Submitted:
03 January 2023
Posted:
05 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Identification of Isolates
2.2.1. Conventional Methods
2.2.2. Molecular Assay
2.3. Antifungal Susceptibility Testing (AFST)
2.4. Antifungal Activity of Farnesol
2.5. Combination Study
2.6. Cytotoxcity Assay
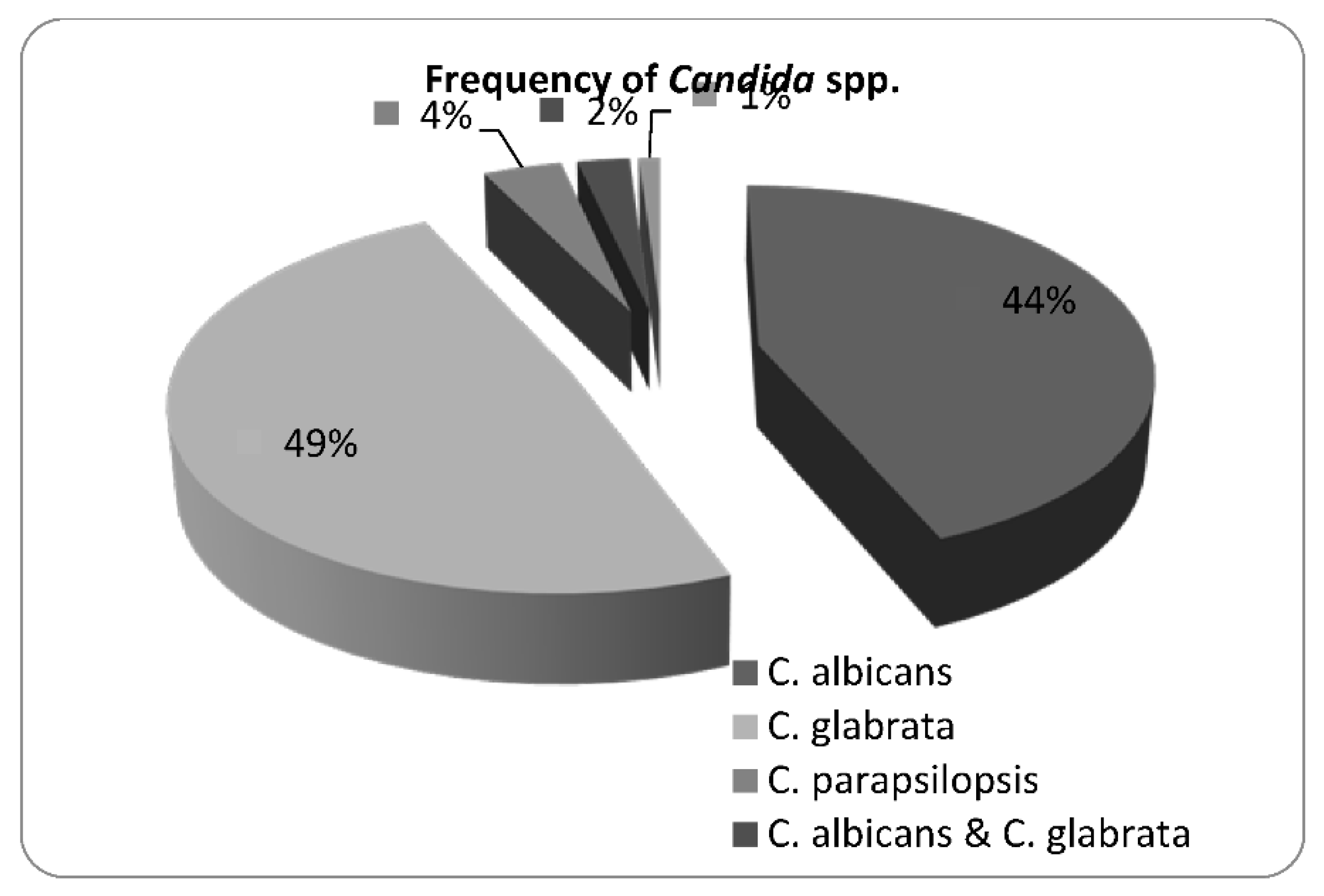
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denning, D.W.; Bromley, M.J. How to Bolster the Antifungal Pipeline: Few Drugs Are Coming to Market, but Opportunities for Drug Development Exist. Science (80-. ). 2015, 347, 1414–1416. [Google Scholar] [CrossRef]
- Eckert L; Hawes S; Stevens c; Koutsky L; Eschenbach D; Holmes K Vulvovaginal Candidiasis: Clinical Manifestations, Risk Factors, Management Algorithm. Obs. Gynecol 1998, 92, 757–765. [CrossRef]
- Pirotta MV; Garland SM Genital Candida Species Detected in Samples from Women in Melbourne, Australia, before and after Treatment with Antibiotics. J. Clin. Microbiol 2006, 44, 3213–3217. [CrossRef]
- Guzel AB; Ilkit M; Burgut R; Urunsak IF; Ozgunen FT An Evaluation of Risk Factors in Pregnant Women with Candida Vaginitis and the Diagnostic Value of Simultaneous Vaginal and Rectal Sampling. Mycopathologia 2011, 172, 25–36. [CrossRef]
- Lema, VM. Recurrent Vulvo-Vaginal Candidiasis: Diagnostic and Management Challenges in a Developing Country Context. Obs. Gynecol Int J 2017, 7, 260. [Google Scholar] [CrossRef]
- Lírio J; Giraldo PC; Amaral RL; Sarmento ACA; Costa APF; Gonçalves AK Antifungal (Oral and Vaginal) Therapy for Recurrent Vulvovaginal Candidiasis: A Systematic Review Protocol. BMJ Open 2019, 9, e027489. [CrossRef]
- Arastehfar A; Kargar ML; Mohammadi SR; Roudbary M; Ghods N; Haghighi L; et al. A High Rate of Recurrent Vulvovaginal Candidiasis and Therapeutic Failure of Azole Derivatives among Iranian Women. Front. Microbiol. 2021, 12. [CrossRef]
- Li C; Xu Z; Liu S; Huang Y; Duan W; Wei X In Vivo Antifungal Activities of Farnesol Combined with Antifungal Drugs against Murine Oral Mucosal Candidiasis. Biofouling 2021, 37, 818–829. [CrossRef]
