | ||
| 10-02 | 10-03 | ||
| 10-02 | 10-03 | ||
| thiamine HCl (V B1) | 10-04 | 10-05 | |
| biotine (V H) | 10-06 | 10-07 | |
| cyanocobalamin (V B12) | 10-07 | 10-08 | |
2.3. Growth experiments
2.4. Sampling and analytical methods
3. Results
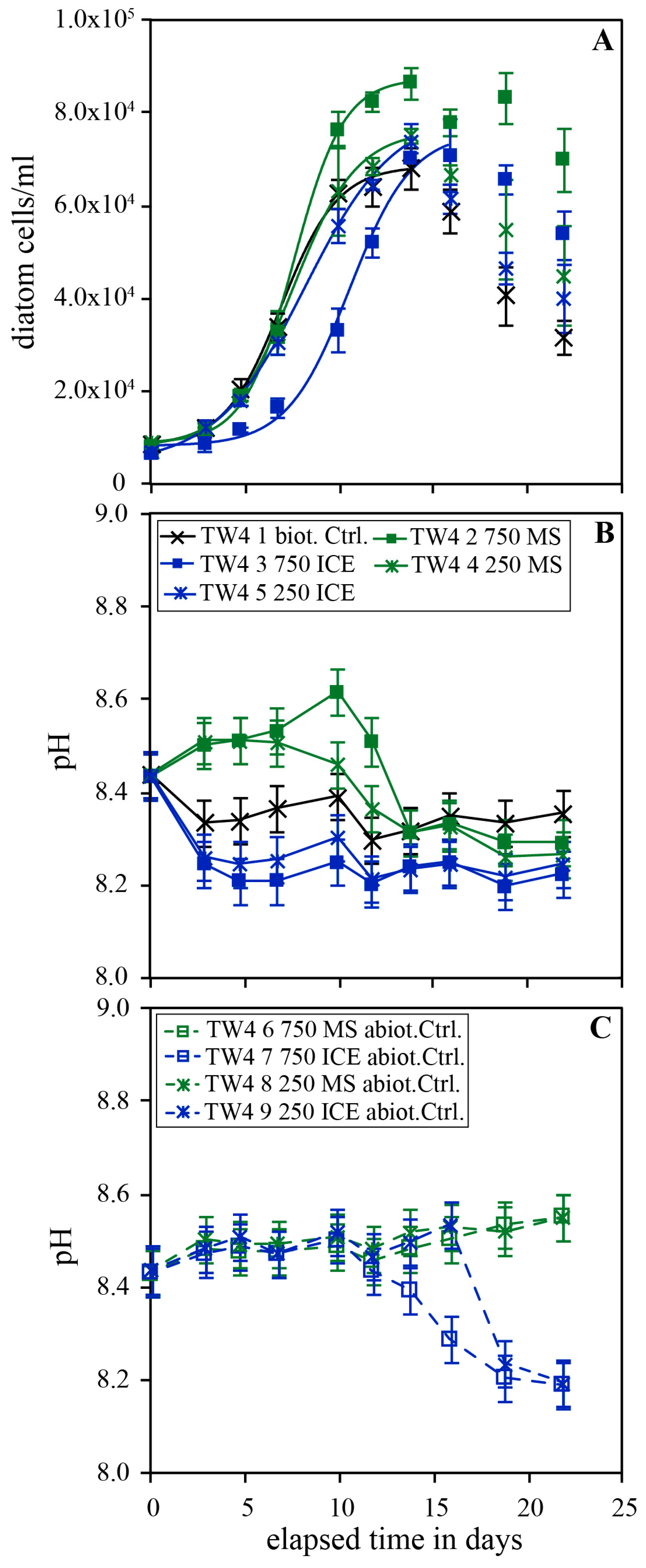
