Submitted:
29 December 2022
Posted:
03 January 2023
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Homology search
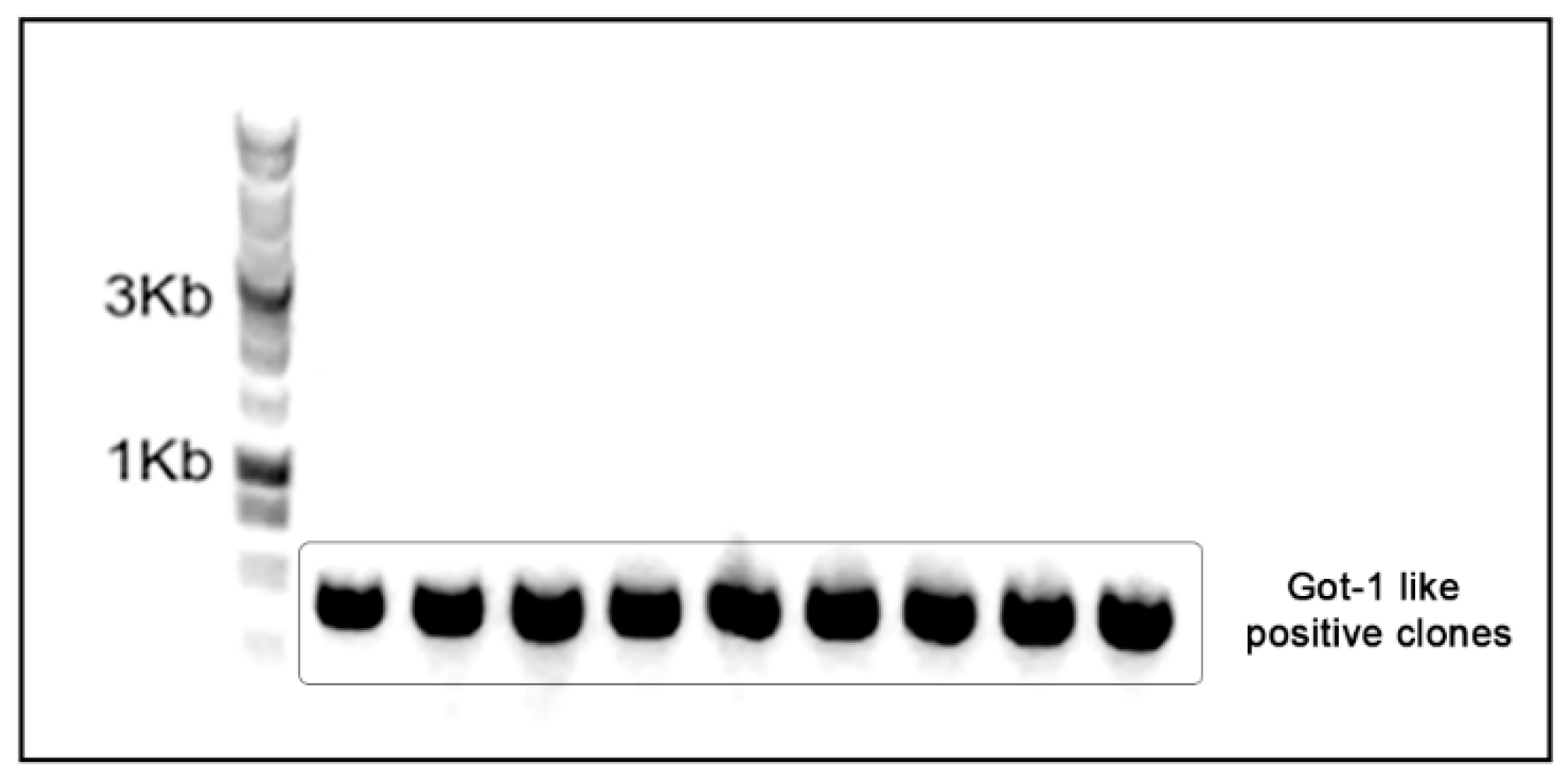
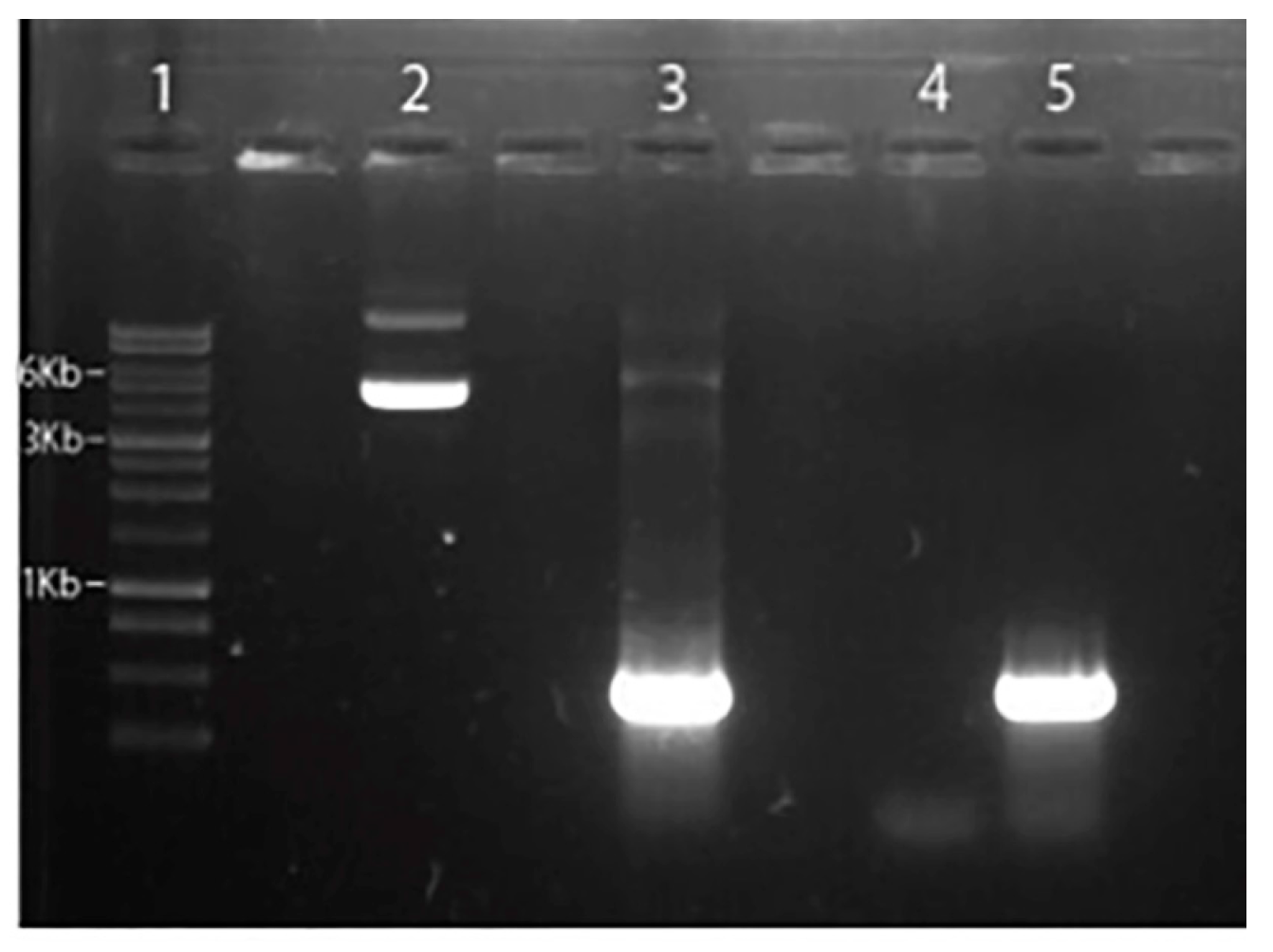
2.2. Genomic DNA extraction and Got-1-like protein cloning
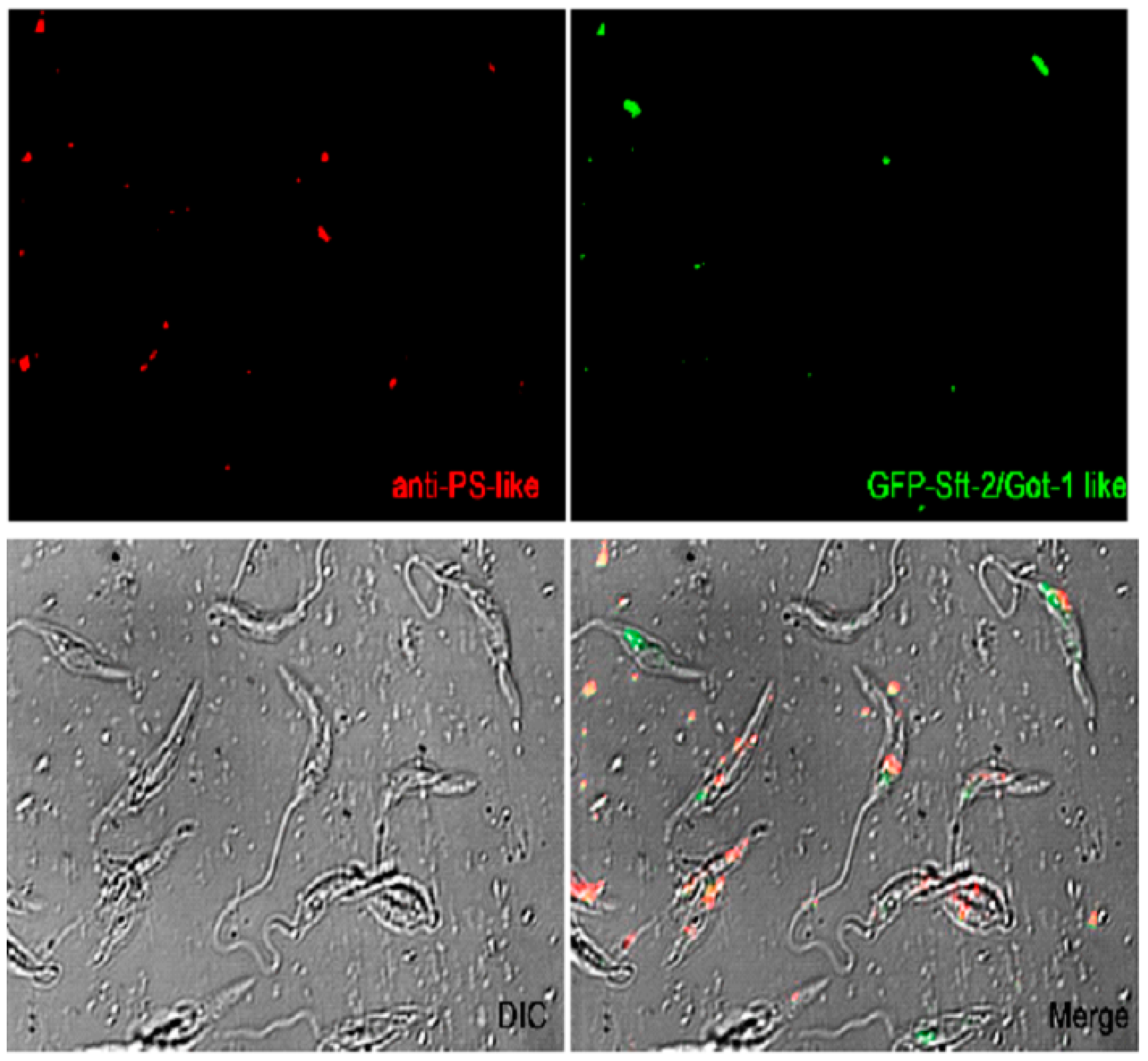
2.2. Cellular localization of PS-like and Got-1-like
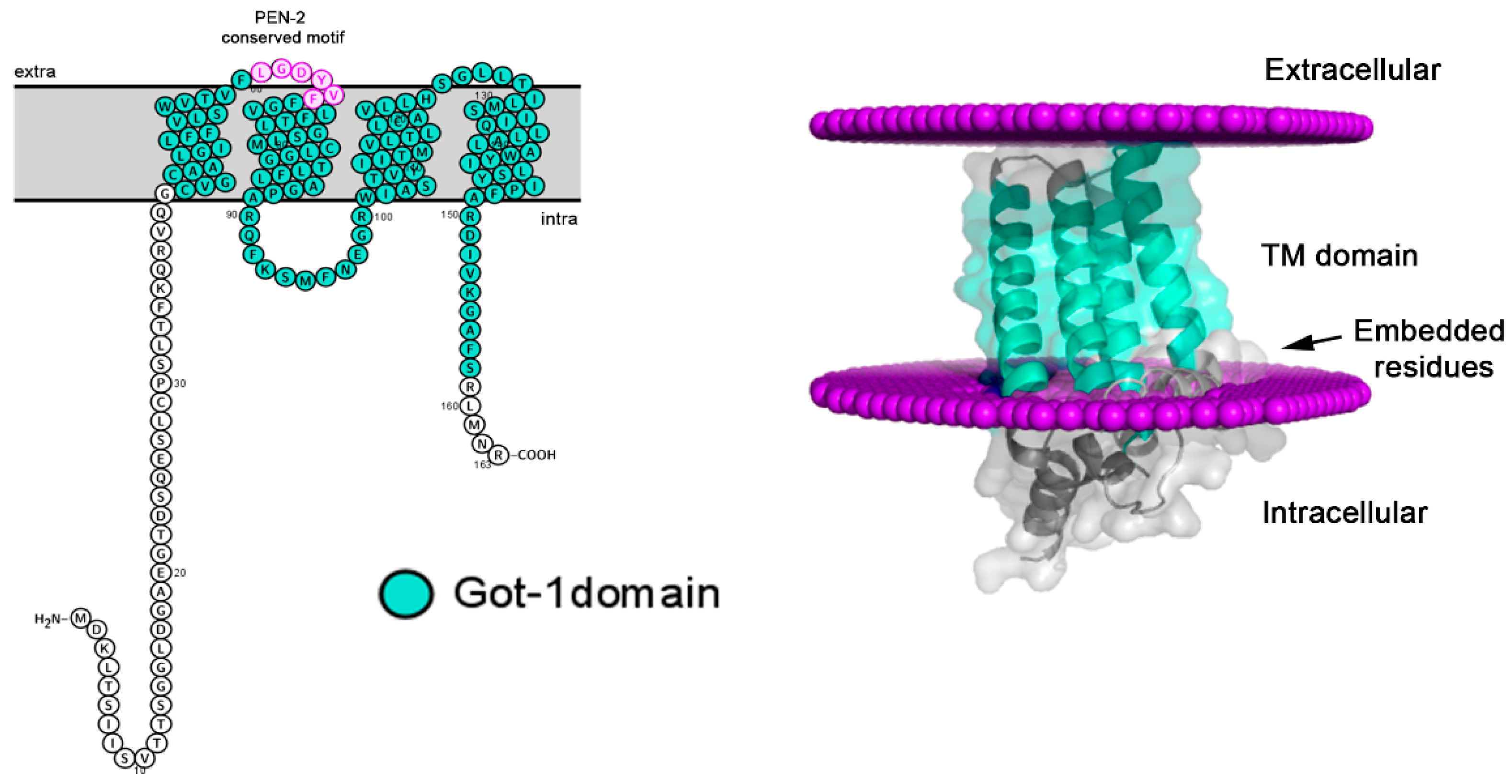
2.3. Topology and conserved motifs of SFT2/Got-1-like
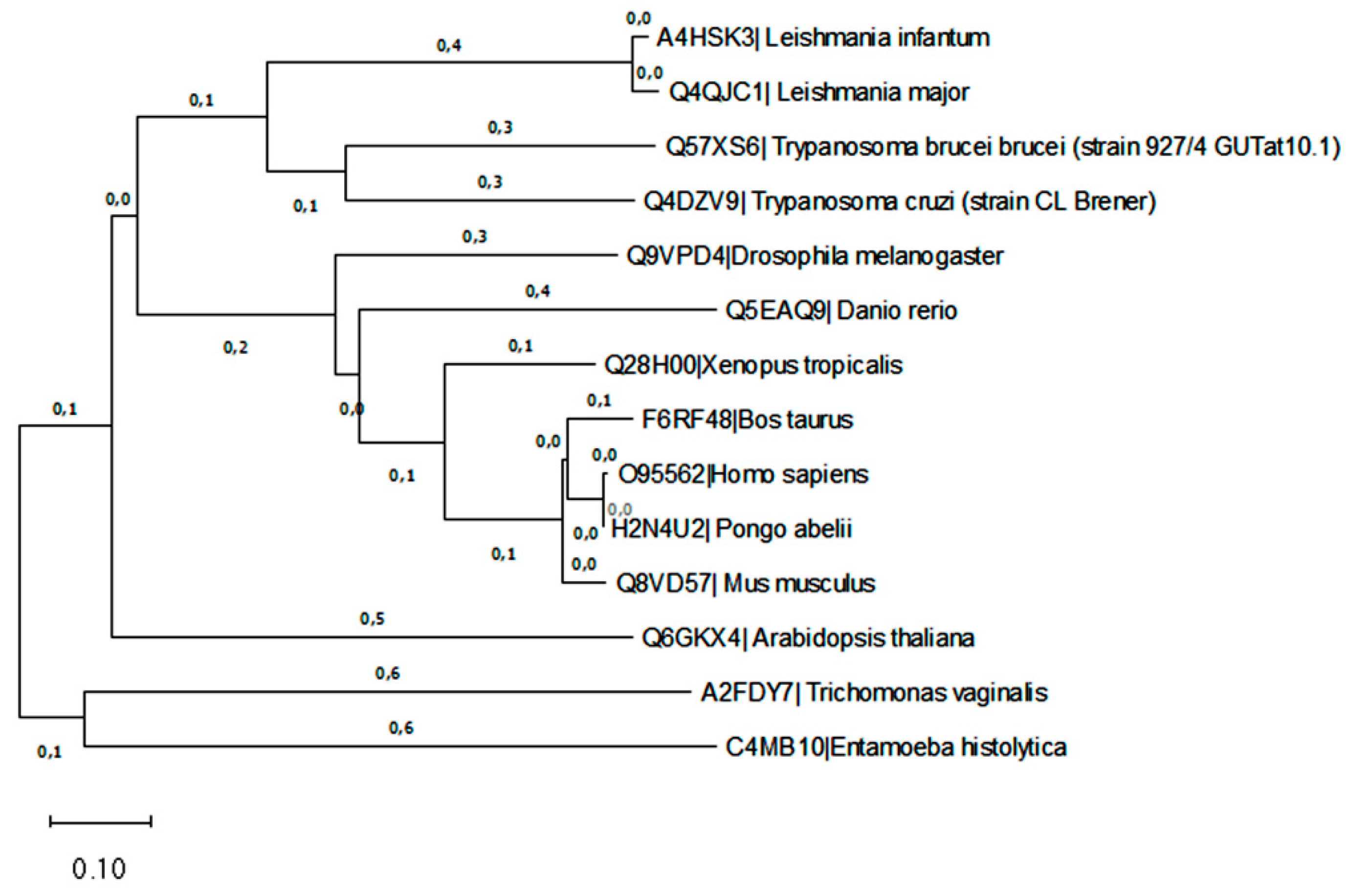
2.4. Phylogenetic analysis
3. Discussion
4. Materials and Methods
4.1. Parasites
4.2. DNA extraction and oligonucleotide synthesis
4.3. Amplification and cloning
4.4. Polyclonal antibodies anti-presenilin like protein
4.5. Fluorescence microscopy
4.6. Phylogenetic analysis
4.7. Bioinformatic analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Leishmaniasis. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (Accessed on 20 June 2022).
- Akpunarlieva, S.; Burchmore, R. The role of membrane transporters in Leishmania virulence. Emerg Top Life Sci 2017, 1, 601-611. [CrossRef]
- Field, M.C.; Natesan, S.K.; Gabernet-Castello, C.; Koumandou, V.L. Intracellular trafficking in the trypanosomatids. Traffic 2007, 8, 629-639. [CrossRef]
- Matte, C.; Descoteaux, A. Exploitation of the host cell membrane fusion machinery by leishmania is part of the infection process. PLoS Pathog. 2016, 12, e1005962. [CrossRef]
- Landfear, S.M.; Ignatushchenko, M. The flagellum and flagellar pocket of trypanosomatids. Mol Biochem Parasitol 2001, 115, 1-17. [CrossRef]