- Sobel J; Sobel R; et al. Current Treatment Options for Vulvovaginal Candidiasis Caused by Azole-Resistant Candida Species. Expert Opin. Pharmacother. 2018, 19, 971–977. [CrossRef]
- Jabra-Rizk MA; Shirtliff M; James C; Meiller T Effect of Farnesol on Candida Dubliniensis Biofilm Formation and Fluconazole Resistance. FEMS Yeast Res. 2006, 6, 1063–1073. [CrossRef]
- Brilhante RSN; de Lima RAC; Caetano EP; Leite JJG; Castelo-Branco DSCM; Riberio JF; et al. Effect of Farnesol on Growth, Ergosterol Biosynthesis, and Cell Permeability in Coccidioides Posadassi. Antimicrob Agent Chemother 2013, 57, 2167–2170. [CrossRef]
- Wang X; Wang Y; Zhou Y; Wei X Farnesol Induces Apoptosis-like Cell Death in the Pathogenic Funus Aspergillus Flavus. Mycologia 2014, 106, 881–888. [CrossRef]
- Delmondes, G.D.A.; Santiago Lemos, I.C.; Dias, D.D.Q.; Cunha, G.L. Da; Araújo, I.M.; Barbosa, R.; Coutinho, H.D.M.; Felipe, C.F.B.; Barbosa-Filho, J.M.; Lima, N.T.R. De; et al. Pharmacological Applications of Farnesol (C15H26O): A Patent Review. Expert Opin. Ther. Pat. 2020, 30, 227–234. [Google Scholar] [CrossRef]
- Katragkou, A.; Mccarthy, M.; Alexander, E.L.; Antachopoulos, C.; Meletiadis, J.; Jabra-rizk, M.A.; Petraitis, V.; Roilides, E.; Walsh, T.J. In Vitro Interactions between Farnesol and Fluconazole, Amphotericin b or Micafungin against Candida Albicans Biofilms. J. Antimicrob. Chemother. 2015, 70, 470–478. [Google Scholar] [CrossRef]
- Onder, S.; Oz, Y. In Vitro Effects of Farnesol Alone and in Combination with Antifungal Drugs against Aspergillus Clinical Isolates. Med. Mycol. J. 2021, 62, 5–10. [Google Scholar] [CrossRef]
- Bozó, A.; Domán, M.; Majoros, L.; Kardos, G.; Varga, I.; Kovács, R. The in Vitro and in Vivo Efficacy of Fluconazole in Combination with Farnesol against Candida Albicans Isolates Using a Murine Vulvovaginitis Model. J. Microbiol. 2016, 54, 753–760. [Google Scholar] [CrossRef]
- Spitzer M; Robbins N; Wright GD Combinatorial Strategies for Combating Invasive Fungal Infections. Virulence 2017, 8, 169–185. [CrossRef]
- Gong Y; Liu W; Huang X; Hao L; Li Y; Sun S Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination with Fluconazole against Candida Albicans. Front. Microbiol. 2019, 10, 1461.