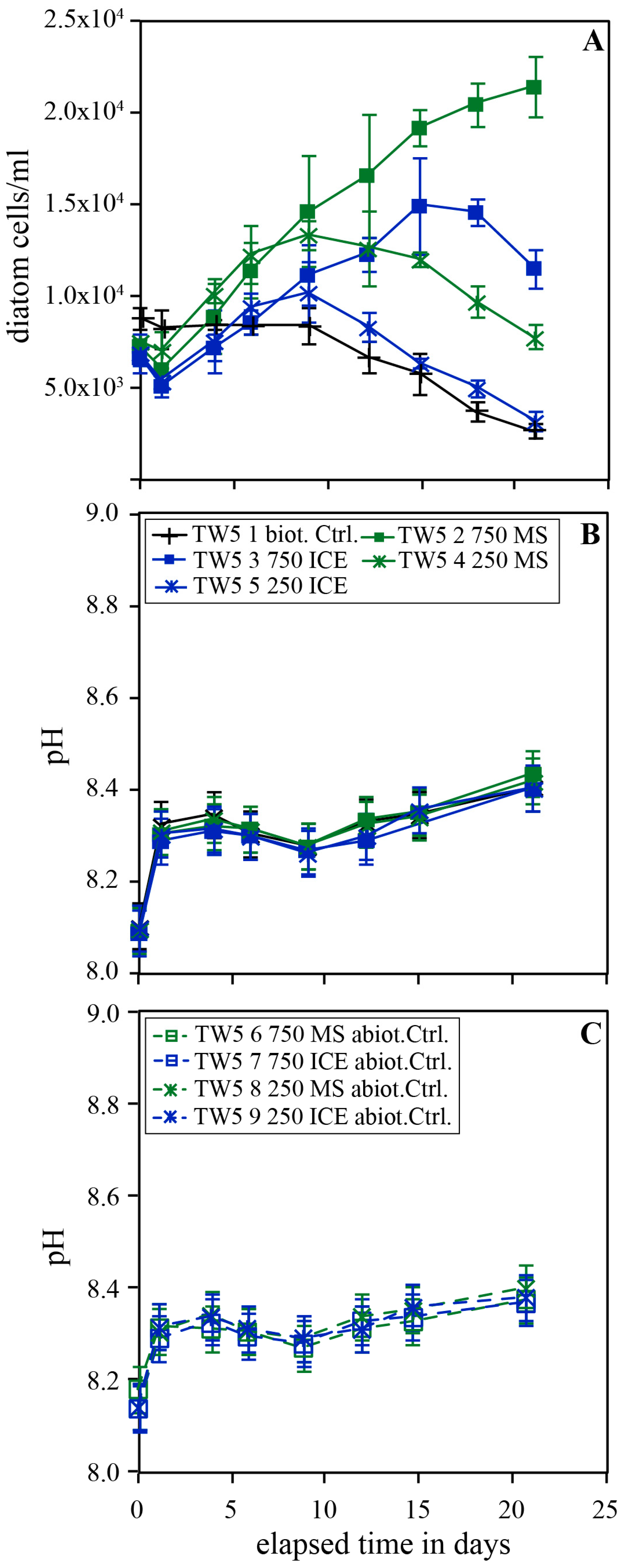
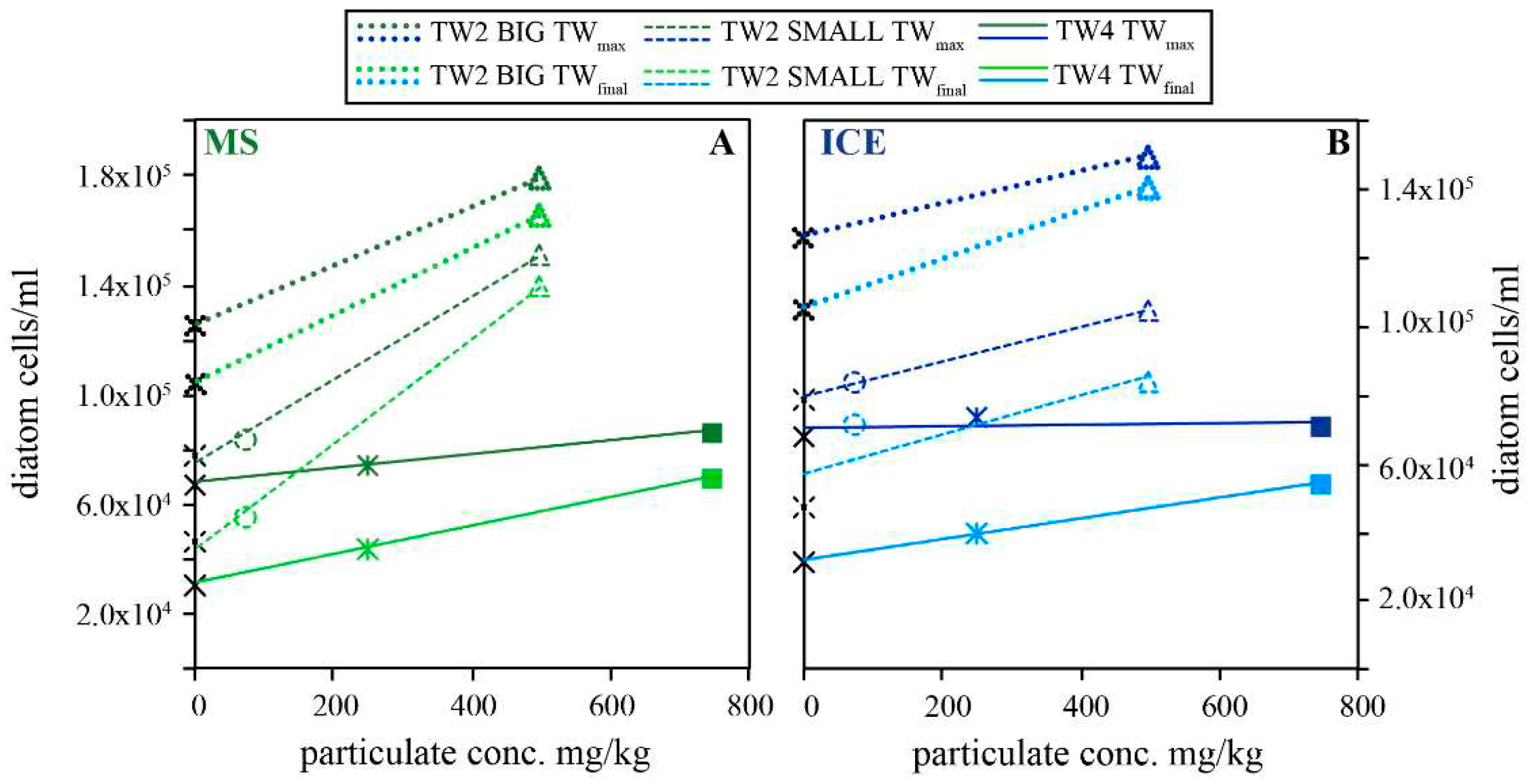
3.1. Temporal evolution of diatom concentrations in experiments carried out in nutrient enriched Instant Ocean© - Experiments TW1, TW2, and TW4
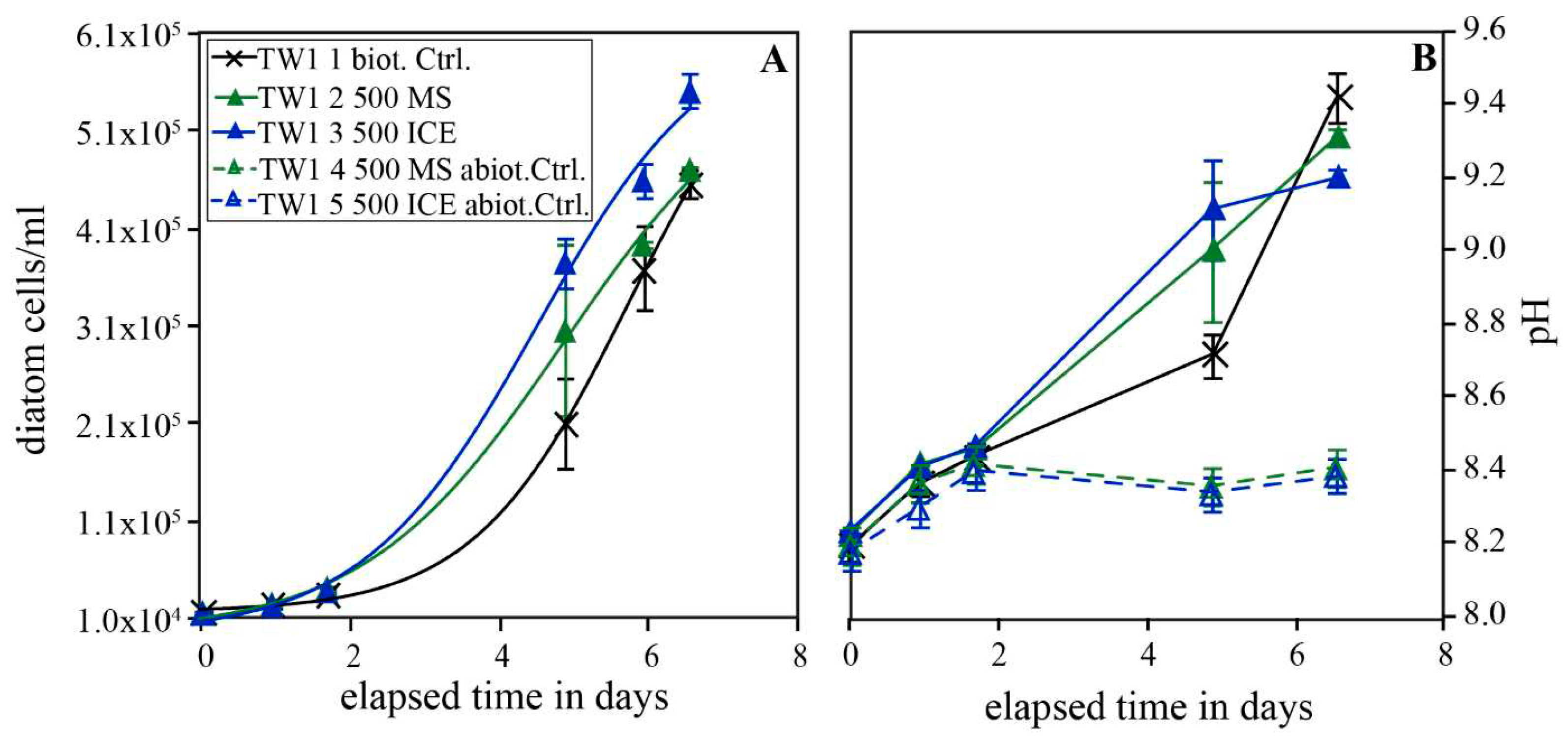
