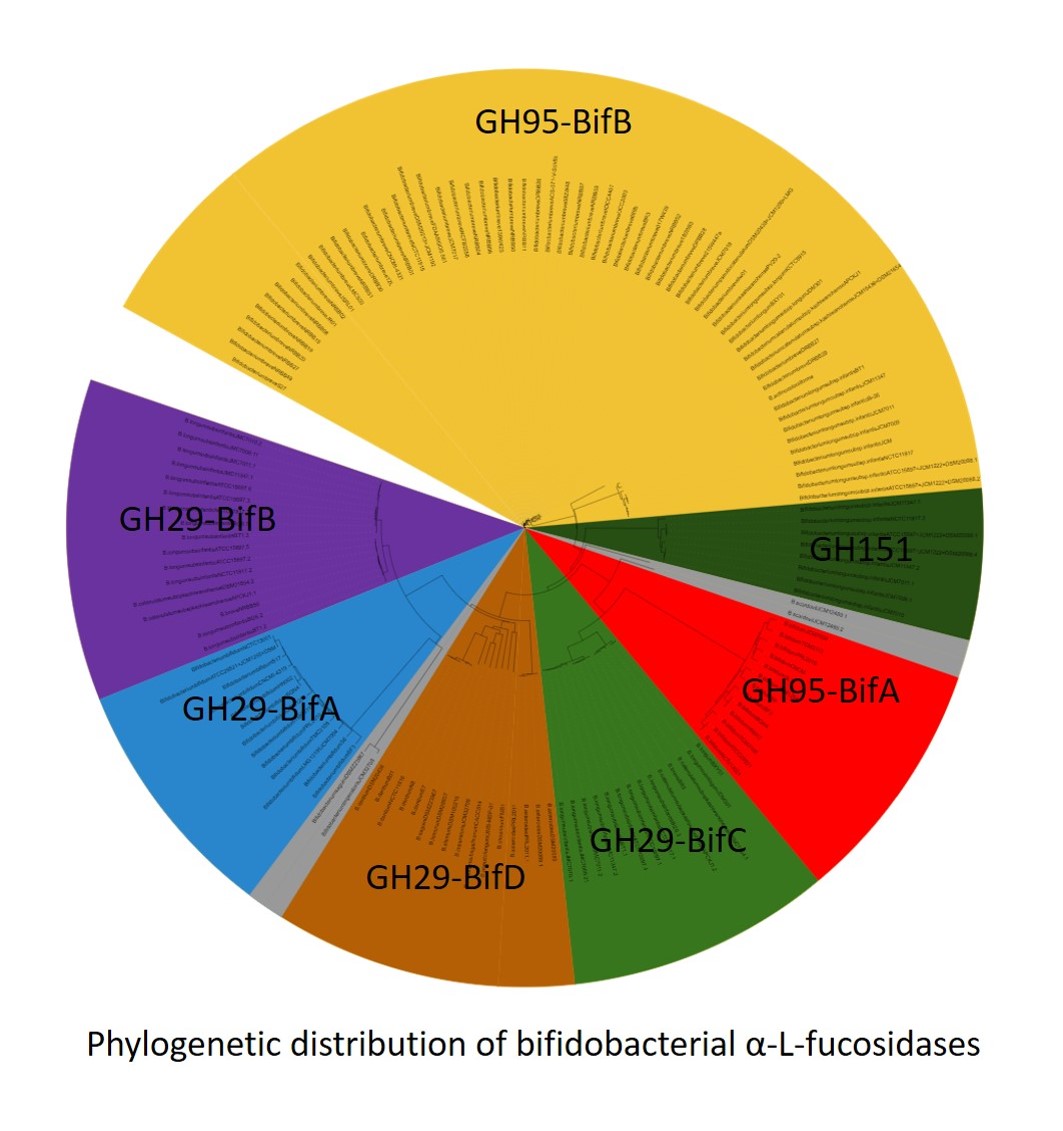
Fucosylated carbohydrates and glycoproteins from human breast milk are essential for the development of the gut microbiota in early life because they are selectively metabolized by bifidobacteria. In this regard, α-L-fucosidases play a key role in this successful bifidobacterial colonization allowing the utilization of these substrates. Although a considerable number of α-L-fucosidases from bifidobacteria have been identified by computational analysis, only a few of them have been characterized. Hitherto, α-L-fucosidases are classified into 3 families, GH29, GH95 and GH151 based on their catalytic structure. However, bifidobacterial α-L-fucosidases belonging to a particular family show significant differences in their sequence. Because this fact could underlie distinct phylogenetic evolves, here extensive similarity searches and comparative analyses of the bifidobacterial α-L-fucosidases identified were carried out with the assistance of previous physicochemical studies available. This work reveals 4 and 2 paralogue bifidobacterial fucosidase groups within GH29 and GH95 families, respectively. Moreover, Bifidobacterium logum subsp. infantis species exhibited the greatest number of phylogenetic lineages in their fucosidases clustered in every family GH29, GH95 and GH151. Since α-L-fucosidases phylogenetically descended from other glycosyl hydrolase families, we hypothesized that could exhibit additional glycosidase activities other than fucosidase, raising the possibility about their application to transfucosylate other substrates than lactose in order to synthesis novel prebiotics.