1. Introduction
Recently, there is a on the rise knowing of the significance of multi-species interface for some emergence and re-emergence of pathogens in wildlife, livestock and humans (Amissah-Reynolds, 2020). Since two-thirds of human diseases being multi-host pathogens and three-quarter of emerging human diseases being zoonotic, there is a strong public health concern in the better understanding the dynamics of multi-species pathogens (Muhammed et al., 2020). In addition, the spill-over of domestic animal pathogens to wildlife caused unpredictable outbreaks with great impact for wildlife conservation, such as pasturellosis and Sierra Nevada outbreak in Bighorn sheep, rabies in Ethiopian wolves, and bovine brucellosis and tuberculosis in bison (Randall et al., 2006; Clifford et al., 2009).
Bovine tuberculosis is one of the chronic bacterial diseases of animals that can take a variable amount of time (from a few weeks to a lifetime) to develop from infection to clinical disease and to be converted into infectious to other animals (Khan and Zahoor, 2018). Bovine tuberculosis disease generally affects cattle and infrequently other species of domestic animals (Hind, 2013).
The common cause of Bovine tuberculosis, Mycobacterium bovis has an outstandingly wide range of mammalian hosts and it affects all age groups of susceptible hosts of domestic, wild animals and human (Amato et al., 2018). The most familiar maintenance hosts for mycobacterium infection which carries the infection to wildlife or to human are cattle (Amissah-Reynolds, 2020). On the other hand, Opossums, badgers and bison are common maintenance hosts in different European countries while in Africa, African buffalo, Kudu, deer, lechwe and wild boar are maintenance hosts for M. bovis (Lekko et al., 2020). The numerous susceptible animals and wildlife species, including man are really spillover hosts in which infection is not self- maintaining (Tenguria et al., 2011).
In addition to domestic animals, BTB can also infect wild animals. The bacterium M. bovis has been isolated from almost more than 40 free-ranging wild animal species around the world (Thoen et al., 2011). In Africa only few countries have detailed data regarding M. bovis in wildlife, these are Southern Africa, Uganda and Tanzania. Nonetheless, the status of BTB in wildlife is still not there and need more studies in most African countries(Borham et al., 2022). Similarly, in Ethiopia, numerous studies conducted in different parts of the country have only confirmed the endemic nature of the disease in Ethiopian cattle populations rather than reporting the Spillover of the disease (Megersa, 2010).
Besides being a potential zoonotic threat through consumption of raw animal products and close animal-human contact, the disease can have major economic impacts on national livestock sector (Khan and Zahoor, 2018). Despite the isolation of M. bovis from domestic animal and human, no infection due to M. bovis was reported in Ethiopian wildlife populations so far and the status of the disease in wildlife populations is yet unknown (WHO, 2012).
Moreover, the information on the epidemiology of the disease in wildlife-livestock- human interface is scarce and not well established at a national level (Mohamed, 2020). This is mainly due to the absence of disease surveillance, insufficient laboratory capacity and the lack of veterinary expertise (Firdessa et al., 2012). Therefore, objectives of this review paper is to elaborate the epidemiological features of M. bovis in wildlife-livestock-human interface, to identify risk factors considered in studies conducted so far in Ethiopia.