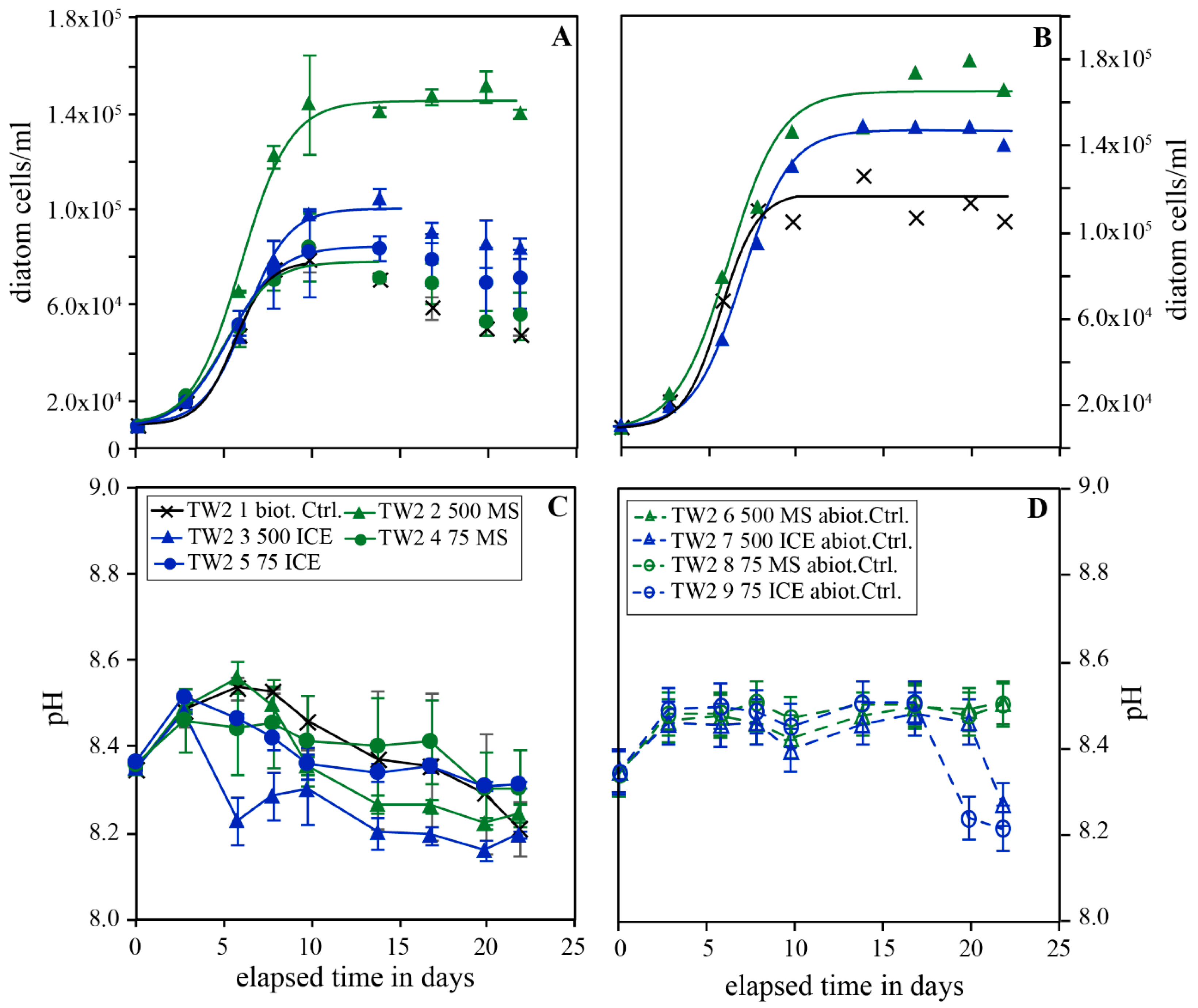
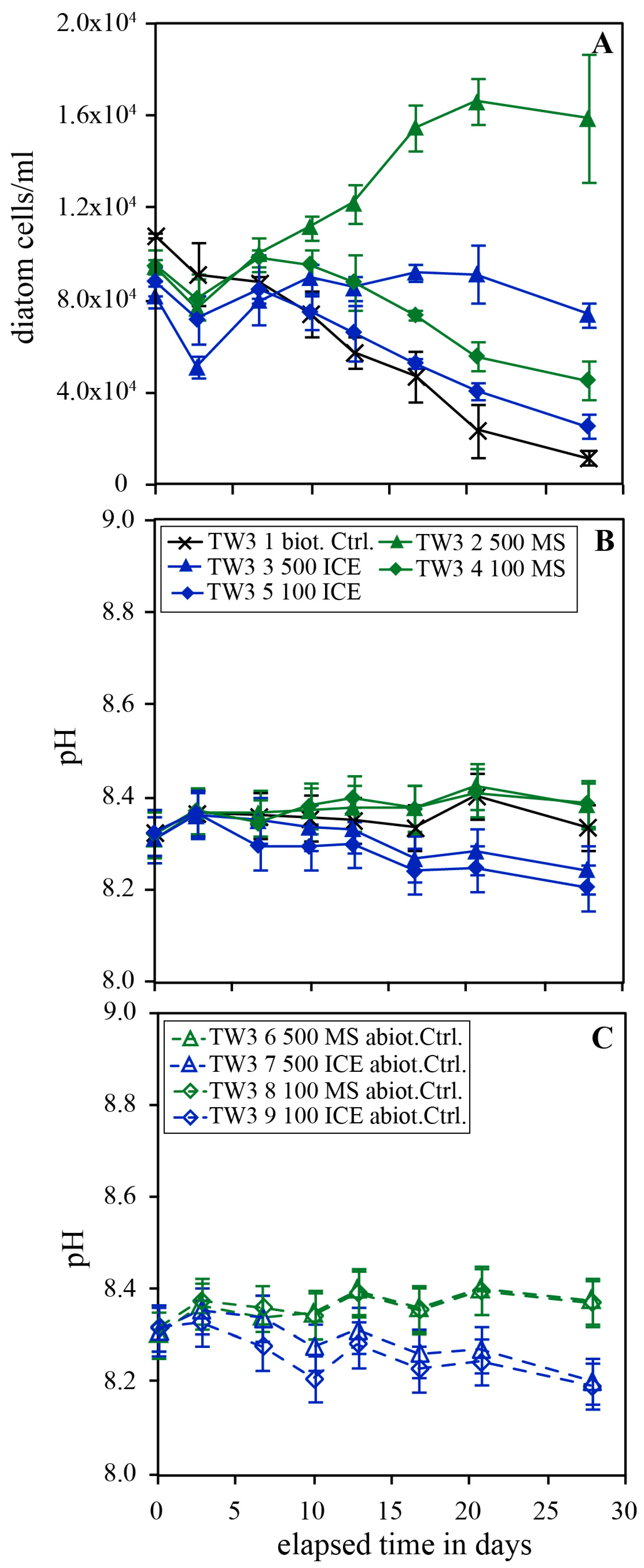
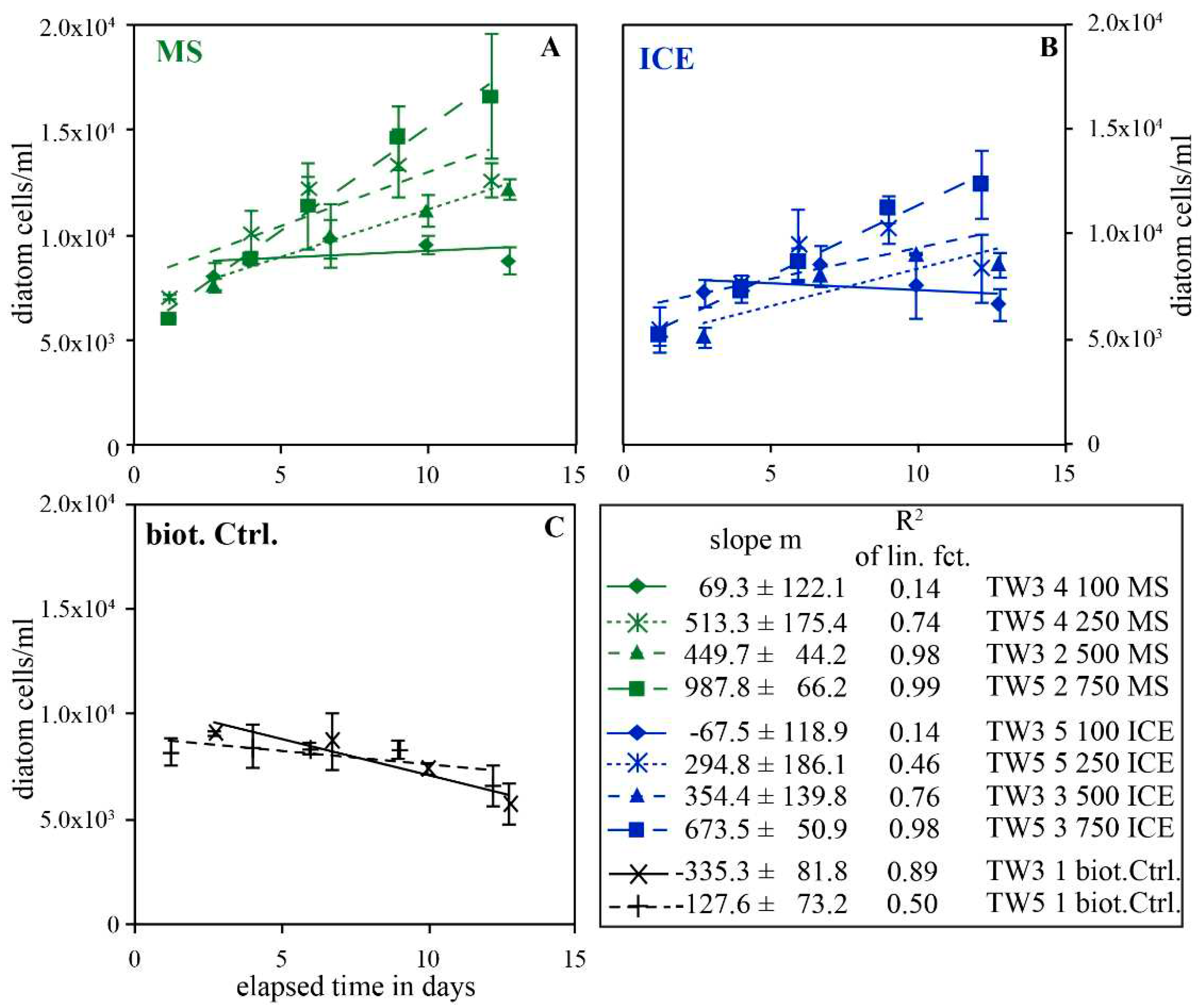
3.2. Temporal evolution of diatom concentrations in Instant Ocean© without added dissolved nutrients - Experiments TW3 and TW5.
