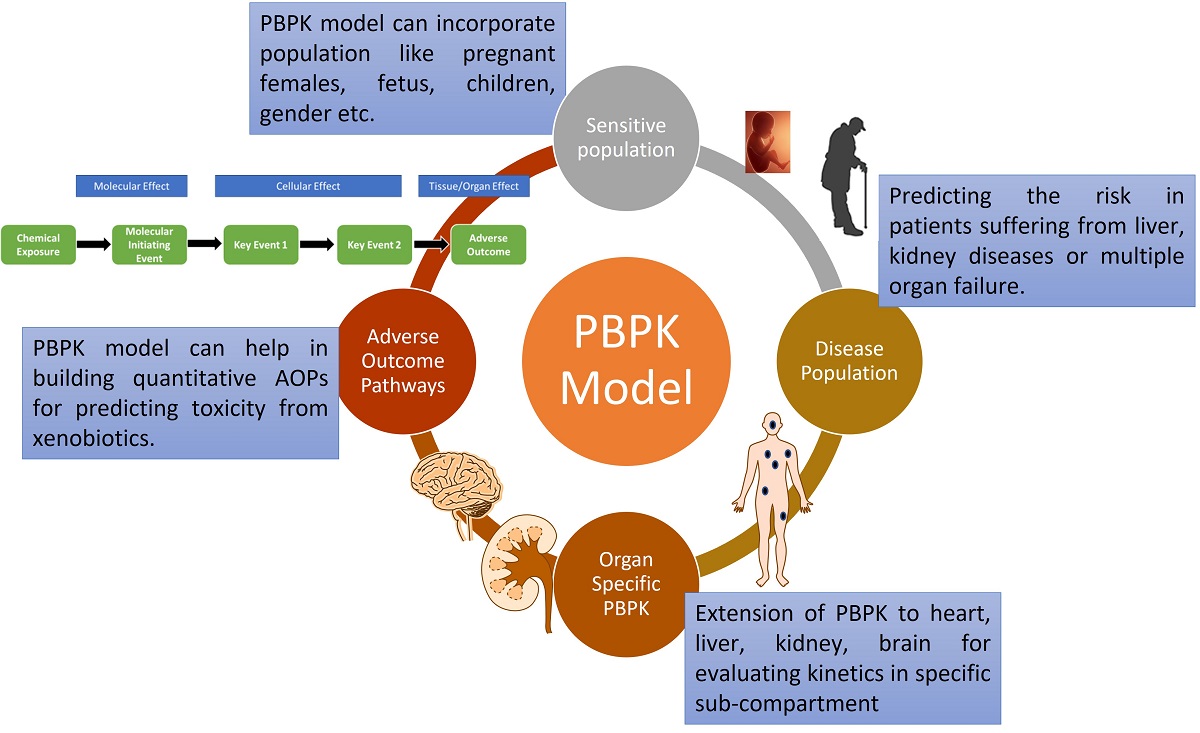
Physiologically Based Pharmacokinetic Models (PBPK) are mechanistical tools generally employed in the pharmaceutical industry and environmental health risk assessment. These models are recognised by regulatory authorities for predicting organ concentration-time profile, pharmacokinetic and daily intake dose of xenobiotics. Extension of PBPK models to capture sensitive populations like pediatric, geriatric, pregnant females, fetus etc. and diseased population like renal impairment, liver cirrhosis etc. is a must. However, the current modelling practice and existing models are not mature enough to confidently predict the risk in these populations. A multidisciplinary collaboration between clinicians, experimental and modeler scientist is vital to improve the physiology, and calculation of biochemical parameters for integrating the knowledge and refining existing PBPK models. Specific PBPK covering compartments like cerebrospinal fluid, and hippocampus are required to gain mechanistic understanding about xenobiotic disposition in these sub-parts. The PBPK model assists in building quantitative adverse outcome pathways (qAOPs) for several endpoints like developmental neurotoxicity (DNT), hepatotoxicity and cardiotoxicity. Machine learning algorithms can predict physicochemical parameters required to develop in-silico models where experimental data is unavailable. Integrating machine learning with PBPK carries the potential to revolutionize the field of drug discovery and development and environmental risk. Overall, this review tried to summarize the recent developments in the in-silico models, building qAOPs, use of machine learning for improving existing models along with a regulatory perspective. This review can act as a guide for toxicologists who wish to build their careers in kinetic modeling.