- Machado, P.A.; Carneiro, M.P.D.; Sousa-Batista, A.J.; Lopes, F.J.P.; Lima, A.P.C.A.; Chaves, S.P.; Sodero, A.C.R.; de Matos Guedes, H.L. Leishmanicidal therapy targeted to parasite proteases. Life Sci 2019, 219, 163-181. [CrossRef]
- Rawlings, N.D.; Barrett, A.J.; Finn, R. Twenty years of the MEROPS database of proteolytic enzymes, their substrates and inhibitors. Nucleic Acids Res 2016, 44, D343-D350. [CrossRef]
- Yang, G.; Zhou, R.; Guo, X.; Yan, C.; Lei, J.; Shi, Y. Structural basis of γ-secretase inhibition and modulation by small molecule drugs. Cell 2021, 184, 521-533.e14. [CrossRef]
- Welander, H.; Tjernberg, L.O.; Karlström, H.; Ankarcrona, M. γ-Secretase complexes containing caspase-cleaved presenilin-1 increase intracellular Aβ(42) /Aβ(40) ratio. J Cell Mol Med 2011, 15, 2150-63. [CrossRef]
- Leissring, M.A.; Yamasaki, T.R.; Wasco, W.; Buxbaum, J.D.; Parker, I.; LaFerla, F.M. Calsenilin reverses presenilin-mediated enhancement of calcium signaling. Proc Natl Acad Sci USA 2000, 97, 8590-8593. [CrossRef]
- Kim, D.Y.; Ingano, L.A.; Kovacs, D.M. Nectin-1 alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem 2002, 277, 49976-49981. [CrossRef]
- Marambaud, P.; Shioi, J.; Serban, G.; Georgakopoulos, A., Sarner, S.; Nagy, V.; Baki, L.; Wen, P.; Efthimiopoulos, S.; Shao, Z.; Wisniewski, T.; Robakis, N.K. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J 2002, 21, 1948-1956. [CrossRef]
