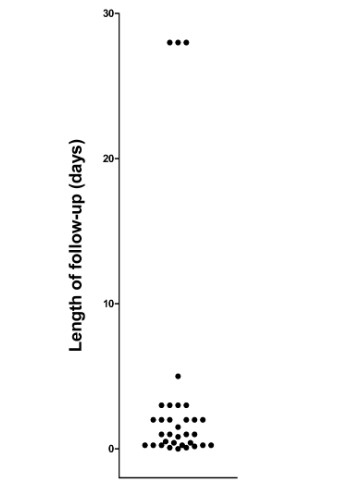
Although widely believed to be safe for use in infants and children when used as directed, increasing evidence indicates that early life exposure to paracetamol (acetaminophen) may cause long-term neurodevelopmental problems. Further, recent studies in animal models demonstrate that cognitive development is exquisitely sensitive to paracetamol exposure during early development. In this study, evidence for the claim that paracetamol is safe was evaluated using a systematic literature search. Publications on PubMed between 1974 and 2017 that contained the keywords “infant” and either “paracetamol” or “acetaminophen” were considered. Of those initial 3096 papers, 218 were identified that made claims that paracetamol was safe for use with infants or children. Of these, a total of 103 papers were identified as sources of authority for the safety claim, and 36 of those contained actual experiments designed to test safety. The 36 experiments described had a median follow-up time of 24 hours, and none monitored neurodevelopment. Further, no trial considered total exposure to drug since birth, eliminating the possibility that the effects of drug exposure on long-term neurodevelopment could be accurately assessed. On the other hand, abundant and sufficient evidence was found to conclude that paracetamol does not induce acute liver damage in babies or children when used as directed.