2. Methods
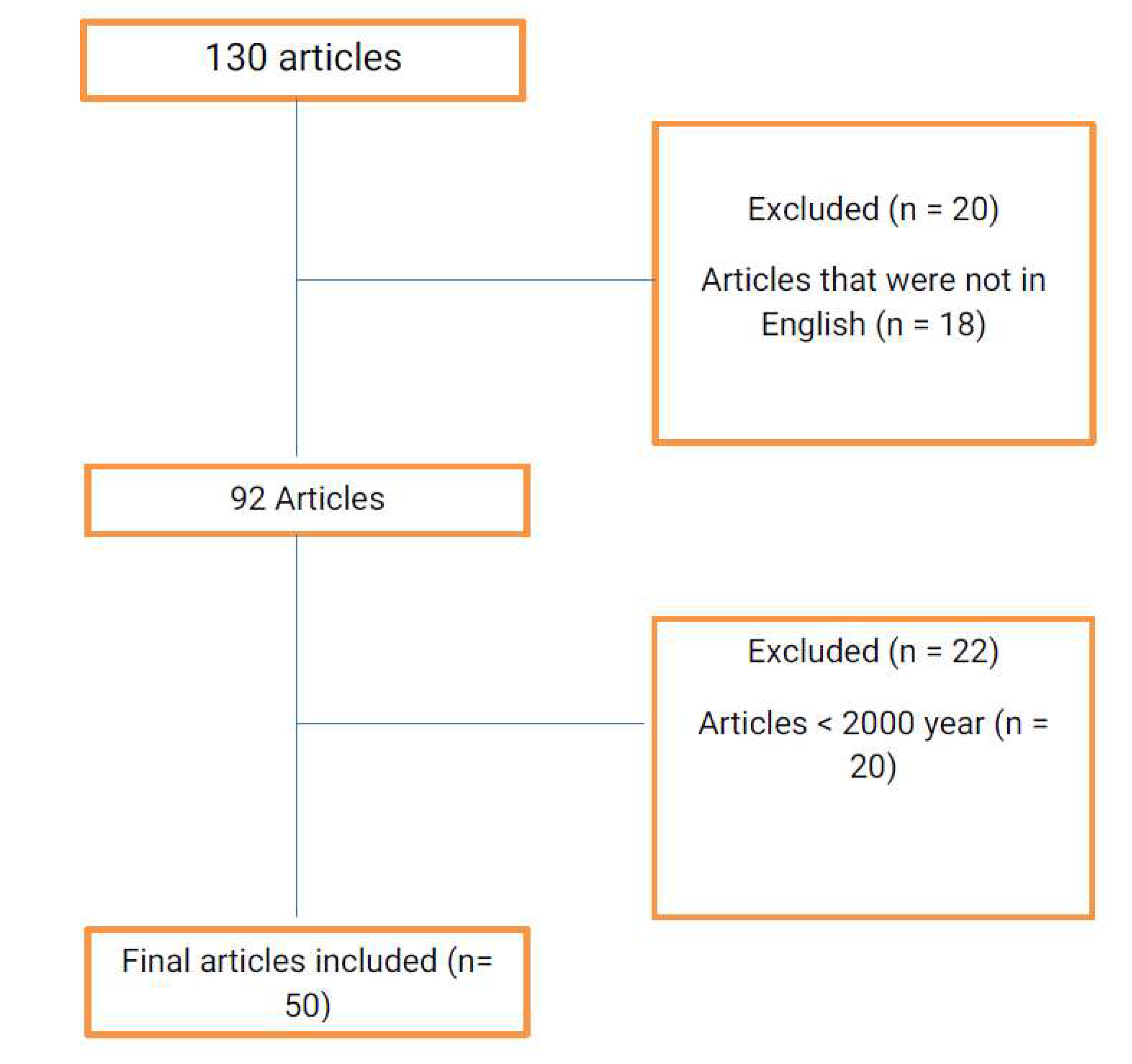
References were obtained from Google scholar database, PubMed and Science direct using keywords “
Bovine tuberculosis” and “interface” and “Wildlife” and “Epidemiological” and “Human”. The articles that were published before 2000, articles that were not written in English, articles from journals with low impact factor and articles with low quality result were excluded during the selection of articles for the review. The flowchart of the literature search can be seen in
Figure 1.
2. Literature Review
2.1. Etiology
Bovine tuberculosis (BTB) is a chronic bacterial disease caused by Mycobacterium bovis, a Gram positive, acid-fast bacterium. This pathogen belongs to the Mycobacterium tuberculosis complex, a group of genetically closely related mycobacteria. Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium bovis BCG, Mycobacterium canettii, Mycobacterium africanum, Mycobacterium pinnipedii, Mycobacterium microti, Mycobacterium caprae, the dassie and the oryx bacillus, and the recently discovered Mycobacterium mungi are closely related species that form the M. tuberculosis comple (MTBC) (Raffo et al., 2017).
Mycobacterium tuberculosis and Mycobacterium bovis are the most important species in the complex which commonly cause human and animal tuberculosis (TB), with concomitant negative consequences for human and animal health and economic costs (Katale et al., 2012). Mycobacterium bovis is a slow growing, facultative intracellular, aerobic and gram - positive bacterium with a dysgonic colony shape when cultured on Löwenstein - Jensen (LJ) medium (Ashmi, 2019). As all Mycobacterium spp., M. bovis has an unusual cell wall surface structure characterized by the dominant presence of mycolic acids and a wide array of lipids (Szewczyk et al., 2013). This waxy lipid envelope confers an extreme hydrophobicity, which renders the bacteria acid - and alcohol - fast, a feature that can be exploited to identify mycobacteria via the Ziehl - Neelsen staining technique (Vilchèze et al., 2014).
The mycobacteria surface lipids also have a potent biologic activity and are thought to play a crucial role in pathogenesis (Falanga et al., 2016). M. bovis can be identified based on specific biochemical and metabolic properties. e.g., M. bovis requires pyruvate as a growth supplement, is negative for niacin accumulation and nitrate reduction, shows microaerophilic growth on Lebek medium and is generally resistant to pyrazinamide (Daniel Wambua, 2015). In contrast, MTB does not require pyruvate as a growth supplement, is positive for niacin accumulation and nitrate reduction, shows aerophilic growth on Lebek medium, and is usually not mono- resistant to pyrazinamide (Daniel Wambua, 2015). M. bovis isolates are resistant to pyrazinamide because the organism does not produce the enzyme pyrazinamidase, which is needed to convert pyrazinamide into pyrazinic acid, the active form of the antimicrobial agent (Barouni et al., 2004). This resistance is one of the basic features, which can be used to distinguish isolates of M. bovis (universally resistant to pyrazinamide) from M. tuberculosis (commonly susceptible).