- Lammich, S.; Okochi, M.; Takeda, M.; Kaether, C.; Capell, A.; Zimmer, A.K.; Edbauer, D.; Walter, J.; Steiner, H.; Haass, C. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an a beta-like peptide. J Biol Chem 2002, 277, 44754-44759. [CrossRef]
- Cai, T.; Tomita, T. Structure-activity relationship of presenilin in γ-secretase-mediated intramembrane cleavage. Semin Cell Dev Biol. 2020, 105, 102-109. [CrossRef]
- Nasamu, A.S.; Polino, A.J.; Istvan, E.S.; Goldberg, D.E. Malaria parasite plasmepsins: More than just plain old degradative pepsins. J Biol Chem 2020, 295, 8425-8441. [CrossRef]
- Johnson, D.S.; Li, Y.M.; Pettersson, M.; St George-Hyslop, P.H. Structural and chemical biology of presenilin complexes. Cold Spring Harb Perspect Med. 2017, 7, a024067. [CrossRef]
- Sarasija, S.; Norman, K.R. A gamma-secretase independent role for presenilin in calcium homeostasis impacts mitochondrial function and morphology in Caenorhabditis elegans. Genetics 2015. 201, 1453-1466. [CrossRef]
- Holmes, O.; Paturi, S.; Selkoe, D.J.; Wolfe, M.S. Pen-2 is essential for γ-secretase complex stability and trafficking but partially dispensable for endoproteolysis. Biochemistry 2014, 53(27):4393-4406. [CrossRef]
- Yoon, T.Y.; Munson, M. SNARE complex assembly and disassembly. Curr Biol. 2018, 28, R397-R401. [CrossRef]
- Besteiro, S.; Coombs, G.H.; Mottram, J.C. The SNARE protein family of Leishmania major. BMC Genomics 2006, 7, 250. [CrossRef]
- Conchon, S.; Cao, X.; Barlowe, C.; Pelham, H.R. Got1p and Sft2p: membrane proteins involved in traffic to the Golgi complex. EMBO J 1999, 18, 3934-3946. [CrossRef]