- Rodrigues, C.F.; Boas, D.V.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida Glabrata. Biomolecules 2018, 8. [Google Scholar] [CrossRef]
- Arastehfar A; Fang W; Pan W; Liao W; Yan L; Boekhout T Identification of Nine Cryptic Species of Candida Albicans, C. Glabrata, and C. Parapsilosis Complexes Using One-Step Multiplex PCR. BMC Infect. Dis. 2018, 18, 1–9.
- Nikoomanesh, F.; Roudbarmohammadi, S.; Khoobi, M.; Haghighi, F.; Roudbary, M. Design and Synthesis of Mucoadhesive Nanogel Containing Farnesol: Investigation of the Effect on HWP1, SAP6 and Rim101 Genes Expression of Candida Albicans in Vitro. Artif. Cells, Nanomedicine Biotechnol. 2019, 47, 64–72. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida Albicans and Emerging Non-Albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Nikoomanesh F.; Roudbarmohammadi S.; Bashardoust B.; Zareei M. Effect of Farnesol on Responsive Gene Expressions in Hyphal Morphogenesis Transformation of Candida Albicans. Infect. Epidemiol. Microbiol. 2018, 4, 73–77.
- Cordeiro, R.A.; Teixeira, C.E.C.; Brilhante, R.S.N.; Castelo-Branco, D.S.C.M.; Paiva, M.A.N.; Giffoni Leite, J.J.; Lima, D.T.; Monteiro, A.J.; Sidrim, J.J.C.; Rocha, M.F.G. Minimum Inhibitory Concentrations of Amphotericin B, Azoles and Caspofungin against Candida Species Are Reduced by Farnesol. Med. Mycol. 2013, 51, 53–59. [Google Scholar] [CrossRef]
- Alipour R; Fatemi A; Alsahebfosul F; Andalib A; Pourazar A Autologous Plasma versus Fetal Calf Serum as a Supplement for the Culture of Neutrophils. BMC Res. Notes 2020, 13, 39. [CrossRef]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 1–49. [Google Scholar] [CrossRef]
- Denning, D.W.; Kneale, M.; Rautemaa-Richardson, R.; Sobel, J.D. Global Burden of Recurrent Vulvovaginal Candidiasis. Lancet Infect Dis 2017, 1–9, in press. [Google Scholar]
- Roudbary M; Roudbarmohammadi SH; Bakhshi B; Farhadi Z; Nikoomanesh F Identification of Candida Species Isolated Form Iranian Women Eith Vaginal Candidasis by PCR-RFLP Method. Eur.J. Exp. Biol. 2013, 3, 365–369.
- Rodrigues CF, Gonçalves B, Rodrigues ME, Silva S, Azeredo J, H.M. The Effectiveness of Voriconazole in Therapy of Candida Glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia 2017, 182, 653–664. [CrossRef]
- Bitew, A.; Abebaw, Y. Vulvovaginal Candidiasis: Species Distribution of Candida and Their Antifungal Susceptibility Pattern. BMC Womens. Health 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Mohammadi-Ghalehbin, B.; Javanpour Heravi, H.; Arzanlou, M.; Sarvi, M. Prevalence and Antibiotic Resistance Pattern of Candida Spp. Isolated from Pregnant Women Referred to Health Centers in Ardabil, Iran. J Ardabil Univ Med Sci 2017, 16, 409–421. [Google Scholar]
- Sustr, V.; Foessleitner, P.; Kiss, H.; Farr, A. Vulvovaginal Candidosis: Current Concepts, Challenges and Perspectives. J. Fungi 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Hornby JM; Jensen EC; Lisec AD; Tasto JJ; Jahnke B; Shoemaker R; et al. Quorum Sensing in the Demorphic Fungus Candida Albicans Is Mediated by Farnesol. Appl Env. Microbiol 2001, 67, 2982–2992. [CrossRef]
- Lorek J; Poggeler S; Weide MR; Breves R; Bokmuhl DP Infeluence of Farnesol on the Morphogenesis of Aspergillus Niger. J Basic Microbiol 2008, 48, 99–103. [CrossRef]
- Semighini CP; Murray N; Harris SD Inhibition of Fusarium Graminearum Growth and Development by Farnesol. FEMS Microbiol. Lett. 2008, 279, 259–264. [CrossRef]
- Semighini CP; Honrry JM; Dumitru R; Nickerson KW; Harris SD Farnesol-Induced Apoptosis in Aspergillus Nidulans Reveals a Possible Mechanism for Antagonistic Interaction between Fungi. Mol Microbiol 2006, 59, 753–764. [CrossRef]
- Nikoomanesh F.; Roudbarmohammadi S.; Bashardoust B.; Zareei M . . Effect of Farnesol on Responsive Gene Expressions in Hyphal Morphogenesis Transformation of Candida Albicans. Infect. Epidemiol. Microbiol. 2018, 4, 73–77.
