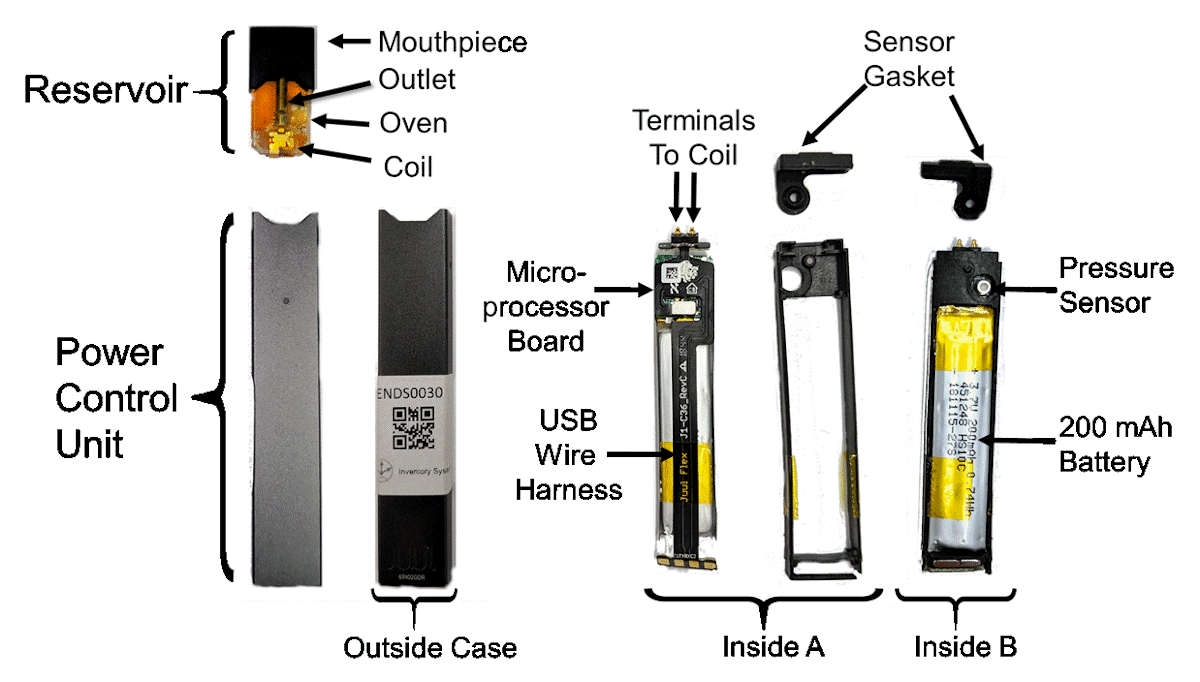
The popularity of electronic cigarettes in the United States and around the world has led to a startling rise in youth nicotine use. The Juul e-cigarette was introduced in the U.S. market in 2015 and had captured approximately 13% of the U.S. market by 2017. Unlike many other contemporary electronic cigarette companies, the founders behind the Juul® e-cigarette approached their product launch like a traditional high-tech start-up company, not like a tobacco company. This article presents a case study of Juul’s corporate and product development history in the context of US regulatory actions. The objective of this article is to demonstrate the value of government-curated archives as leading indicators which can (a) provide insight into emergent technologies and (b) inform emergent regulatory science research questions. A variety of sources were used to gather data about the Juul e-cigarette and the corporations that surround it. Sources included government agencies, published academic literature, non-profit organizations, corporate and retail websites, and the popular press. Data was disambiguated, authenticated, and categorized prior to being placed on a timeline of events. A timeline of four significant milestones, nineteen corporate filings and events, twelve US regulatory actions, sixty four patent applications, eighty seven trademark applications, twenty three design patents and thirty two utility patents related to Juul Labs and its associates is presented spanning the years 2004 thought 2020. This work demonstrates the probative value of findings from patent, trademark, and SEC filing literature in establishing a premise for emergent regulatory science research questions which may not yet be supported by traditional archival research literature. The methods presented here can be used to identify key aspects of emerging technologies before products actually enter the market, this shifting policy formulation and problem identification from a paradigm of being reactive in favor of becoming proactive. Such a proactive approach may permit anticipatory regulatory science research and ultimately shorten the elapsed time between market technology innovation and regulatory response.