- Banfield, D.K.; Lewis, M.J.; Pelham, H.R.; A SNARE-like protein required for traffic through the Golgi complex. Nature 1995, 375, 806-809. [CrossRef]
- Suga, K.; Saito, A.; Tomiyama, T.; Mori, H.; Akagawa, K. Syntaxin 5 interacts specifically with presenilin holoproteins and affects processing of betaAPP in neuronal cells. J Neurochem 2005, 94, 425-439. [CrossRef]
- Lechuga, G.C.; Bottino, C.C.G.; Pinho, R.T.; Souza, A.L.A.; Provance-Jr, D.W.; De-Simone, S.G. Trypanosoma cruzi presenilin-like transmembrane aspartyl protease: Characterization and cellular localization. Biomolecules 2020, 10, 1564-1584. [CrossRef]
- Lechuga, G.C.; Napoleão-Pêgo, P.; Gomes, L.R.; Durans, A.M.; Provance-Jr, D.W.; De-Simone, S.G. Nicastrin-like, a novel transmembrane protein from Trypanosoma cruzi associated to the flagellar pocket. Microorganisms 2021, 9, 1750. [CrossRef]
- De-Simone, S.G.; Napoleão-Pêgo, P.; Gonçalves, P.S.; Lechuga, G.C.; Mandonado-Jr, A.; Graeff-Teixeira, C.; Provance-Jr, D.W. Angiostrongylus cantonensis an atypical presenilin: epitope mapping, characterization, and development of an ELISA peptide assay for specific diagnostic of angiostrongyliasis. Membranes 2022, 12, 108. [CrossRef]
- Wolfe, M.S.; Miao, Y. Structure and mechanism of the γ-secretase intramembrane protease complex. Curr Opin Struct Biol. 2022, 74,102373. [CrossRef]
- Zhao, G.; Cui, M.Z.; Mao, G.; Dong, Y.; Tan, J.; Sun, L.; Xu, X. gamma-Cleavage is dependent on zeta-cleavage during the proteolytic processing of amyloid precursor protein within its transmembrane domain. J Biol Chem 2005, 280, 37689-37697. [CrossRef]
