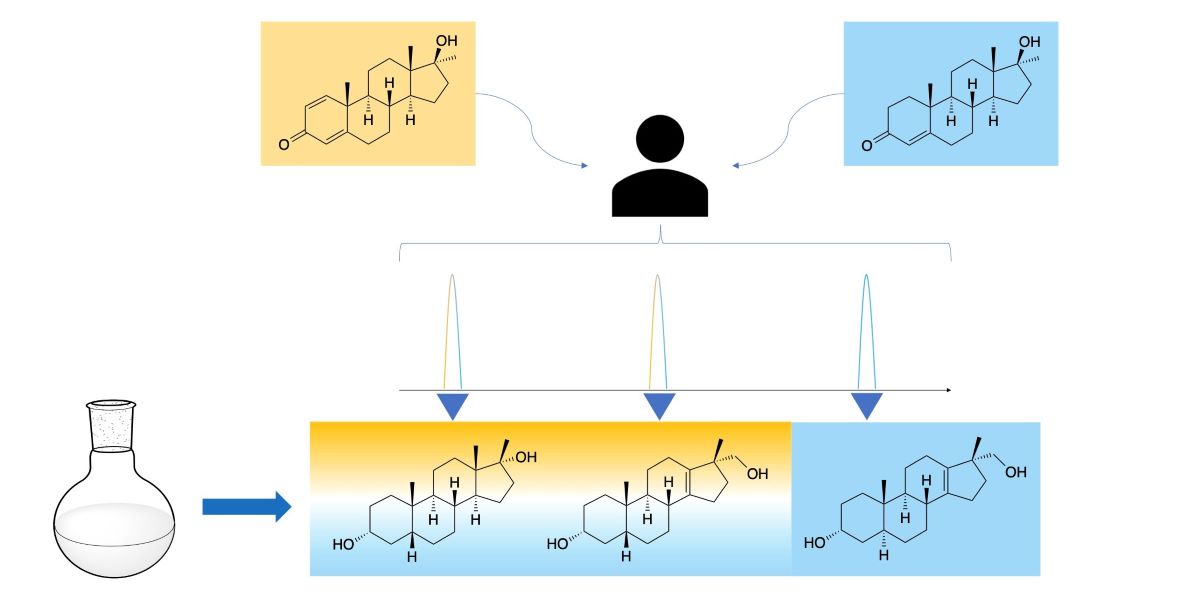
Metandienone and methyltestosterone are orally available anabolic-androgenic steroids with a 17α-methyl structure that are prohibited in sports but are frequently detected in anti-doping analysis. After the previously reported detection of long-term metabolites with a 17ξ-hydroxymethyl-17ξ-methyl-18-nor-5ξ-androst-13-en-3ξ-ol structure in the chlorinated metandienone analog dehydrochloromethyltestosterone (“Oral Turinabol”), in this study we investigated the formation of similar metabolites of metandienone and 17α-methyltestosterone with a rearranged D-ring and a fully reduced A-ring. Using a semi-targeted approach including synthesis of reference compounds, two diastereomeric substances, viz. 17α-hydroxymethyl-17β-methyl-18-nor-5β-androst-13-en-3α-ol and its 5α-analog, were identi-fied following an administration of methyltestosterone. In post-administration urines of metandienone, only the 5β-metabolite was detected. Additionally, 3α,5β-tetrahydro-epi-methyltestosterone was identified in the urines of both administrations besides the classical metabolites included in the screening procedures. Besides their applicability for anti-doping analysis, the results provide new hypotheses on the metabolism of 17α-methyl steroids with respect to the order of reductions in the A-ring and the participation of different enzymes.