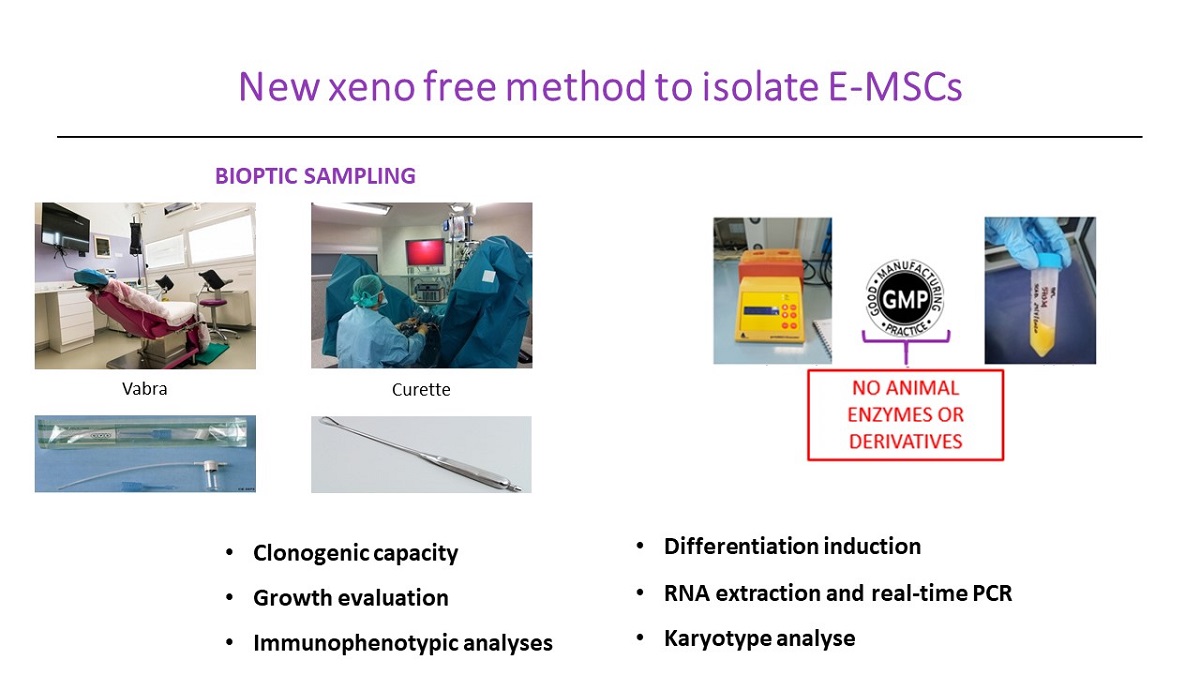
The cyclic regeneration of human endometrium is guaranteed by the proliferative capacity of Endometrial Mesenchymal Stromal Cells (E-MSCs). Due to this, the autologous infusion of E-MSCs has been proposed to support endometrial growth in a wide range of gynecological diseases. We aimed to compare two different endometrial sampling methods, the surgical curettage and the Vacuum Aspiration Biopsy Random Assay, and to validate a novel xeno-free method to culture human E-MSCs. Six E-MSCs cell lines were isolated after a mechanical tissue homogenization and cultured using human platelet lysate. E-MSCs were characterized for the colony formation capacity, proliferative potential and multilineage differentiation. The expression of mesenchymal and stemness markers was tested by FACS analysis and Real-Time PCR, respectively. Chromosomal alterations were evaluated by karyotype analysis, whereas tumorigenic capacity and invasiveness were tested by soft agar assay. Both endometrial sampling techniques allowed to efficiently isolate and expand E-MSCs using a xeno-free method preserving their mesenchymal and stemness phenotype, proliferative potential and multi-lineage differentiation ability during the culture. No chromosomal alterations and invasive/tumorigenic capacity were observed. Herein we report the first evidence of efficient E-MSCs isolation and culture in Good Manufacturing Practice compliance conditions, suggesting Vabra endometrial sampling as alternative to surgical curettage.