- Rossignol, T.; Logue, M.E.; Reynolds, K.; Grenon, M.; Lowndes, N.F.; Butler, G. Transcriptional Response of Candida Parapsilosis Following Exposure to Farnesol. Antimicrob. Agents Chemother. 2007, 51, 2304–2312. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Černáková, L. Farnesol and Tyrosol: Secondary Metabolites with a Crucial Quorum-Sensing Role in Candida Biofilm Development. Genes (Basel). 2020, 11. [Google Scholar] [CrossRef]
- Xia J; Qian F; Xu W; Zhang Z; Wei X In Vitro Inhibitory Effects of Farnesol and Interaction between Farnesol and Antifungals against Biofilm of C Andida Albicans Resistance Strains. Biofouling 2017, 33, 283–293. [CrossRef]
- Nagy F, Vitális E, Jakab Á, Borman AM, Forgács L, Tóth Z, Majoros L, K.R. In Vitro and in Vivo Effect of Exogenous Farnesol Exposure Against Candida Auris. Front Microbiol 2020, 20, 957. [CrossRef]
- Sebaa S, Boucherit-Otmani Z, C.P. Effects of Tyrosol and Farnesol on Candida Albicans Biofilm. Mol Med Rep 2019, 19, 3201–3209. [CrossRef]
- Décanis N, Tazi N, Correia A, Vilanova M, R.M. Farnesol, a Fungal Quorum-Sensing Molecule Triggers Candida Albicans Morphological Changes by Downregulating the Expression of Different Secreted Aspartyl Proteinase Genes. Open Microbiol J 2011, 5, 119–126. [CrossRef]
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin b in the Treatment of Candida Glabrata Biofilms. Microorganisms 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Bezerra, C.F.; de Alencar Júnior, J.G.; de Lima Honorato, R.; dos Santos, A.T.L.; Pereira da Silva, J.C.; Gusmão da Silva, T.; Leal, A.L.A.B.; Rocha, J.E.; de Freitas, T.S.; Tavares Vieira, T.A.; et al. Antifungal Activity of Farnesol Incorporated in Liposomes and Associated with Fluconazole. Chem. Phys. Lipids 2020, 233, 33058818. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Prasad, R. The Quorum-Sensing Molecule Farnesol Is a Modulator of Drug Efflux Mediated by ABC Multidrug Transporters and Synergizes with Drugs in Candida Albicans. Antimicrob. Agents Chemother. 2011, 55, 4834–4843. [Google Scholar] [CrossRef] [PubMed]
- Černáková, L.; Dižová, S.; Gášková, D.; Jančíková, I.; Bujdáková, H. Impact of Farnesol as a Modulator of Efflux Pumps in a Fluconazole-Resistant Strain of Candida Albicans. Microb. Drug Resist. 2019, 25. [Google Scholar] [CrossRef] [PubMed]
- Dekkerová, J.; Černáková, L.; Kendra, S.; Borghi, E.; Ottaviano, E.; Willinger, B.; Bujdáková, H. Farnesol Boosts the Antifungal Effect of Fluconazole and Modulates Resistance in Candida Auris through Regulation of the CDR1 and ERG11 Genes. J. Fungi 2022, 8, 783. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum MA; Rice LB Antifungal Agents: Mode of Action Mechnism of Resistance, and Correlation of These Mechanisms with Bacterial Resistance. Clin Microbiol Rev 1999, 12, 501–517. [CrossRef] [PubMed]
- Fana, Y.S.Y.Z.S.; Liuc, A.V.X. Clinical Characteristics and Antifungal Susceptibility of Candida Nivariensis from Vulvovaginal Candidiasis. 2019.
- Öztürk BY; Feyzullazade N; Dağ I; Şengel T The Investigation of in Vitro Effects of Farnesol at Different Cancer Cell Lines. Microsc. Res. Tech. 2022.