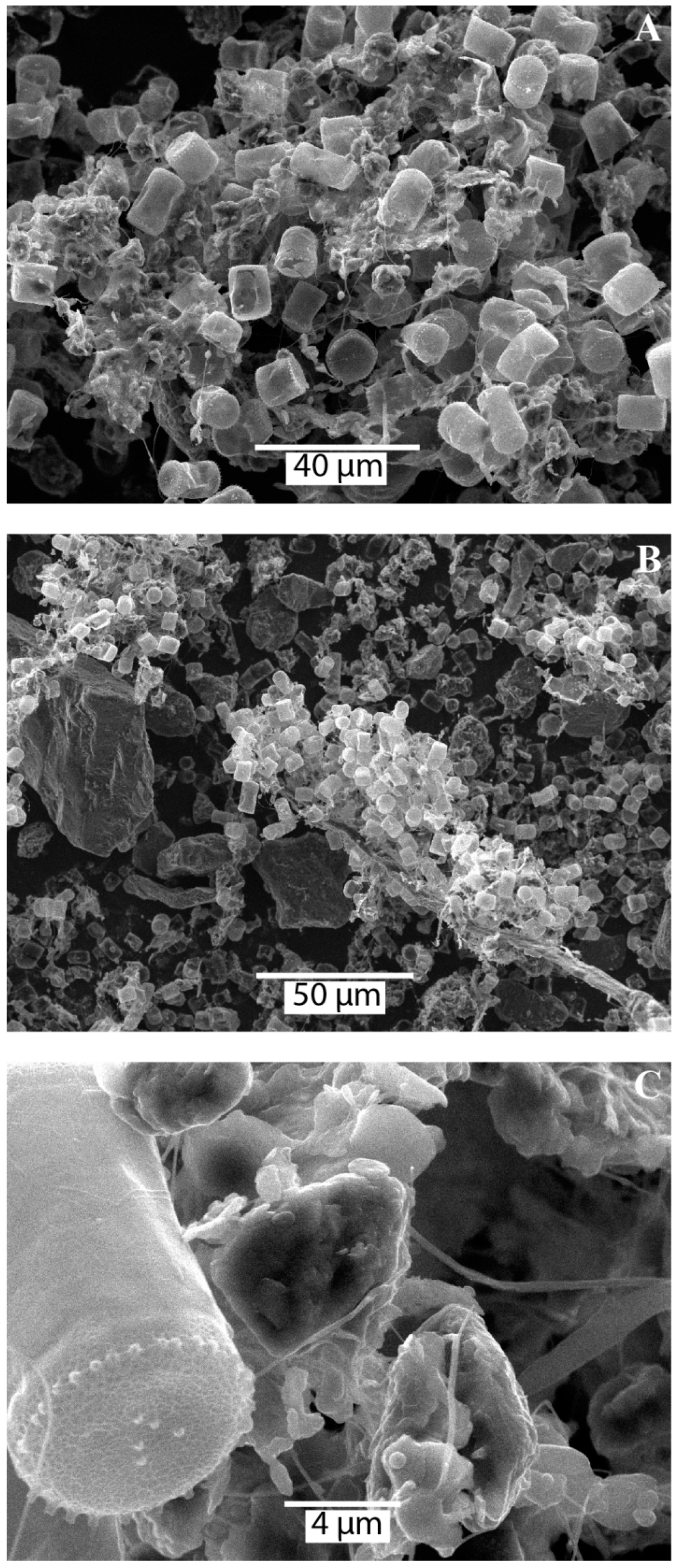
3.3. SEM and optical microscopy
4. Discussion
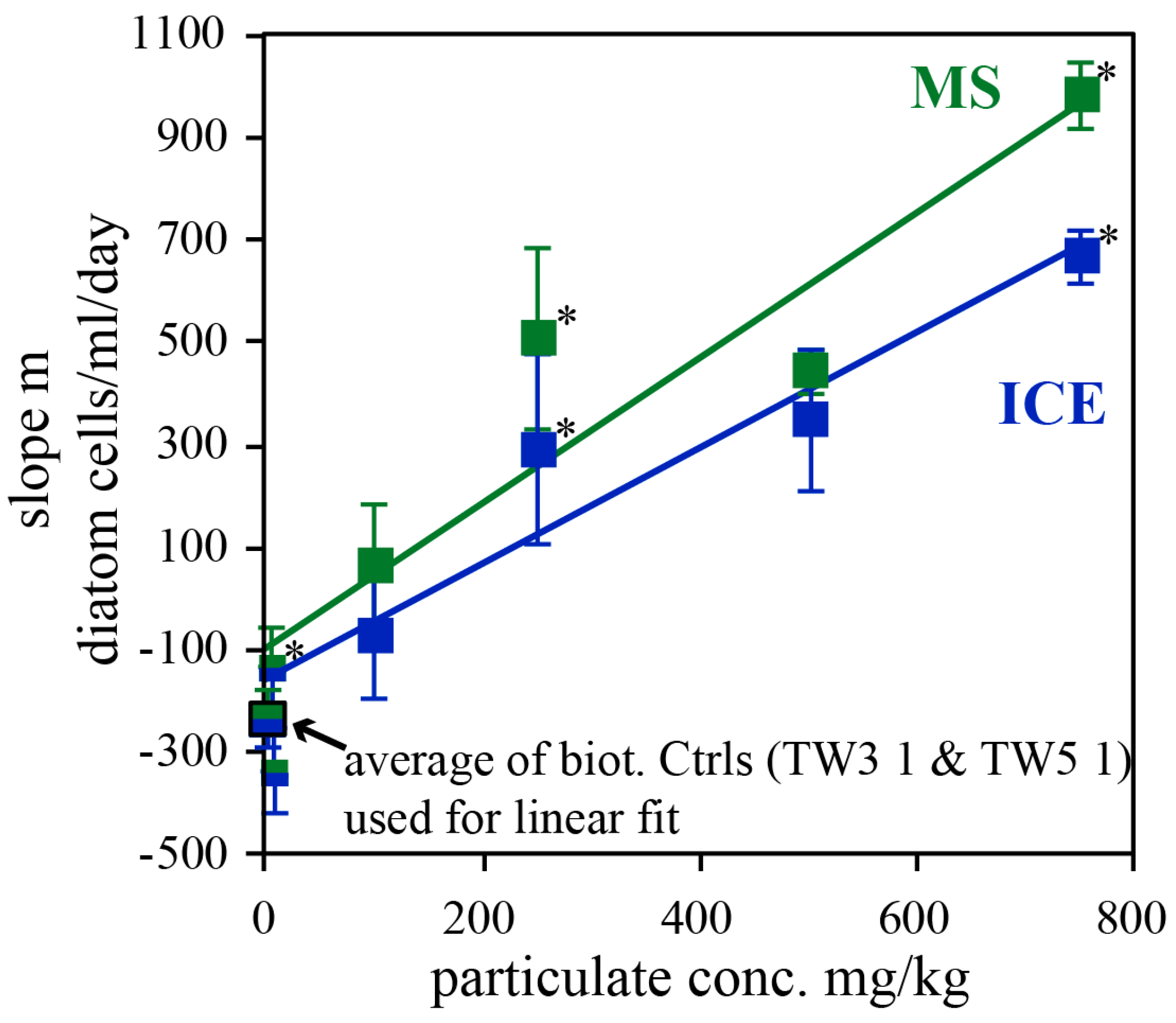
4.1. Summary of the effect of MS and ICE riverine particulate material on diatom growth
4.2. Potential role of riverine particulate material in natural systems
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petit, J.R.; Jouzel, J.; Raynaud, D.; Barkov, N. I.; Barnola, J.-M.; Basile, I.; Bender, M.; Chappellaz, J.; Davis, M.; Delaygue, G.; Delmotte, M.; Kotlyakov, V. M.; Legrand, M.; Lipenkov, V.Y.; Lorius, C.; Pépin, L.; Ritz, C.; Saltzman, E.; Stievenard, M. Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica. Nature 1999, 399, 429–436. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Cole, D.R. Carbon Dioxide Sequestration A Solution to a Global Problem. Elements 2008, 4, 305–310. [Google Scholar] [CrossRef]
- IPCC 2018. Summary for Policymakers. In: Global warming of 1.5°C. An IPCC Special Report on the impacts of global warming of 1.5°C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global response to the threat of climate change, sustainable development, and efforts to eradicate poverty [V. Masson-Delmotte; P. Zhai, H.O.; Pörtner, D.; Roberts, J., Skea, P. R. Shukla, A.; Pirani, W.; Moufouma-Okia, C., Péan, R., Pidcock, S., Connors, J.B.R.; Matthews, Y.; Chen, X.; Zhou, M.I.; Gomis, E.; Lonnoy, T.; Maycock, M.; Tignor, T.; Waterfield (eds.)]. 2018. World Meteorological Organization, Geneva, Switzerland, 32 pp.