- Wolfe, M.S. Structure and function of the γ-secretase complex. Biochemistry 2019, 58, 2953-2966. [CrossRef]
- Chen, F.; Hasegawa, H.; Schmitt-Ulms, G.; Kawarai, T.; Bohm, C.; Katayama, T.; Gu, Y.; Sanjo, N.; Glista, M.; Rogaeva, E.; et al.. TMP21 is a presenilin complex component that modulates gamma-secretase but not epsilon-secretase activity. Nature 2006, 440, 1208-1212. [CrossRef]
- Wolfe, M.S. Probing mechanisms and therapeutic potential of γ-secretase in Alzheimer's disease. Molecules 2021, 26, 388. [CrossRef]
- Svedružić, Ž.M.; Vrbnjak, K.; Martinović, M.; Miletić, V. Structural Analysis of the simultaneous activation and inhibition of γ-secretase activity in the development of drugs for Alzheimer's disease. Pharmaceutics 2021, 13, 514. [CrossRef]
- Marambaud, P.; Robakis, N.K. Genetic and molecular aspects of Alzheimer's disease shed light on new mechanisms of transcriptional regulation. Genes Brain Behav, 2005, 4, 134-146. [CrossRef]
- Besteiro, S.; Williams, R.A.; Coombs, G.H.; Mottram, J.C. Protein turnover and differentiation in Leishmania. Int J Parasitol 2007, 37, 1063-1075. [CrossRef]
- Arango Duque, G.; Jardim, A.; Gagnon, É.; Fukuda, M.; Descoteaux, A. The host cell secretory pathway mediates the export of Leishmania virulence factors out of the parasitophorous vacuole. PLoS Pathog 2019, 15, e1007982. [CrossRef]