2.2. Epidemiology of Bovine tuberculosis
2.2.1. Risk Factors of BTB
Risk Factors in Cattle
In cattle, risk factors for bovine TB are cattle breeds, genetic resistance, physiological state of the animal, age, sex, stress, concurrent infection, immune status and body condition score (BCS) (Vlasova and Saif, 2021). Several past and recent studies have shown that susceptibility to bovine TB can vary between cattle breeds with suggestions that indigenous zebu cattle are more resistant to BTB than exotic breeds (Callaby et al., 2020). This fact is substantiated by the lower prevalence recorded in several studies and it is evident where European breeds of cattle have been used to establish a dairy industry. Genetically improved cattle may suffer more severely from deficient housing and malnutrition and thus be more prone to infection (Vlasova and Saif, 2021).
Studies conducted in different areas of Ethiopia also confirm variation in susceptibility to bovine TB among cattle breeds. One of these studies have reported that, there is statistically significant difference (P<0.05) prevalence of bovine TB among exotic, cross and zebu breed where exotic and cross breeds were observed with high prevalence of BTB as compared to zebu cattle breed (Zeru et al., 2014). One of the main animal risk factor identified by numerous studies in both developed and developing countries is the age of animals. The duration of exposure increases with age (Dinka and Duressa, 2011). Several studies carried out in Tanzania, Zambia and Chad have shown that older animals are more likely to have been exposed than younger ones (Humblet et al., 2009). Similarly in Ethiopia, also there were studies found that, statistically significant difference prevalence among age groups where higher prevalence of BTB was observed in older animals than younger ones (Almaw et al., 2021).
Gender mostly appears as a risk factor of Bovine tuberculosis in many published African studies. Gender-linked factors are probably related to management practices or behavioral habits. Males and females are managed differently, in both developed and developing countries (Muhammed et al., 2020). Males have potentially more contact with other herds during breeding, which may increase their risk (Muhammed et al., 2020). A study conducted in Tanzania revealed that male cattle were significantly more affected by bovine TB than female animals; because they are mostly used as oxen and kept longer in the herd than females (Humblet et al., 2009). However, in developed countries, dairy cows usually reach an older age than males because of their role in calving and milk production. Female cattle are usually confined in a barn and kept long for production purpose which may facilitate infection and acquisition of the disease (Humblet et al., 2009). Moreover, dairy cows experience greater production stress and gathering of cattle during milking increases the risk of transmission (Zeru et al., 2014). Similarly, a studies conducted in Uganda and Ethiopia revealed significantly more females positive to the skin test than males (Regassa et al., 2008) (Daniel Wambua, 2015).
Factors associated with bovine TB also differed statistically according to body condition categories. In Zambia and Tanzania, studies conducted indicated that low BCS was associated with increased risk of tuberculin reactivity (Lekko et al., 2020) (Moiane et al., 2014). Similarly, study conducted in Ethiopia reported a higher prevalence of BTB in animals with poor body condition score (Biratu et al., 2014). This could be related that animal’s resistance to tuberculosis is reduced by a shortage of feed and/or unbalanced diet, attributable to a deficiency of proteins, minerals and vitamins in the diet (Zeru et al., 2014). In contrast to the above studies, a study found higher prevalence of the disease in animals with good body condition than poor body conditioned animals (Zeru et al., 2014).
Studies in Ethiopia indicated that the physiological and immunological state of an animal, including the degree of environmental stress being experienced at the time, could strongly influence the course of tuberculosis (Demelash et al., 2009).
Risk Factors in Wild Life
Although no M. bovis infections have been reported in Ethiopian wildlife populations so far, reports from different parts of the world have demonstrated several risk factors for the presence of the disease in wildlife. Direct contact or sharing of environment with domestic cattle, the extent of the disease prevalence within the region/country or domestic animal reservoir host, herd size (wildlife densities) and previous history of M. bovis in the wildlife populations are among the potential risk factors (Muhammed et al., 2020). The presence of the aforementioned animals in different wildlife reserves may have an epidemiological role in the spread of the disease among other wild and domestic animal (Mohamed, 2020)
On the other hand, in Ethiopia, as wildlife habitats are not fenced, there is intensive interaction between a fast-growing human population and livestock and wildlife competing for scarce grazing land. Wildlife and, in particular, herbivores sharing pastures with cattle might therefore be at risk for bovine TB transmission (Dejene et al., 2016).
Risk Factors in Human
Nowadays many developing countries have intensified their livestock production to meet the growing demand for food security. This intensification promotes close physical contact between the owner and his or her cattle, especially at night and thus facilitates the transmission of bovine TB as zoonosis. The main risk factors which contribute to the acquisition M. bovis infections in both urban and rural human populations are poverty, malnutrition, HIV infection, illiteracy, the consumption of raw milk (unpasteurized milk), uncooked or poorly cooked meat, work condition and close contact to livestock and using cow dung for plastering wall or floor (Muhammed et al., 2020).
In Ethiopia, the habit and tradition of consumption raw milk and meat in societies is the main risk factors for M. bovis infection in human (Deneke et al., 2022). In addition, there is a habit of chewing and spitting tobacco to their cattle among Ethiopian farmers. This led to a higher risk of transmission for M. tuberculosis as well as for M. bovis at the human–livestock interface through inhalation of the cough spray from infected animals or transmission of M. tuberculosis from human to cattle as the organism can spread to the animal (Desta et al., 2022).