- Falkowski, P.G.; Barber, R.T.; Smetacek, V. Biogeochemical Controls and Feedbacks on Ocean Primary Production. Science 1998, 281, 200–206. [Google Scholar] [CrossRef]
- Falkowski, P.; Scholes, R.J.; Boyle, E.; Canadell, J.; Canfield, D.; et al. The Global Carbon Cycle. Science 2000, 290, 291–296. [Google Scholar] [CrossRef]
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Bakken, L.R.; Baylis, M. , et al. Scientists’ warning to humanity: microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586. [Google Scholar] [CrossRef]
- Walker, J.C.; Hays, P.B.; Kasting, J.F. A negative feedback mechanism for the long-term stabilization of Earth's surface temperature. J. Geophys. Res.-Oceans 1981, 86, 9776–9782. [Google Scholar] [CrossRef]
- Berner, R.A. Burial of organic carbon and pyrite sulfur in the modern ocean: Its geochemical and environmental significance. Am. J. Sci. 1982, 282, 451–473. [Google Scholar] [CrossRef]
- Berner, R.A.; Lasaga, A.C.; Garrels, R.M. The carbonate-silicate geochemical cycle and its effect on atmospheric carbon dioxide over the past 100 million years. Am. J. Sci. 1983, 283, 641–683. [Google Scholar] [CrossRef]
- Berner, R.A.; Kothavala, Z. Geocarb III. Am. J. Sci. 2001, 301, 182–204. [Google Scholar] [CrossRef]
- Wallmann, K. Controls on the cretaceous and cenozoic evolution of seawater composition, atmospheric CO2 and climate. Geochim. Cosmochim. Acta 2001, 65, 3005–3025. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H.; Eiriksdottir, E.S.; Kardjilov, M.I.; Gisladottir, G.; Sigfusson, B.; Snorrason, A.; Elefsen, S.; Hardardottir, J.; Torssander, P.; Oskarsson, N. Direct evidence of the feedback between climate and weathering. Earth Planet. Sci. Lett. 2009, 277, 213–222. [Google Scholar] [CrossRef]
- Labat, D.; Goddéris, Y.; Probst, J.L.; Guyot, J.L. Evidence for global runoff increase related to climate warming. Adv. Water Resour. 2004, 27, 631–642. [Google Scholar] [CrossRef]
- Gedney, N.; Cox, P.M.; Betts, R.A.; Boucher, O.; Huntingford, C.; Stott, P.A. Detection of a direct carbon dioxide effect in continental river runoff records. Nature 2006, 439, 835–838. [Google Scholar] [CrossRef] [PubMed]
- Gislason, S.R.; Oelkers, E.H.; Snorrason, Á. Role of river-suspended material in the global carbon cycle. Geology 2006, 34, 49–52. [Google Scholar] [CrossRef]
- Hein, M.; Sand-Jensen, K. CO2 increases oceanic primary production. Nature 1997, 388, 526–527. [Google Scholar] [CrossRef]
- Clark, D.R.; Flynn, K.J. The relationship between the dissolved inorganic carbon concentration and growth rate in marine phytoplankton. P. Roy. Soc. Lond. B Bio. 2000, 267, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Riebesell, U.; Zondervan, I.; Rost, B.; Tortell, P.D.; Zeebe, R.E.; Morel, F.M. Reduced calcification of marine plankton in response to increased atmospheric CO2. Nature 2000, 407, 364–367. [Google Scholar] [CrossRef]
- Tortell, P.D.; DiTullio, G.R.; Sigman, D.M.; Morel, F.M. CO₂ effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002, 236, 37–43. [Google Scholar] [CrossRef]
- Riebesell, U.; Schulz, K.G.; Bellerby, R.G.; Botros, M.; Fritsche, P.; Meyerhöfer, M.; Neill, C.; Nondal, G.; Oschlies, A.; Wohlers, J.; Zöllner, E. Enhanced biological carbon consumption in a high CO2 ocean. Nature 2007, 450, 545–548. [Google Scholar] [CrossRef]
- Rost, B.; Zondervan, I.; Wolf-Gladrow, D. Sensitivity of phytoplankton to future changes in ocean carbonate chemistry. Mar. Ecol. Prog. Ser. 2008, 373, 227–238. [Google Scholar] [CrossRef]
- Feng, Y.; Hare, C.E.; Leblanc, K.; Rose, J.M.; Zhang, Y.; DiTullio, G.R.; et al. Effects of increased pCO2 and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 2009, 388, 13–25. [Google Scholar] [CrossRef]
- Passow, U.; Carlson, C.A. The biological pump in a high CO2 world. Mar. Ecol. Prog. Ser. 2012, 470, 249–271. [Google Scholar] [CrossRef]
- Engel, A.; Borchard, C.; Piontek, J.; Schulz, K.G.; Riebesell, U.; Bellerby, R. CO2 increases 14C primary production in an Arctic plankton community. Biogeosciences 2013, 10, 1291–1308. [Google Scholar] [CrossRef]
- Boyd, P.W. Toward quantifying the response of the oceans' biological pump to climate change. Front. Mar. Sci. 2015, 13, 77. [Google Scholar] [CrossRef]
- Arrigo, K.R. Carbon cycle: Marine manipulations. Nature 2007, 450, 491–492. [Google Scholar] [CrossRef]
- Passow, U.; Laws, E.A. Ocean acidification as one of multiple stressors. Mar. Ecol. Prog. Ser. 2015, 541, 75–90. [Google Scholar] [CrossRef]
- Seebah, S.; Fairfield, C.; Ullrich, M.S.; Passow, U. Aggregation and Sedimentation of Thalassiosira weissflogii (diatom) in a Warmer and More Acidified Future Ocean. PLOS ONE 2014, 9, e112379. [Google Scholar] [CrossRef] [PubMed]
- Frogner, P.; Gíslason, S.R.; Óskarsson, N. Fertilizing potential of volcanic ash in ocean surface water. Geology 2001, 29, 487–490. [Google Scholar] [CrossRef]
- Jickells, T.D.; An, Z.S.; Andersen, K.K.; Baker, A.R.; Bergametti, G.; et al. Global Iron Connections Between Desert Dust, Ocean Biogeochemistry, and Climate. Science 2005, 308, 67–71. [Google Scholar] [CrossRef]
- Jones, M.T.; Gislason, S.R. Rapid releases of metal salts and nutrients following the deposition of volcanic ash into aqueous environments. Geochim. Cosmochim. Acta 2008, 72, 3661–3680. [Google Scholar] [CrossRef]
- Olsson, J.; Stipp, S.L.S.; Dalby, K.N.; Gislason, S.R. Rapid release of metal salts and nutrients from the 2011 Grímsvötn, Iceland volcanic ash. Geochim. Cosmochim. Acta 2013, 123, 134–149. [Google Scholar] [CrossRef]
- Eiriksdottir, E.S.; Gislason, S.R.; Oelkers, E.H. Direct evidence of the feedback between climate and nutrient, major, and trace element transport to the oceans. Geochim. Cosmochim. Acta 2015, 166, 249–266. [Google Scholar] [CrossRef]
- Eiriksdottir, E.S.; Oelkers, E.H.; Hardardottir, J.; Gislason, S.R. The impact of damming on riverine fluxes to the ocean. Water Res. 2017, 113, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Gaillardet, J.; Dupré, B.; Allègre, C.J. Geochemistry of large river suspended sediments. Geochim. Cosmochim. Acta 1999, 63, 4037–4051. [Google Scholar] [CrossRef]
- Gaillardet, J.; Viers, J.; Dupré, B. Chapter 5.09 - Trace Elements in River Waters. In Treatise on Geochemistry (eds. H. D. Holland and K. K. Turekian). Pergamon. Oxford, 2003, pp. 225–272.