- Hasegawa, H.; Sanjo, N.; Chen, F.; Gu, Y.J.; S,hier, C.; Petit, A.; Kawarai, T.; Katayama, T.; Schmidt, S.D.; Mathews, P.M. et al.. Both the sequence and length of the C terminus of PEN-2 are critical for intermolecular interactions and function of presenilin complexes. J Biol Chem 2004, 279, 46455-46463. [CrossRef]
- Crystal, A.S.; Morais, V.A.; Pierson, T.C.; Pijak, D.S.; Carlin, D.; Lee, V.M.; Doms, R.W. Membrane topology of gamma-secretase component PEN-2. J Biol Chem 2003, 278, 20117-20123. [CrossRef]
- Wheeler, R.J.; Sunter, J.D.; Gull, K. Flagellar pocket restructuring through the Leishmania life cycle involves a discrete flagellum attachment zone. J Cell Sci 2016, 129, 854-867. [CrossRef]
- Brickman, M.J.; Balber, A.E.Trypanosoma brucei rhodesiense: membrane glycoproteins localized primarily in endosomes and lysosomes of bloodstream forms. Exp Parasitol 1993, 76, 329-344. [CrossRef]
- Müller, M.T.; Schempp, R.; Lutz, A.; Felder, T.; Felder, E.; Miklavc, P. Interaction of microtubules and actin during the post-fusion phase of exocytosis. Sci Rep 2019, 9, 11973. [CrossRef]
- McConville, M.J.; Mullin, K.A.; Ilgoutz, S.C.; Teasdale, R.D. Secretory pathway of trypanosomatid parasites. Microbiol Mol Biol Rev 2002, 66, 122-54. [CrossRef]
- Clayton, C.; Hausler,T.; Blattner, J. Protein trafficking in kinetoplastid protozoa. Microbiol Rev 1995, 59, 325-344. [CrossRef]
- Zoltowska, K.M.; Maesako, M.; Lushnikova, I; Takeda, S.; Keller, L.J.; Skibo, G.; Hyman, B.T.; Berezovska, O. Dynamic presenilin 1 and synaptotagmin 1 interaction modulates exocytosis and amyloid β production. Mol Neurodegener 2017, 12, 15. [CrossRef]