Professional occupation or workers such as, abattoir workers, veterinarians and laboratory technicians, animal caretaker in zoos and those who are working in animals reservations and at national parks can acquire the infection in due course of regular work (Mekonnen et al., 2022).
2.2.2. Conceptual Model of bTB Transmission at the Wildlife–Livestock–Human Interface
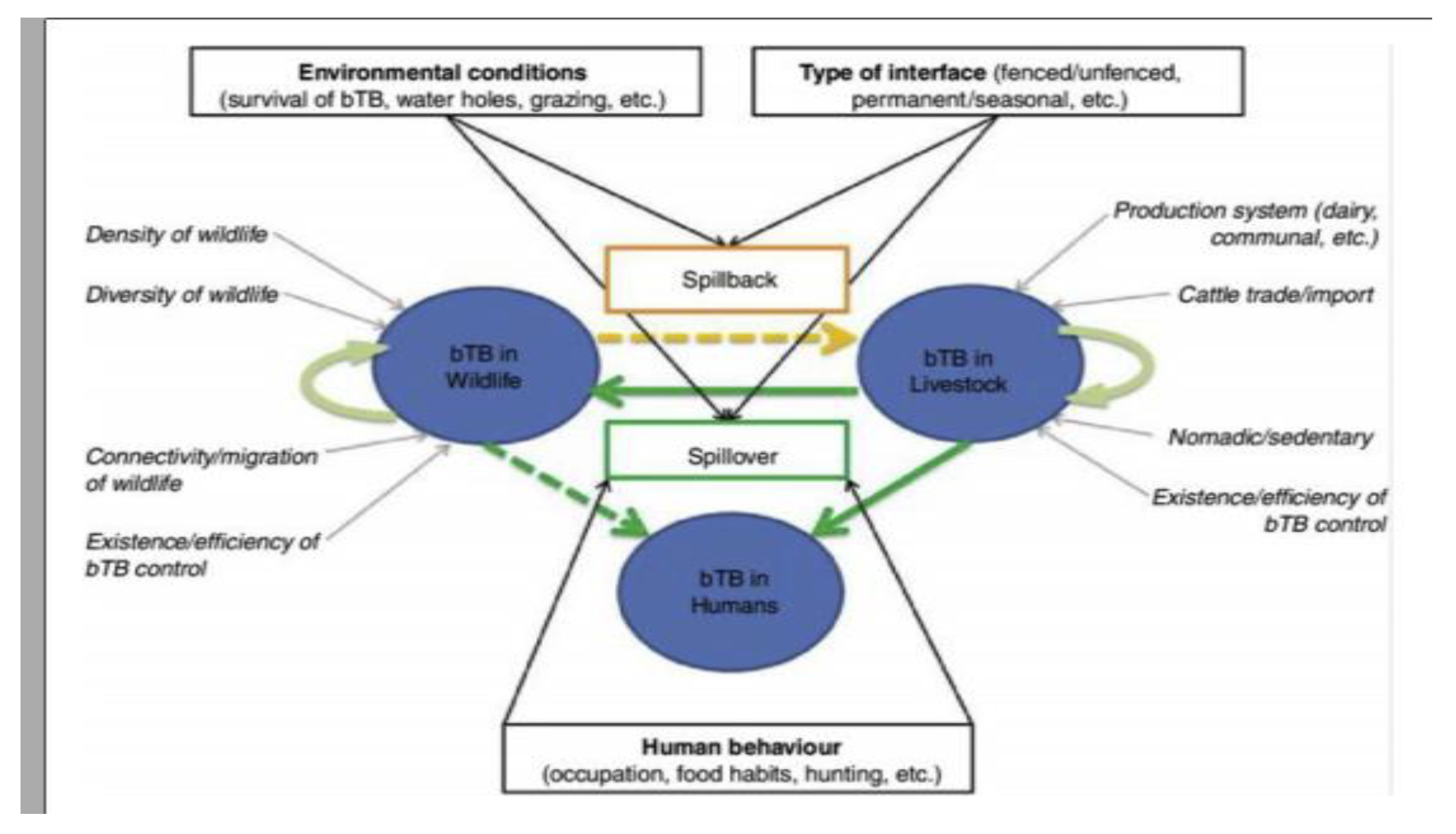
A disease reservoir may consist in a maintenance population or community (i.e., with more than one species involved) within which a given pathogen can be maintained and which acts as a potential source of infection for a target species (Palmer, 2013). In the case of bTB in Africa, maintenance populations have been identified as cattle, lechwe and buffalo. The existence of a maintenance community remains unknown (De Garine-Wichatitsky et al., 2013). The role played by other wild ungulate species, such as the greater kudu and the common warthog, is still debated although in theory, they could connect reservoir (e.g., buffalo) and target (e.g., naive cattle) populations, and so contribute to the maintenance community of bTB. Similarly, the maintenance community created by sympatric cattle and buffalo populations in frequent contacts may allow bTB spillover or spillback, and could act as a source of bTB for other target populations (De Garine-Wichatitsky et al., 2013).
Within this maintenance community framework, bTB epidemiology may be represented as a dynamic multi-host system with three main ‘compartments’, namely wildlife, livestock and humans (
Figure 2). Bovine TB infections may be maintained (independently or not) within livestock populations and within wildlife populations, whereas human infections result from pathogen spillover from animals (De Garine-Wichatitsky
et al., 2013), and very rarely from human-to-human transmission (Palmer, 2013).