- Meybeck, M.; Laroche, L.; Dürr, H.H.; Syvitski, J.P.M. Global variability of daily total suspended solids and their fluxes in rivers. Global Planet. Change 2003, 39, 65–93. [Google Scholar] [CrossRef]
- Syvitski, J.P.M.; Peckham, S.D.; Hilberman, R.; Mulder, T. Predicting the terrestrial flux of sediment to the global ocean: A planetary perspective. Sediment. Geol. 2003, 162, 5–24. [Google Scholar] [CrossRef]
- Walling, D.E. Human impact on land–ocean sediment transfer by the world's rivers. Geomorphology 2006, 79, 192–216. [Google Scholar] [CrossRef]
- Viers, J.; Dupré, B.; Gaillardet, J. Chemical composition of suspended sediments in World Rivers. Sci. Total Environ. 2009, 407, 853–868. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R.; Eiriksdottir, E.S.; Jones, M.; Pearce, C.R.; Jeandel, C. The role of riverine particulate material on the global cycles of the elements. Appl. Geochem. 2011, 26, S365–S369. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Jones, M.T.; Pearce, C.R.; Jeandel, C.; Eiriksdottir, E.S.; Gislason, S.R. Riverine particulate material dissolution in seawater and its implications for the global cycles of the elements. C. R. Geosci. 2012, 344, 646–651. [Google Scholar] [CrossRef]
- Jeandel, C.; Oelkers, E.H. The influence of terrigenous particulate material dissolution on ocean chemistry and global element cycles. Chem. Geol. 2015, 395, 50–66. [Google Scholar] [CrossRef]
- De La Rocha, C.L.; Passow, U. Recovery of Thalassiosira weissflogii from nitrogen and silicon starvation. Limnol. Oceanogr. 2004, 49, 245–255. [Google Scholar] [CrossRef]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T; Falkowski, P. Primary Production of the Biosphere. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, E. The life of diatoms in the world's oceans. Nature 2009, 459, 185–192. [Google Scholar] [CrossRef]
- Malviya, S.; Scalco, E.; Audic, S.; Vincent, F.; Veluchay, A.; Poulain, J.; Wincker, P.; Lucicone, D.; de Vargas, C.; Bittner, L.; Zingone, A.; Bowler, C. Insights into global distribution and diversity in the worlds ocean. Proc. Nat. Acad. Sci. 2016, 113, E1516–E1525. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Campbell, D.A. Photophysiological responses of marine diatoms to elevated CO2 and decreased pH: a review. Functional Plant Biology 2014, 41, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Tréguer, P.; Bowler, C.; Moriceau, B.; Dutkiewicz, S.; Gehlen, M.; Aumont, O.; Bittner, L.; Dugdale, R.; Finkel, Z.; Ludicone, D.; Jahn, O.; Guidi, L.; Lasblei, M.; Leblanc, K.; Levy, M.; Pomndaven, P. Influence of diatom diversity on the ocean biological carbon pump. Nature Geosci. 2018, 11, 27–37. [Google Scholar] [CrossRef]
- Bach, L.T.; Taucher, J. CO2 effects on diatoms: a synthesis of more than a decade of ocean acidification experiments with natural communities. Ocean Sci. 2019, 15, 1159–1175. [Google Scholar] [CrossRef]
- Petrou, K.; Baker, K.G.; Nielsen, D.A.; Hancock, A.M.; Schulz, K.G.; Davidson, A.T. Acidification diminishes diatom silica production in the Southern Ocean. Nat. Clim. Chang. 2019, 9, 781–786. [Google Scholar] [CrossRef]
- Edwards, M.; Beaugrand, G.; Kléparski, L.; Helaouet, P.; Reid, P.C. Climate variability and multi-decadal diatom abundance in the Northeast Atlantic. Commun Earth Environ. 2022, 3, 162. [Google Scholar] [CrossRef]
- Taucher, J.; Bach, L.T.; Prowe, A.E.F.; Boxhammer, T.; Kvale, K.; Riebesell, U. Enhanced silica export in a future ocean triggers global diatom decline. Nature 2022, 605, 696–700. [Google Scholar] [CrossRef]
- Boyd, P.W.; Claustre, H.; Levy, M.; Siegel, D.A.; Weber, T. Multi-faceted particle pumps drive carbon sequestration in the ocean. Nature 2019, 568, 327–335. [Google Scholar] [CrossRef]
- Le Moigne, F.A.C. Pathways of organic carbon downward transport by the ocean biological carbon pump. Front. Mar. Sci. 2019, 6, 634. [Google Scholar] [CrossRef]
- Jackson, G.A.; Lochmann, S.E. Effect of coagulation on nutrient and light limitation of an algal bloom. Limnol. Oceanogr. 1992, 37, 77–89. [Google Scholar] [CrossRef]
- Alldredge, A.L.; Gotschalk, C.; Passow, U.; Riebesell, U. Mass aggregation of diatom blooms. Deep-Sea Res. Pt II, 1995; 42, 9–27. [Google Scholar]
- Alldredge, A.L.; Jackson, G.A. Preface: Aggregation in marine system. Deep-Sea Res. Pt II, 1995; 42, 1–7. [Google Scholar]
- Laufkötter, C.; Vogt, M.; Gruber, N.; Aumont, O.; Bopp, L.; Doney, S.C.; Dunne, J.P.; Hauck, J.; John, J.G.; Lima, I.D.; Seferian, R.; Völker, C. Projected decreases in future marine export production: the role of the carbon flux through the upper ocean ecosystem. Biogeosciences 2016, 13, 4023–4047. [Google Scholar] [CrossRef]
- McCave, I.N. Size spectra and aggregation of suspended particles in the deep ocean. Deep-Sea Res. 1984, 31, 329–352. [Google Scholar] [CrossRef]
- Smetacek, V.S. Role of sinking in diatom life-history cycles: Ecological, evolutionary and geological significance. Mar. Biol. 1985, 84, 239–251. [Google Scholar] [CrossRef]
- Hill, P.S. Reconciling aggregation theory with observed vertical fluxes following phytoplankton blooms. J. Geophys. Res.-Oceans 1992, 97, 2295–2308. [Google Scholar] [CrossRef]
- Jackson, G.A. A model of the formation of marine algal flocs by physical coagulation processes. Deep-Sea Res. 1990, 37, 1197–1211. [Google Scholar] [CrossRef]
- Avnimelech, Y.; Troeger, B.W.; Reed, L.W. Mutual Flocculation of Algae and Clay. Science 1982, 216, 63. [Google Scholar] [CrossRef]
- Ding, X.; Henrichs, S.M. Adsorption and desorption of proteins and polyamino acids by clay minerals and marine sediments. Mar. Chem. 2002, 77, 225–237. [Google Scholar] [CrossRef]
- Beaulieu, S.E.; Sengco, M.R.; Anderson, D.M. Using clay to control harmful algal blooms. Harmful Algae 2005, 4, 123–138. [Google Scholar] [CrossRef]
- Verspagen, J.M.; Visser, P.M.; Huisman, J. Aggregation with clay causes sedimentation of the buoyant cyanobacteria Microcystis spp. Aquat. Microb. Ecol. 2006, 44, 165–174. [Google Scholar] [CrossRef]
- Passow, U.; De La Rocha, C.; Fairfield, C.; Schmidt, K. Aggregation as a function of and mineral particles. Limnol. Oceanogr. 2014, 59, 532–547. [Google Scholar] [CrossRef]
- Ploug, H.; Iversen, M.H.; Fischer, G. Ballast, sinking velocity, and apparent diffusivity within marine snow and zooplankton fecal pellets: Implications for substrate turnover by attached bacteria. Limnol. Oceanogr. 2008, 53, 1878–1886. [Google Scholar] [CrossRef]
