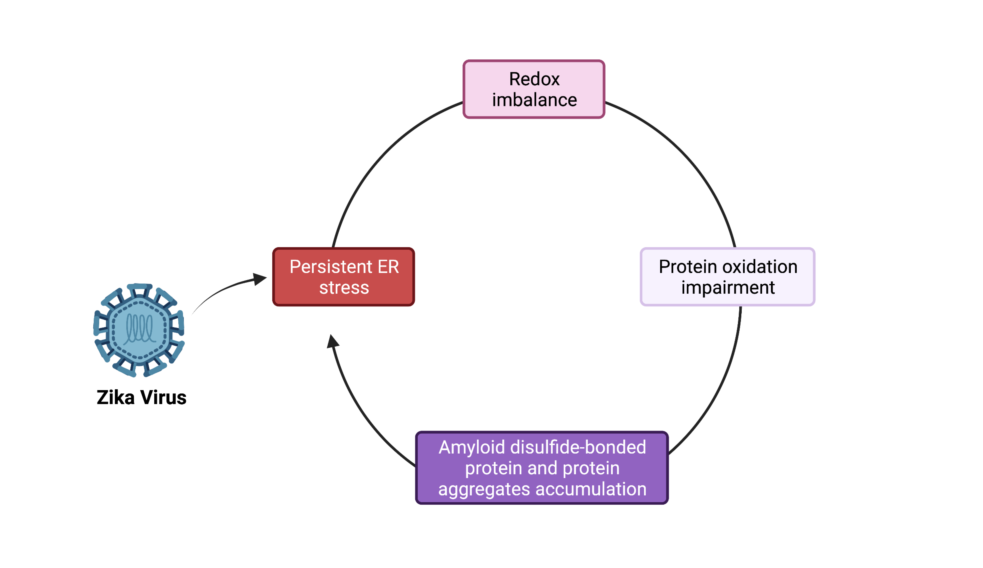
Flaviviruses replicate in membrane factories associated with the endoplasmic reticulum (ER). Significant levels of flavivirus viral protein accumulation contribute to ER stress. As a consequence, the host cell exhibits an Unfolded Protein Response (UPR), subsequently stimulating appropriate cellular responses such as adaptation, autophagy or apoptosis. The correct redox conditions of this compartment are essential to form native disulfide bonds in proteins. ZIKA virus (ZIKV) has ability to induce persistent ER stress leading to activation of UPR pathways. In this study, we wondered whether ZIKV affects the redox balance and consequently the oxidative protein folding in the ER. We found that ZIKV replication influences redox state leading to aggregation of viral envelope protein as amyloid-like structures in the infected cells.