2.3. Diagnosis
Tubercullosis can be diagnosed clinically, but usually only in the later stages of the disease. The tuberculin skin test is universally recognized and is generally used for preliminary diagnosis in BTB control programs. However, in countries with low disease prevalence or disease free status, meat inspection is used for diagnosis and surveillance. Other tests, such as an antibody enzyme -linked immunoassay (ELISA) and the gamma interferon assay, have been used as supplementary tests in eradication and control (Gormley et al., 2006).
Bovine tuberculosis infection in cattle is usually diagnosed in the live animal on the basis of delayed hypersensitivity reactions (tuberculin skin testing). In cattle, infection is often sub clinical; when present, clinical signs are not specifically distinctive to other disease caused conditions and might include weakness, anorexia, emaciation, dyspnoea, enlargement of lymph nodes, and cough, particularly with advanced TB (Aman, Dessalegn and Masrie, 2017). Post - mortem, infection is diagnosed by necropsy and histo-pathological and bacteriological techniques. Rapid nucleic acid methodologies such as the polymerase chain reaction (PCR) may also be used. These are demanding techniques and only validated procedures should be used. Classical mycobacteria l culture remains the routine method for confirmation of infection (Medley et al., 2009).
2.4. Control of Tuberculosis
Control and eradication programs for BTB, human TB and zoonotic TB of humans due to M. bovis are based on early accurate detection and removal of infected animals, chemotherapy of infected humans and vaccination of target populations to attenuate or prevent the manifestation of the disease (Arnot and Michel, 2019).
The test- and- slaughter policy is the basis for international BTB control and eradication programs using the TST to detect affected herds (and re - test) periodically and removing reacting cattle that may shed the infective organism (Arnot and Michel, 2019). In many industrialized countries there are effective compulsory reporting of M.bovis infection of all animals, quarantine of infected herds, tracing and re- testing of animals in contact with BTB skin positive reactors. as well as movement restrictions of cattle herds not yet tested for TB as well as controlled animal movement out of known TB infected herds and endemic areas (Schiller et al., 2010).
However, the test- and segregation program, a modified form of the test- and- slaughter policy, may be more useful for developing countries, where the test- and- slaughter policy cannot be practicable for the whole cattle population, thus, interim measures to segregate infected herds and phased slaughter of reactors are done (Dibaba and Kriek, 2019). In most countries with strict TB eradication program, the test- and segregation strategy made up the early stages followed by the test- and slaughter methods in the final stage (Schiller et al., 2010) and infected slaughter/meat cases during inspection are traced back to the originating farms. Informed farm management decisions such as proper sanitation and disinfection are also important to reduce the spread of Mycobacterium, within and between herds as well as the risks of exposure and transmission of BTB infection to humans (Phipps et al., 2019).
The occurrence of M. bovis in wildlife reservoir hosts complicates eradication efforts. Culling to reduce population density can decrease animal TB transmission but the situation must be assessed carefully to avoid unanticipated effects such as the economic benefit and increase scattering members of the infected species (Roex et al., 2016). The development of TB vaccines for wildlife reservoirs and use in situations where the test- and- slaughter policy is totally impracticable is also being considered as an alternative (Buddle et al., 2018). Also, human TB due to M. bovis is rare in countries where raw and poorly cooked meat are not consumed and pasteurization of milk and milk products are components of BTB eradication programs (Arnot and Michel, 2019).
Mycobacterium Tubercullosis infection and zoonotic TB of humans can be treated successfully with antimicrobial drugs but there is widespread drug resistance and untreated infections are usually fatal (Sharma et al., 2021).
2.5. Economic Importance of Tuberculosis
Mycobaterium bovis has been widely distributed throughout the world and it represents a very significant economic and public health problem in numerous countries in both developed and the developing world (Sandeep et al., 2018). Consequently, most developed nations have embarked on campaigns to eradicate M. bovis from the cattle population or at least to control the spread of the infection (Bhuachalla, Corner and More, 2015).
In developed countries, although tuberculosis is eliminated in cattle, the disease still has a major economic impact, mainly due to the existence of a permanent wildlife reservoir that reduces the efficiency of control strategies. For instance, in the United Kingdom, where badger and other wildlife such as deer remain an important source of infection for livestock, approximately £100 million is spent annually in efforts to control the disease. Republic of Ireland and New Zealand also spent approximately 35 and 13 million US $ annually for disease control (Bhuachalla, Corner and More, 2015). In Argentina, the annual loss due to bovine TB is approximately US$63 million (Zinsstag and Stephan, 2018). Although the disease has zoonotic threat, economic and financial burden to society, its cost has rarely been assessed and is largely unknown for Africa (Ayele et al., 2004).
Although the economic importance and public health significance of tuberculosis has been established in many countries, the economic impact of M. bovis on cattle productivity, bovine TB control program and other related economic effects of the disease are not yet well documented or studied in Ethiopia (Ayele et al., 2004).