- Ploug, H.; Iversen, M.H.; Koski, M.; Buitenhuis, E.T. (2008b) Production, oxygen respiration rates, and sinking velocity of copepod fecal pellets: Direct measurements of ballasting by opal and calcite. Limnol. Oceanogr. 2008, 53, 469–476. [Google Scholar] [CrossRef]
- Passow, U.; De La Rocha, C.L. Accumulation of mineral ballast on organic aggregates. Global Biogeochem. Cycles 2006, 20, GB1013. [Google Scholar] [CrossRef]
- De La Rocha, C.L.; Passow, U. Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep-Sea Res. Pt II 2007, 54, 639–658. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, P.; Liu, D.; Zhang, W.; Tian, Q.; Bu, H.; Wei, Y.; Xia, J.; Wang, Y.; Zhou, J. Insight into cyanobacterial preservation in shallow marine environments from experimental simulation of cyanobacteria-clay co-aggregation. Chem. Geol. 2021, 577, 120285. [Google Scholar] [CrossRef]
- Smetacek, V. Biological oceanography: Diatoms and the silicate factor. Nature 1998, 391, 224–225. [Google Scholar] [CrossRef]
- Tréguer, P.; Pondaven, P. Silica control of carbon dioxide. Nature 2000, 406, 338–359. [Google Scholar] [CrossRef]
- Mayer, L.M. Surface area control of organic carbon accumulation in continental shelf sediments. Geochim. Cosmochim. Acta 1994, 58, 1271–1284. [Google Scholar] [CrossRef]
- Lalonde, K.; Mucci, A.; Ouellet, A.; Gélinas, Y. Preservation of organic matter in sediments promoted by iron. Nature 2012, 483, 198–200. [Google Scholar] [CrossRef]
- Eiriksdottir, E.S.; Louvat, P.; Gislason, S.R.; Óskarsson, N.; Hardardóttir, J. Temporal variation of chemical and mechanical weathering in NE Iceland. Earth Planet. Sci. Lett. 2008, 272, 78–88. [Google Scholar] [CrossRef]
- Milliman, J.D.; Syvitski, J.P. Geomorphic/Tectonic Control of Sediment Discharge to the Ocean. J. Geol. 1992, 100, 525–544. [Google Scholar] [CrossRef]
- Guillard, R.R. Culture of Phytoplankton for Feeding Marine Invertebrates. In Culture of Marine Invertebrate Animals: Proceedings — 1st Conference on Culture of Marine Invertebrate Animals Greenport (eds. W. L. Smith and M. H. Chanley); Springer US.: Boston, MA, 1975; pp. 29–60. [Google Scholar]
- Johansen, J.R.; Theriot, E. The relationship between valve diameter and number of central flutoportulae in Thalassiosira weissflogii (Barcillariophyceae). J. Phycol. 1987, 23, 663–665. [Google Scholar] [CrossRef]
- Atkinson, M.J.; Barnett, H.; Aceves, H.; Langdon, C.; Carpenter, S.J.; McConnaughey, T.; Hochberg, E.; Smith, M.; Marino, B.D.V. The Biosphere 2 coral reef biome. Ecol. Eng. 1999, 13, 147–172. [Google Scholar] [CrossRef]
- Roberts, S.B.; Lane, T.W.; Morel, F.M. Carbonic anhydrase in the marine diatom Thalassiosira weissflogii (Barcillariophyceae). J. Phycol. 1997, 33, 845–850. [Google Scholar] [CrossRef]
- De La Rocha, C.L.; Terbrüggen, A.; Völker, C.; Hohn, S. Response to and recovery from nitrogen and silicon starvation in Thalassiosira weissflogii: growth rates, nutrient uptake and C, Si and N content per cell. Mar. Ecol. Prog. Ser. 2010, 412, 57–68. [Google Scholar] [CrossRef]
- Sorhannus, U.; Ortiz, J.D.; Wolf, M.; Fox, M.G. Microevolution and Speciation in Thalassiosira weissflogii (Bacillariophyta). Protist 2010, 161, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Vella, F.M.; Sardo, A.; Gallo, C.; Landi, S.; Fontana, A.; d'Ippolito, G. Annual outdoor cultivation of the diatom Thalassiosira weissflogii: productivity, limits and perspectives. Algal Research 2019, 42, 101553. [Google Scholar] [CrossRef]
- Ernst, A.; Deicher, M.; Herman, P.M.; Wollenzien, U.I. Nitrate and Phosphate Affect Cultivability of Cyanobacteria from Environments with Low Nutrient Levels. Appl. Environ. Microb. 2005, 71, 3379–3383. [Google Scholar] [CrossRef]
- Waite, A.; Fisher, A.; Thompson, P.A.; Harrison, P.J. Sinking rate versus cell volume relationships illuminate sinking rate control mechanisms in marine diatoms. Mar. Ecol. Prog. Ser. 1997, 157, 97–108. [Google Scholar] [CrossRef]
- Brady, P.V.; Walther, J.V. Kinetics of quartz dissolution at low temperatures. Chem. Geol. 1990, 82, 253–264. [Google Scholar] [CrossRef]
- Gislason, S.R.; Oelkers, E.H. Mechanism, rates, and consequences of basaltic glass dissolution. Geochim. Cosmochim. Acta 2003, 67, 3817–3832. [Google Scholar] [CrossRef]
- Gélabert, A.; Pokrovsky, O.S.; Schott, J.; Boudou, A.; Feurtet-Mazel, A. Cadmium and lead interaction with diatom surfaces: A combined thermodynamic and kinetic approach. Geochim. Cosmochim. Acta 2007, 71, 3698–3716. [Google Scholar] [CrossRef]
- Poulton, S.W.; Raiswell, R. Chemical and physical characteristics of iron oxides in riverine and glacial meltwater sediments. Chem. Geol. 2005, 218, 203–221. [Google Scholar] [CrossRef]
- Palenik, B.; Morel, F.M.M. Comparison of cell-surface L-amino acid oxidases from several marine phytoplankton. Mar. Ecol. Progr. Ser. 1990, 59, 195–201. [Google Scholar] [CrossRef]
- Cermeno, P.; Falkowski, P.G.; Romero, O.E.; Schaller, M.F.; Vallina, S. M. Continental erosion and the Cenozoic rise of marine diatoms. Proc. Nat. Acad. Sci. 2015, 112, 4239–4244. [Google Scholar] [CrossRef] [PubMed]
- Gordeev, V.V.; Martin, J.M.; Sidorov, I.S.; Sidorova, M. A reassessment of the Eurasian river input of water, sediment, major elements, and nutrients to the Arctic Ocean. Am. J. Sci. 1996, 296, 664–691. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Viers, J.; Dupré, B.; Chabaux, F.; Gaillardet, J.; Audry, S.; Prokushkin, A.S.; Shirokova, L. S.; Kirpotin, S.N.; Lapitsky, S.A.; Shevchenko, V.P. Biogeochemistry of carbon, major and trace elements in watersheds of northern Eurasia: The change of fluxes, sources and mechanisms under the climate warming prospectivedrained to the Arctic Ocean. C. R. Geosci. 2012, 344, 663–677. [Google Scholar] [CrossRef]
- Gordeev, V.V.; Dara, O.M.; Filippov, A.S.; Belorukov, S.K.; Lokhov, A.S.; Kotova, E.I.; Kochenkova, A.I. Mineralogy of Particulate Suspended Matter of the Severnaya Dvina River (White Sea, Russia). Minerals 2022, 12, 1600. [Google Scholar] [CrossRef]
- Baisre, J.A.; Arboleya, Z. Going against the flow. Fish. Res. 2006, 81, 283–292. [Google Scholar] [CrossRef]
