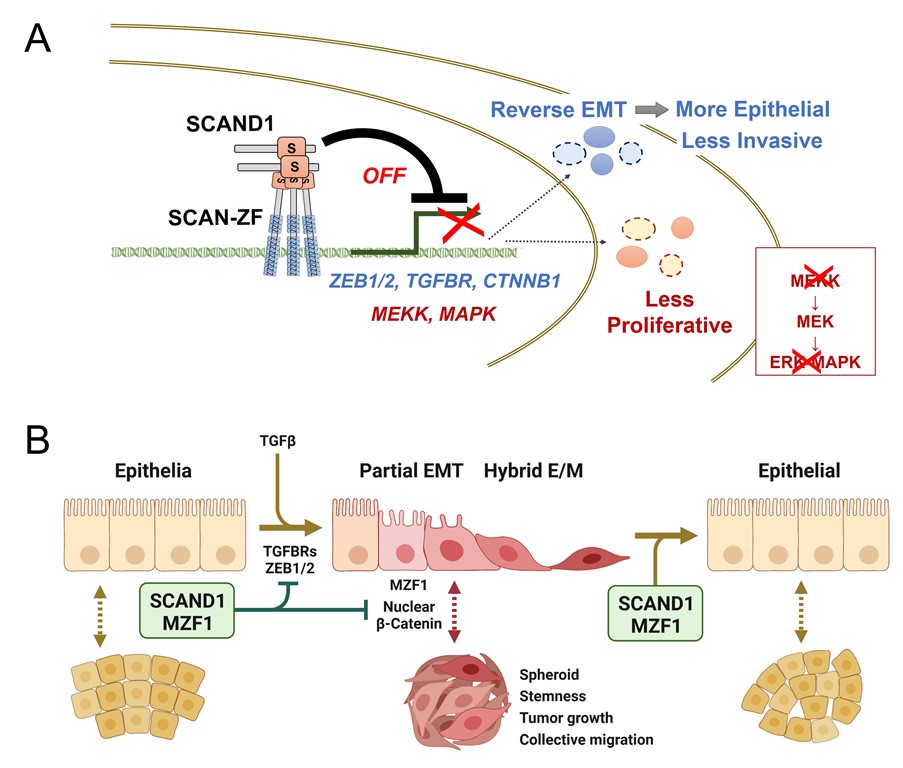
Epithelial-mesenchymal transition (EMT) is a reversible cellular program that transiently places epithelial (E) cells into pseudo-mesenchymal (M) cell states. The malignant progression and resistance of many types of carcinomas depends on EMT activation, partial EMT and hybrid E/M status in neoplastic cells. EMT is activated by tumor microenvironmental TGFβ signal and EMT-inducing transcription factors, such as ZEB1/2 in tumor cells. However, reverse EMT factors are less studied. We demonstrate that transcription factor SCAND1 can revert mesenchymal and hybrid E/M phenotype of cancer cells to a more epithelial, less invasive status and inhibit their proliferation and migration. SCAND1 is a SCAN domain-containing protein and hetero-oligomerizes with SCAN-zinc finger transcription factors, such as MZF1, for accessing DNA and transcriptional co-repression of target genes. We found that SCAND1-MZF1 co-expression and interaction correlated with maintaining epithelial features, whereas the simultaneous loss of SCAND1 and MZF1 correlated with mesenchymal features of tumor cells. Overexpression of SCAND1 over endogenous MZF1 in DU-145 prostate cancer cells reverted their hybrid E/M status into cobblestone morphology with increased epithelial adhesion by E-cadherin and β-catenin relocation. Consistently, co-expression analysis in TCGA PanCancer Atlas revealed that both SCAND1 and MZF1 co-express and are negatively correlated with EMT driver genes, including CTNNB1, ZEB1, ZEB2 and TGFBR, in prostate tumor specimens. In addition, SCAND1 overexpression suppressed tumor cell proliferation by reducing the MAP3K-MEK-ERK signaling pathway. Of note, SCAND1-overexpressing DU-145 cells migrated slower than control cells with decreased lymph node metastasis of prostate cancer in a mouse tumor xenograft model. Kaplan-Meyer analysis showed high expression of MZF1 and SCAND1 to correlate with better prognoses in pancreatic cancer and head and neck cancers, although with poorer prognosis in kidney cancer. Overall, these data suggest that the combination of SCAND1-MZF1 complexes may revert the EMT mechanism in cancer to establish an epithelial phenotype. These effects seem to include co-repression of EMT-driver genes and suppression of tumor cell proliferation via inhibition of the MAP3K-MEK-ERK signaling pathway.