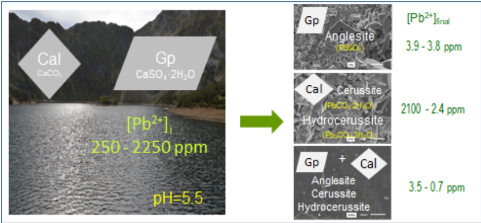
Anthropogenic lead pollution is an environmental problem that threatens the quality of soils and waters and endangers living organisms in numerous surface and subsurface habitats. Lead coprecipitation on mineral surfaces through dissolution-recrystallization processes has long term effects on lead bioavailability. Gypsum and calcite are among the most abundant and reactive rock forming minerals present in numerous geological settings. In this work, we study the interaction of slightly acidic (pHi = 5.5) Pb-bearing aqueous solutions ([Pb]i = 1 mM and 10 mM) with crystals of gypsum and /or calcite under atmospheric conditions. This interaction results in a reduction of the concentration of lead in the liquid phase due to the precipitation of newly formed Pb-bearing solid phases. The extent of this Pb removal mainly depends on the nature of the primary mineral phase involved in the interaction. Thus, when gypsum is the only solid phase initially present in the system the Pb-bearing liquid-gypsum interaction results in Pb removals in the 98-99.8 % range, regardless of [Pb]i. In contrast, when the interaction takes place with calcite, Pb removal strongly depends on [Pb]i. It reaches 99% when [Pb]i = 1 mM while it is much more modest (⁓13%) when [Pb]i = 10 mM. Interestingly, Pb-removal is maximized for both [Pb]i (99.9 % for solutions with [Pb]i = 10 mM and 99.7% for solutions with [Pb]i = 1 mM) when Pb-polluted solutions simultaneously interact with gypsum and calcite crystals. Despite the large Pb removals found in most of the cases studied, the final Pb concentration ([Pb]f) in the liquid phase always is well above the maximum permitted in drinking water (0.1 ppm), with the minimum ([Pb]f = 0.7 ppm) being obtained for solutions with [Pb]i =1 mM after their interaction with mixtures of gypsum and calcite crystals. This result suggests that integrating the use of mixtures of gypsum-calcite crystals might help to develop more efficient strategies for in-situ decontaminating Pb-polluted waters through mineral coprecipitation processes.