- Jones, J.I.; Duerdoth, C.P.; Collins, A.L.; Naden, P.S.; Sear, D.A. Interactions between diatoms and fine sediment. Hydrol. Processes 2012, 28, 1226–1237. [Google Scholar] [CrossRef]
- Jiang, Z.; Liu, J.; Chen, J.; Chen, Q.; Yan, X.; Xuan, J.; Zeng, J. Responses of summer phytoplankton community to drastic environmental changes in the Changjiang (Yangtze River) estuary during the past 50 years. Water Res. 2014, 54, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mao, Z.; Tang, F.; Han, G.; Jiang, Y. Declining riverine sediment input impact on spring phytoplankton bloom off the Yangtze River Estuary from 17-year satellite observation. Cont. Shelf. Res. 2017, 135, 86–91. [Google Scholar] [CrossRef]
- Burd, A.B.; Jackson, G.A. Particle Aggregation. Ann. Rev. Marine Sci. 2009, 1, 65–90. [Google Scholar] [CrossRef]
- Richardson, T.L. Mechanisms and pathways of small-phytoplankton export from the surface ocean. Ann. Rev. Marine Sci. 2019, 11, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Rixen, T.; Gaye, B.; Eeis, K.-C.; Ramasway, V. The ballast effect of lithogenic matter and its influences on the carbon fluxes in the Indian Ocean. Biosciences 2019, 16, 486–503. [Google Scholar] [CrossRef]
- Armstrong, R.A.; Lee, C.; Hedges, J.I.; Honjo, S.; Wakeham S., G. A new, mechanistic model for organic carbon fluxes in the ocean based on the quantitative association of POC with ballast minerals. Deep-Sea Res. Pt II 2001, 49, 219–236. [Google Scholar] [CrossRef]
- Iversen, M.H.; Robert, M.L. Ballasting effects of smectite on aggregate formation and export from a natural plankton community. Mar. Chem. 2015, 175, 18–27. [Google Scholar] [CrossRef]
- Anderson, M.A.; Morel, F.M. The influence of aqueous iron chemistry on the uptake of iron by the coastal diatom Thalassiosira weissflogii. Limnol. Oceanogr. 1982, 27, 789–813. [Google Scholar] [CrossRef]
- Milligan, A.J.; Mioni, C.E.; Morel, F.M.M. Response of cell surface pH to pCO2 and iron limitation in the marine diatom Thalassiosira weissflogii. Mar. Chem. 2009, 114, 31–36. [Google Scholar] [CrossRef]
- Wolf-Gladrow, D.; Riebesell, U. Diffusion and reactions in the vicinity of plankton: A refined model for inorganic carbon transport. Mar. Chem. 1997, 59, 17–34. [Google Scholar] [CrossRef]
- Shaked, Y.; Kustka, A.B.; Morel, F.M. A general kinetic model for iron acquisition by eukaryotic phytoplankton. Limnol. Oceanogr. 2005, 50, 872–882. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C.; Choi, W.J. Feldspars as a source of nutrients for microorganisms. Am. Mineral. 1998, 83, 1532–1540. [Google Scholar] [CrossRef]
- Rogers, J.R.; Bennett, P.C. Mineral stimulation of subsurface microorganisms: Release of limiting nutrients from silicates. Chem. Geol. 2004, 203, 91–108. [Google Scholar] [CrossRef]
- Bailey, B.; Templeton, A.; Staudigel, H.; Tebo, B.M. Utilization of Substrate Components during Basaltic Glass Colonization by Pseudomonas and Shewanella Isolates. Geomicrobiol. J. 2009, 26, 648–656. [Google Scholar] [CrossRef]
- Sudek, L.A.; Wanger, G.; Templeton, A.S.; Staudigel, H.; Tebo, B.M. Submarine Basaltic Glass Colonization by the Heterotrophic Fe(II)-Oxidizing and Siderophore-Producing Deep-Sea Bacterium Pseudomonas stutzeri VS-10: The Potential Role of Basalt in Enhancing Growth. Front. Microbiol. 2017, 8, 363. [Google Scholar] [CrossRef] [PubMed]
- Mayer, L.M.; Keil, R.G.; Macko, S.A.; Joye, S.B.; Ruttenberg, K.C.; Aller, R.C. Importance of suspended participates in riverine delivery of bioavailable nitrogen to coastal zones. Global Biogeochem. Cycles 1998, 12(4), 573–570. [Google Scholar] [CrossRef]
- Jones, M.T.; Pearce, C.R.; Oelkers, E.H. An experimental study of basaltic riverine particulate material and seawater. Geochim. Cosmochim. Acta 2011, 77, 108–120. [Google Scholar] [CrossRef]
- Jones, M.T.; Pearce, C.R.; Jeandel, C.; Gislason, S.R.; Eiriksdottir, E.; Mavromatis, V.; Oelkers, E.H. Riverine particulate material dissolution as a significant flux of strontium to the oceans. Earth Planet. Science Lett. 2012, 355, 51–59. [Google Scholar] [CrossRef]
- Jones, M.T.; Gislason, S.R.; Burton, K.W.; Pearce, C.R.; Mavromatis, V.; Pogge Van Strandmann, P.A.E.; Oelkers, E.H. Quantifying the impact of riverine particulate dissolution in seawater on ocean chemistry. Earth Planet. Sci. Lett. 2014, 395, 91–100. [Google Scholar] [CrossRef]
- Alley, R.B.; Cuffey, K.M.; Evenson, E.B.; Strasser, J.C.; Lawson, D.E.; Larson, G.J. How glaciers entrain and transport basal sediment. Quaternary Sci. Rev. 1997, 16, 1017–1038. [Google Scholar] [CrossRef]
- Broecker, W.S. Massive iceberg discharges as triggers for global climate change. Nature 1994, 372, 421–424. [Google Scholar] [CrossRef]
- Hemming, S.R. Heinrich events: Massive late Pleistocene detritus layers of the North Atlantic and their global climate imprint. Rev. Geophys. 2004, 42, RG1005. [Google Scholar] [CrossRef]
- Raiswell, R.; Hawkings, J.R.; Benning, L.G.; Baker, A.R.; Death, R.; Albani, S.; Mahowald, N.; Krom, M. D.; Poulton, S.W.; Wadham, J.; Tranter, M. Potentially bioavailable iron delivery by iceberg-hosted sediments and atmospheric dust to the polar oceans. Biogeosciences 2016, 13, 3887–3900. [Google Scholar] [CrossRef]
- Hawkings, J.R.; Wadham, J.L.; Benning, L.G.; Hendry, K.R.; Tranter, M.; Tedstone, A.; Nienow, P.; Raiswell, R. Ice sheets as a missing source of silica to the polar oceans. Nat. Commun. 2017, 8, 14198. [Google Scholar] [CrossRef]
- Holland, H.D. Volcanic gases, black smokers, and the great oxidation event. Geochim. Cosmochim. Acta 2002, 66, 3811–3826. [Google Scholar] [CrossRef]
- Shields-Zhou, G.; Och, L. The case for a Neoproterozoic Oxygenation Event. GSAT 2011, 21, 4–11. [Google Scholar] [CrossRef]
- Tang, H.; Chen, Y. Global glaciations and atmospheric change at ca. 2.3 Ga. Geosci. Front. 2013, 4, 583–596. [Google Scholar] [CrossRef]
- Arnaud, E.; Halverson, G.P.; Shields-Zhou, G. The geological record of Neoproterozoic ice ages, Chapter 1. Geol. Soc., London, Mem. 2011, 36, 1–16. [Google Scholar] [CrossRef]
- Rooney, A.D.; Strauss, J.V.; Brandon, A.D.; Macdonald, F.A. A Cryogenian chronology: Two long-lasting synchronous Neoproterozoic glaciations. Geology 2015, 43, 459–462. [Google Scholar] [CrossRef]
- Holland, H.D. The oxygenation of the atmosphere and oceans. Philos. T. Roy. Soc. B Bio. 2006, 361, 903–915. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