3. bTB at the Human-Livestock-Wildlife Interface in Ethiopia
Human–livestock–wildlife interface is not a standard concept but rather one that varies tremendously across Sub-Saharan African pastoralists depending on human, livestock and Wildlife densities and their movements (e.g. Migration, transhumance), wildlife species, environmental factors and anthropogenic land-use change (Tschopp, 2015).
Mycobacterium bovis is an example of a pathogen shared at the human–livestock–wildlife interface (Mohamed, 2020). The list of wildlife species around the world from which M. bovis has been isolated is long and reports in the literature of new susceptible species have increased in recent years. Some wildlife species have long been known to be maintenance hosts (i.e., wildlife species that can maintain the disease in the absence of infected cattle) (Palmer, 2013).
Classic examples of maintenance hosts include; the brush tail possum (Trichosurus vulpecula) in New Zealand, the white-tailed deer (Odocoileus virginianus) in the USA; the Eurasian badger (Meles meles) in the UK and Ireland; the African buffalo (Syncerus caffer) in South Africa (Palmer, 2013). In addition to these the more recently described, the wild boar (Sus scrofa) in Spain and wood bison (Bison bison) in Canada (Wobeser, 2009). These maintenance hosts are a source of infection for livestock and can also be described as a source for BTB in humans that have close contact with infected animals, such as hunters and game farmers (Wobeser, 2009).
Rainfall and water shortages are the main drivers and constraining factors for the distribution and abundance of wildlife species and thus for contact opportunity between species. Interaction between different wildlife species around natural and artificial water sources have been described (Mohamed, 2020); but how wildlife and livestock interact is poorly known.
However, intermediate species such as impala, kudu and warthog (Phacochoerus africanus), which are less affected by livestock presence, could play a role as disease ‘vector’ by having close physical contact with BTB buffalo reactors, that stay within the park, and with livestock in the agricultural land outside the park (Muhammed et al., 2020).
Common use of pastureland is another potential risk for BTB transmission between wildlife and livestock. Mainly wildlife grazer species (as opposed to browser species) are likely to compete with cattle. There seems to be a species-specific tolerance level for cattle presence (Tschopp, 2015). Many grazer species favor grazing in old pastoral places where grass cover is rich due to the cattle manure (Mohamed, 2020). As M. bovis can be excreted in cattle manure and survive in the environment over days and months, it is worth remembering that disease transmission can still occur even with a temporally asymmetric interface (with no direct animal contact) (Sharma et al., 2021).
Cattle have historically played a key role in the introduction of BTB into wildlife conservation areas in many countries. Once endemic, the possibility for spillback from wildlife to livestock and humans constitutes an animal and public health risk to communities neighboring infected wildlife reserves (De Garine-Wichatitsky et al., 2013). Mycobacterium bovis transmission at the wildlife–livestock-human interface is driven by contact with infected animals or through sharing water and grazing resources, following which the infection in livestock may pose a zoonotic risk to farmers and consumers of infected milk (Rudo et al., 2020).
Most of the rural areas of Ethiopia, people drink raw milk and do have extremely close attachment with their animals and that intensifies the transmission of BTB from livestock to human beings. Detection of M. bovis from raw milk (Kiros, 1998) confirms the existing problem and the potential risk of the infection in humans. Transmission of M. bovis at the livestock wildlife or human-animal interface occurs essentially because of overlap in their territories (Alelign, 2019).
4. Conclusion and Recommendations
Although there are many studies reports that the prevalence of BTB in Ethiopia is reported to be high similar to other developing countries. However, no studies concerning the burden of the disease in wildlife and human beings were undertaken and this indicates that BTB is not well studied in the country. Human-livestock-wildlife interaction is dynamic. There are no sufficient studies clearly indicating the role of humans, livestock, wildlife and their environment with respect to the transmission dynamics of Mycobacterium bovis in the Ethiopian pastoral areas.
Mycobacterium bovis is an example of a pathogen shared at the human-livestock-wildlife interface. Therefore, studies concerning the burden of the diseases in wildlife, livestock and human beings should be undertaken.
References
- Alelign, A. (2019) Tuberculosis in Farmers and Their Cattle in Smallholder Farming System in South Gondar Zone of Northwest Ethiopi... Farming System in South Gondar Zone of Northwest.
- Alemayehu Regassa, Girmay Medhin and Gobena Ameni (2008) ‘Bovine tuberculosis is more prevalent in cattle owned by farmers with active tuberculosis in central Ethiopia’, Veterinary Journal, 178(1), pp. 119–125. [CrossRef]
- Almaw, G. et al. (2021) ‘The variable prevalence of Bovine tuberculosis among dairy herds in Central Ethiopia provides opportunities for targeted intervention’, PLoS ONE, 16(7 July), pp. 1–17. [CrossRef]
- Aman, E., Dessalegn, B. and Masrie, O. (2017) ‘HSOA Archives of Zoological Studies A Review on: Current Diagnostic Techniques of Bovine tuberculosis’, pp. 1–6. [CrossRef]
- Amato, B. et al. (2018) ‘Molecular epidemiology of Mycobacterium tuberculosis complex strains isolated from livestock and wild animals in Italy suggests the need for a different eradication strategy for Bovine tuberculosis’, Transboundary and Emerging Diseases, 65(2), pp. e416–e424. [CrossRef]
- Amissah-Reynolds, P.K. (2020) ‘Zoonotic Risks from Domestic Animals in Ghana’, International Journal of Pathogen Research, 4(3), pp. 17–31. [CrossRef]
- Arnot, L.F. and Michel, A. (2019) ‘Challenges for controlling Bovine tuberculosis in South Africa’, pp. 1–8.
