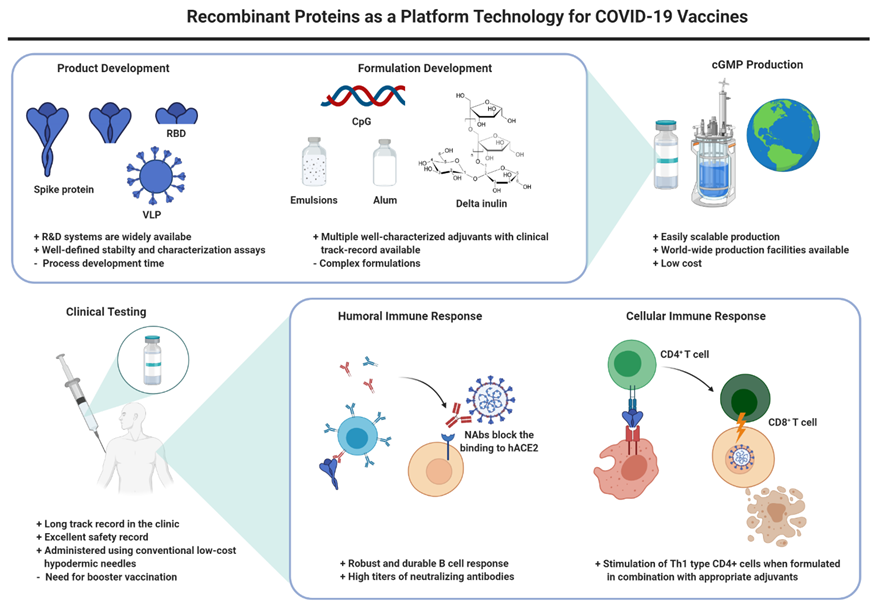
With the COVID-19 pandemic now ongoing for close to a year, people all over the world are still waiting for a vaccine to become available. The initial focus of accelerated global research and development efforts to bring a vaccine to market as soon as possible was on novel platform technologies that promised speed but had limited history in the clinic. In contrast, recombinant protein vaccines, with numerous examples in the clinic for many years, missed out on the early wave of investments from government and industry. Emerging data are now surfacing suggesting that recombinant protein vaccines indeed might offer an advantage or complement to the nucleic acid or viral vector vaccines that will likely reach the clinic faster. Here, we summarize the current public information on the nature and on the development status of recombinant subunit antigens and adjuvants targeting SARS-CoV-2 infections.