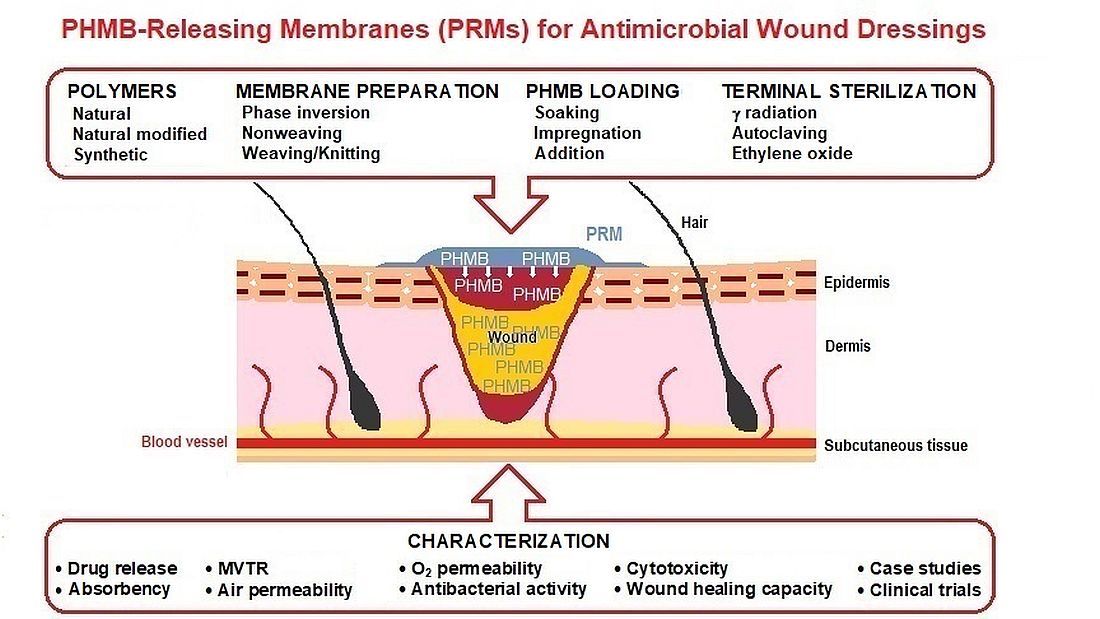
The prevalence for chronic, non-healing skin wounds in the general population, most notably diabetic foot ulcers, venous leg ulcers and pressure ulcers, is approximately 2% and is expected to increase, driven mostly by an aging population and the steady rise in obesity and diabetes. Non-healing wounds often become infected, increasing the risk of life-threatening complications, which poses a significant socioeconomic burden. Aiming at an improved management of infected wounds, a variety of wound dressings incorporating antimicrobials (AMDs), namely polyhexanide (poly(hexamethylene biguanide); PHMB), have been introduced in the wound care market. However, many wound care professionals agree that none shows comprehensive and optimal antimicrobial activity. This manuscript summarizes and discusses studies on novel PHMB-releasing membranes (PRMs) for wound dressings, detailing their preparation, physical properties relevant in the context of AMDs, drug loading and release, antibacterial activity, biocompatibility, wound healing capacity, and clinical trials conducted. Some of these PRMs were able to improve wound healing in in vivo models, with no associated cytotoxicity, but significant differences in study design make it difficult to compare overall effi-cacies. It is hoped that this review, which includes, whenever available, international standards for testing AMDs, will provide a framework for future studies.their preparation, physical properties relevant in the context of AMDs, drug loading and release, antibacterial activity, biocompatibility, wound healing capacity, and clinical trials conducted. Some of these PRMs were able to improve wound healing in in vivo models, with no associated cytotoxicity, but significant differences in study design make it difficult to compare overall efficacies. It is hoped that this review, which includes, whenever available, international standards for testing AMDs, will provide a framework for future studies.