- Ashmi, C. (2019) ‘Evaluation of Role of Xpert MTB/RIF in Diagnosis of Extra Pulmonary Tuberculosis in Patients attending Tertiary Care Hospital’, p. 123. Available at: http://repository-tnmgrmu.ac.in/11125/.
- Ayele, W.Y. et al. (2004) ‘Bovine tuberculosis: an old disease but a new threat to Africa’, 8(February), pp. 924–937.
- Bhuachalla, D.N., Corner, L.A.L. and More, S.J. (2015) ‘The role of badgers in the epidemiology of Mycobacterium bovis infection (tuberculosis) in cattle in the United Kingdom and the Republic of Ireland: current perspectives on control strategies’, pp. 27–38.
- Biratu, N. et al. (2014) ‘Epidemiology of Bovine tuberculosis in Butajira, Southern Ethiopia: A cross-sectional abattoir-based study’, African Journal of Microbiology Research, 8(33), pp. 3112–3117. [CrossRef]
- Borham, M. et al. (2022) ‘Review on Bovine tuberculosis: An Emerging Disease Associated with Multidrug-Resistant Mycobacterium Species’, Pathogens, 11(7), p. 715. [CrossRef]
- Buddle, B.M., Vordermeier, H.M. and Chambers, M.A. (2018) ‘Efficacy and Safety of BCG Vaccine for Control of Tuberculosis in Domestic Livestock and Wildlife’, 5(October), pp. 1–17. [CrossRef]
- Callaby, R. et al. (2020) ‘Genetic Diversity of Cameroon Cattle and a Putative Genomic Map for Resistance to Bovine tuberculosis’, Frontiers in Genetics, 11(November), pp. 1–17. [CrossRef]
- Daniel Wambua, M. (2015) ‘ESTIMATING THE PREVALENCE OF BOVINE TUBERCULOSIS (BTB) USING INDIRECT ELISA TEST IN SELECTED COUNTIES OF KENYA A Thesis Submitted in Partial Fulfilment of Requirements for Master’s degree in Veterinary Medicine’.
- Dejene, S.W. et al. (2016) ‘Risk factors for Bovine tuberculosis (bTB) in cattle in Ethiopia’, PLoS ONE, 11(7), pp. 1–16. [CrossRef]
- Demelash, B. et al. (2009) ‘Prevalence of Bovine tuberculosis in Ethiopian slaughter cattle based on post-mortem examination’, Tropical Animal Health and Production, 41(5), pp. 755–765. [CrossRef]
- Deneke, T.T. et al. (2022) ‘Milk and meat consumption patterns and the potential risk of zoonotic disease transmission among urban and peri-urban dairy farmers in Ethiopia’, BMC Public Health, 22(1), pp. 1–17. [CrossRef]
- Desta, G.B. et al. (2022) ‘Factors associated with zoonosis and reverse zoonosis of mycobacterium tuberculosis and mycobacterium bovis in Ethiopia’, International journal of health sciences, 6(April), pp. 1630–1653. [CrossRef]
- Dibaba, A.B. and Kriek, N.P.J. (2019) The Control of Bovine tuberculosis in Africa.
- Dinka, H. and Duressa, A. (2011) ‘Prevalence of Bovine tuberculosis in Arsi Zones of Oromia, Ethiopia’, African Journal of Agricultural Research, 6(16), pp. 3853–3858. [CrossRef]
- Falanga, A. et al. (2016) ‘Marine antimicrobial peptides: Nature provides templates for the design of novel compounds against pathogenic bacteria’, International Journal of Molecular Sciences, 17(5). [CrossRef]
- De Garine-Wichatitsky, M. et al. (2013) ‘A review of Bovine tuberculosis at the wildlife-livestock-human interface in sub-Saharan Africa’, Epidemiology and Infection, 141(7), pp. 1342–1356. [CrossRef]
- Gormley, E. (no date) ‘Diagnosis of Mycobacterium bovis infection in cattle by use of the gamma-interferon (Bovigam ®) assay’. [CrossRef]
- Gudina Mekonnen, A. and Wakgari Oljira, F. (2022) ‘Review on epidemiology of bovine hemoparasites in Ethiopia’, Insights in Veterinary Science, 6(1), pp. 013–016. [CrossRef]
- Hind, T. (2013) ‘THE NATIONAL FARMERS ’ UNION STONELEIGH PARK, UK BOVINE TUBERCULOSIS AND BADGER CONTROL RESEARCH’, (June).