- Arango Duque, G.; Jardim, A.; Gagnon, É.; Fukuda, M.; Descoteaux, A. The host cell secretory pathway mediates the export of Leishmania virulence factors out of the parasitophorous vacuole. PLoS Pathog 2019, 15, e1007982. [CrossRef]
- Saboia-Vahia, L.; Cuervo, P.; Wiśniewski, J.R; Dias-Lopes, G.; Pinho, N.; Padrón, G.; de Pilla Varotti, F.; Murta, S.M.F. In-depth quantitative proteomics characterization of in vitro selected miltefosine resistance in Leishmania infantum. Proteomes 2022, 10,10. [CrossRef]
- Alsford, S.; Turner, D.J.; Obado, S.O.; Sanchez-Flores, A.; Glover, L.; Berriman, M.; Hertz-Fowler, C.; Horn, D. High-throughput phenotyping using parallel sequencing of RNA interference targets in the African trypanosome. Genome Res 2011, 21, 915-924. [CrossRef]
- Oliveira, I.H.R.; Figueiredo, H.C.P.; Rezende, C.P.; Verano-Braga, T.; Melo-Braga, M.N.; Reis Cunha, J.L.; de Andrade, H.M. Assessing the composition of the plasma membrane of Leishmania (Leishmania) infantum and L. (L.) amazonensis using label-free proteomics. Exp Parasitol 2020, 218, 107964. [CrossRef]
- Venkatesh, D.; Boehm, C.; Barlow, L.D.; Nankissoor, N.N.; O'Reilly, A.; Kelly, S.; Dacks, J.B.; Field, M.C. Evolution of the endomembrane systems of trypanosomatids-conservation and specialisation. J Cell Sci 2017, 130, 1421-1434. [CrossRef]
- Gossage, S.M.; Roger, M.E.; Bates, P.A. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int J Parasitol 2003, 33, 1027-1034. [CrossRef]