- Černáková L, Jordao L, B.H. Impact of Farnesol and Corsodyl ® on Candida Albicans Forming Dual Biofilm with Streptococcus Mutans. Oral Dis 2018, 24, 1126–1131. [CrossRef] [PubMed]
| Candida species | Sequences (5' 3' ) 3' ) |
Amplicons |
|
C. albicans |
F-5'AGATTATTGCCATGCCCTGAG3' R5'CCATGTCGAACGTAGCGTATGC3' |
606bp |
| C. glabrata | F5'ACCGTGCTTGCCTCTACA3' R5'GACATCTGAGCCTCGTCTGA3' |
212bp |
| C. tropicalis | F5'AGAACAAGAAAACAGTGAAGCAA3' R5'CCATGTCGAACGTAGCGTATGC3 |
126bp |
| C. parapsiolosis | F5'TACACCAAGCGACTCAGC3' R5'ACCAGCTGCTTTGACTTG3' |
490bp |
| C. krusei | F5'GGCGTTGTCCATCCAATG3' R5'CAGGAGAATTGCTGTTCCC3' |
1159bp |
| C. dubliniensis | F5'GTCGGACATATACCTCCAACTC3' R5'CCATGTCGAACGTAGCGTAT3' |
718bp |
| Candida species | Antifungal drug | Sensitive(S) | Dose-dependent | Resistance(R) | |||
| n | % | n | % | n | % | ||
|
C. albicans n=35 |
FLU | 11 | 31.4 | 1 | 2.1 | 23 | 65.7 |
| ITZ | 18 | 51.4 | - | - | 17 | 48.5 | |
| VOR | 17 | 48.5 | - | - | 18 | 51.4 | |
| AMB | 35 | 100 | - | - | - | - | |
| CTZ | 13 | 37.1 | - | - | 22 | 62.8 | |
|
C. glabrata n=39 |
FLU | 9 | 23 | 2 | 5.1 | 28 | 71.8 |
| ITZ | 16 | 41 | - | - | 23 | 59 | |
| VOR | 14 | 35.9 | - | - | 25 | 64.1 | |
| AMB | 35 | 89.7 | - | - | 4 | 10.2 | |
| CTZ | 13 | 33.3 | - | - | 26 | 66.6 | |
|
C. parapsilosis n=3 |
FLU | 2 | 66.6 | - | - | 1 | 33.3 |
| ITZ | 3 | 100 | - | - | - | - | |
| VOR | 2 | 66.6 | - | - | 1 | 33.3 | |
| AMB | 1 | 33.3 | - | - | 2 | 66.6 | |
| CTZ | 1 | 33.3 | - | - | 2 | 66.6 | |
| Isolates | Median MIC values | Interaction analysis | ||||
| MIC alone | MIC in combination | Median FICI | Type of interaction | |||
| FLU (µg/L) | FAR (µM | FLU (µg/L) | FAR (µM) | |||
| C. albicans | 64(8-64) | 300 | 8(2-8) | 150 | 0.5 | Synergy |
| C. glabrata | 64(8-64) | 300 | 8(2-16) | 300 | 0.9 | Indifferent |
| C.parapsilosis | 32(8-32) | 300 | 4(2-8) | 150 | 0.35 | Synergy |
| ITRA (µg/L) | FAR (µM | ITRA (µg/L) | FAR (µM) | |||
| C. albicans | 8(1-8) | 300 | 4(1-8) | 150 | 0.5 | Synergy |
| C. glabrata | 8(2-8) | 300 | 8(2-8) | 300 | 1.01 | Indifferent |
| C.parapsilosis | 8(2-8) | 300 | 4(1-4) | 150 | 0.25 | Synergy |
| VOR(µg/L) | FAR (µM | VOR(µg/L) | FAR (µM) | |||
| C. albicans | 16(2-16) | 300 | 8(1-8) | 150 | 0.75 | Indifferent |
| C. glabrata | 16(2-16) | 300 | 8(2-16) | 300 | 0.75 | Indifferent |
| C.parapsilosis | 8(2-16) | 300 | 4(1-4) | 150 | 0.5 | Synergy |
| AmB (µg/L | FAR (µM | AmB(µg/L) | FAR (µM) | |||
| C. albicans | 2(0.031-2) | 300 | 2(0.031-2) | 150 | - | - |
| C. glabrata | 2(0.031-2) | 300 | 1(0.031-2) | 300 | 1.25 | Indifferent |
| C.parapsilosis | 2(0.031-2) | 300 | 1(0.031-2) | 150 | 0.35 | Synergy |
| CTZ(µg/L) | FAR (µM | CTZ(µg/L) | FAR (µM) | |||
| C. albicans | 16(2-16) | 300 | 4(1-4) | 150 | 1.75 | Indifferent |
| C. glabrata | 16(2-16) | 300 | 8(2-16) | 300 | 0.9 | Indifferent |
| C.parapsilosis | 8(2-16) | 300 | 2(0.5-4) | 150 | 1.25 | Indifferent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