- Humblet, M.F., Boschiroli, M.L. and Saegerman, C. (2009) ‘Classification of worldwide Bovine tuberculosis risk factors in cattle: A stratified approach’, Veterinary Research, 40(5). [CrossRef]
- Katale, B.Z. et al. (2012) ‘Bovine tuberculosis at the human-livestock-wildlife interface: Is it a public health problem in Tanzania?: A review’, Onderstepoort Journal of Veterinary Research, 79(2), pp. 1–8. [CrossRef]
- Khan, M.Z. and Zahoor, M. (2018) ‘An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies’, Tropical Medicine and Infectious Disease, 3(2). [CrossRef]
- Lekko, Y.M. et al. (2020) ‘Mycobacterium tuberculosis complex in wildlife: Review of current applications of antemortem and postmortem diagnosis’, Veterinary World, 13(9), pp. 1822–1836. [CrossRef]
- Medley, G.F. et al. (2009) ‘Herd and individual animal risks associated with Bovine tuberculosis skin test positivity in cattle in herds in south west England’, 92, pp. 188–198. [CrossRef]
- Megersa, B.B. (2010) ‘An epidemiological study of major camel diseases in the Borana lowland, Southern Ethiopia’, (58).
- Mohamed, A. (2020) ‘Bovine tuberculosis at the human – livestock – wildlife interface and its control through one health approach in the Ethiopian Somali Pastoralists: A review’, One Health, 9(August 2019), p. 100113. [CrossRef]
- Moiane, I. et al. (2014) ‘Prevalence of Bovine tuberculosis and risk factor assessment in cattle in rural livestock areas of Govuro district in the southeast of Mozambique’, PLoS ONE, 9(3). [CrossRef]
- Muhammed, C., Seboka, F. and Tibesso, G. (2020) ‘Journal of Epidemiology and Infectious Diseases Review on Epidemiological Features of Mycobaterium bovisat the Human, Cattle and Wildlife Interface in Ethiopia’, 1(1), pp. 1–15. [CrossRef]
- Palmer, M.V. (2013) ‘Mycobacterium bovis: Characteristics of Wildlife Reservoir Hosts’, 60, pp. 1–13. [CrossRef]
- Phipps, E. et al. (2019) ‘Bovine tuberculosis in working foxhounds: lessons learned from a complex public health investigation’.
- Raffo, E. et al. (2017) ‘Effect of Mycobacterium avium subsp. paratuberculosis (MAP) infection on the diagnostic accuracy for Mycobacterium bovis (M. bovis) infection under field conditions in cattle belonging to low M. bovis prevalence herds’, Tropical Animal Health and Production, 49(4), pp. 771–775. [CrossRef]
- Roex, N. et al. (2016) ‘Disease Control in Wildlife: Evaluating a Test and Cull Programme for Bovine tuberculosis in African Buffalo’, 63, pp. 647–657. [CrossRef]
- Rudo, P., Id, S. and Kelen, C. Vander (2020) ‘Risk practices for Bovine tuberculosis transmission to cattle and livestock farming communities living at wildlife-livestock-human interface in northern KwaZulu Natal, South Africa’, pp. 1–18. [CrossRef]
- Sandeep, P. et al. (2018) ‘Zoonotic Tuberculosis: Tuberculosis: A A Concern Concern and and Strategies Strategies to to Combat’. [CrossRef]
- Schiller, I. et al. (2010) ‘Bovine tuberculosis: A Review of Current and Emerging Diagnostic Techniques in View of their Relevance for Disease Control and Eradication’, 57, pp. 205–220. [CrossRef]
- Sharma, A. et al. (2021) ‘Tuberculosis: An Overview of the Immunogenic Response, Disease Progression, and Medicinal Chemistry E ff orts in the Last Decade toward the Development of Potential Drugs for Extensively Drug- Resistant Tuberculosis Strains’. [CrossRef]
- Szewczyk, R. et al. (2013) ‘Rapid method for Mycobacterium tuberculosis identification using electrospray ionization tandem mass spectrometry analysis of mycolic acids’, Diagnostic Microbiology and Infectious Disease, 76(3), pp. 298–305. [CrossRef]
- Tschopp, R. (2015) ‘Bovine tuberculosis at the Human – Livestock – Wildlife Interface in Sub-Saharan Africa’, pp. 163–175.
- Vilchèze, C. et al. (2014) ‘Phosphorylation of KasB Regulates Virulence and Acid-Fastness in Mycobacterium tuberculosis’, PLoS Pathogens, 10(5). [CrossRef]
- Vlasova, A.N. and Saif, L.J. (2021) ‘Bovine Immunology: Implications for Dairy Cattle’, Frontiers in Immunology, 12(June), pp. 1–18. [CrossRef]
- Wobeser, G. (2009) ‘Review Article Compte rendu Bovine tuberculosis in Canadian wildlife: An updated history’, 50(November), pp. 1169–1176.
- Zeru, F. et al. (2014) ‘Prevalence of Bovine tuberculosis and assessment of C attle owners ’ awareness on its public health implication in and around Mekelle, Northern Ethiopia’, 6(June), pp. 159–167. [CrossRef]
- Zinsstag, J. and Stephan, R. (2018) ‘Prevalence, Molecular Characterization, Transmission Dynamics and Cost Analysis of Bovine tuberculosis in Morocco INAUGURALDISSERTATION zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universitat Basel von Hind Yahyaoui-Azami Aus Morocco Basel, 2018’.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).