- Mesquita-Rodrigues, C.; Menna-Barreto, R.F.; Sabóia-Vahia, L.; Da-Silva, S.A.; de Souza, E.M.; Waghabi, M.C.; Cuervo, P.; De Jesus, J.B. Cellular growth and mitochondrial ultrastructure of Leishmania (Viannia) braziliensis promastigotes are affected by the iron chelator 2,2-dipyridyl. PLoS Negl Trop Dis 2013, 7, e2481. [CrossRef]
- Taylor-Brown, E.; Hurd, H. The first suicides: a legacy inherited by parasitic protozoans from prokaryote ancestors. Parasit Vectors. 2013, 6, 108. [CrossRef]
- Sharma, B.; Pal, D.; Sharma, U.; Kumar, A. Mitophagy: An emergence of new player in Alzheimer's disease. Front Mol Neurosci. 2022,15, 921908. [CrossRef]
- Planelles, L.; Marañón, C.; Requena, J.M.; López, M.C. Phage recovery by electroporation of naked DNA into host cells avoids the use of packaging extracts. Anal Biochem 1999, 267, 234-235. [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for Bigger Datasets. Mol Biol Evol 2016, 33, 1870–1874. [CrossRef]
- Lomize, M.A.; Pogozheva, I.D.; Joo, H.; Mosberg, H.I.; Lomize, A.L. OPM database and PPM web server: Resources for positioning of proteins in membranes. Nucleic Acids Res 2011, 40, D370–D376. [CrossRef]
| Code (UNIPROT) | Human protein | L. infantum protein | Identity (%) | L. infantum code |
| P49768 | Presenilin-1 | - | - | - |
| P49810 | Presenilin-2 | Putative presenilin-like aspartic peptidase | 31 | A4HWP2 |
| Q92542 | Nicastrin | - | - | - |
| Q96BI3/Q8WW43 | Aph1-a/b | APH-1 family | No significant | A4HV58 |
| - | - | - | ||
| Q9NZ42 | Pen-2 | Putative Sft2/Got-1 like family | 35 | A4HSK3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




