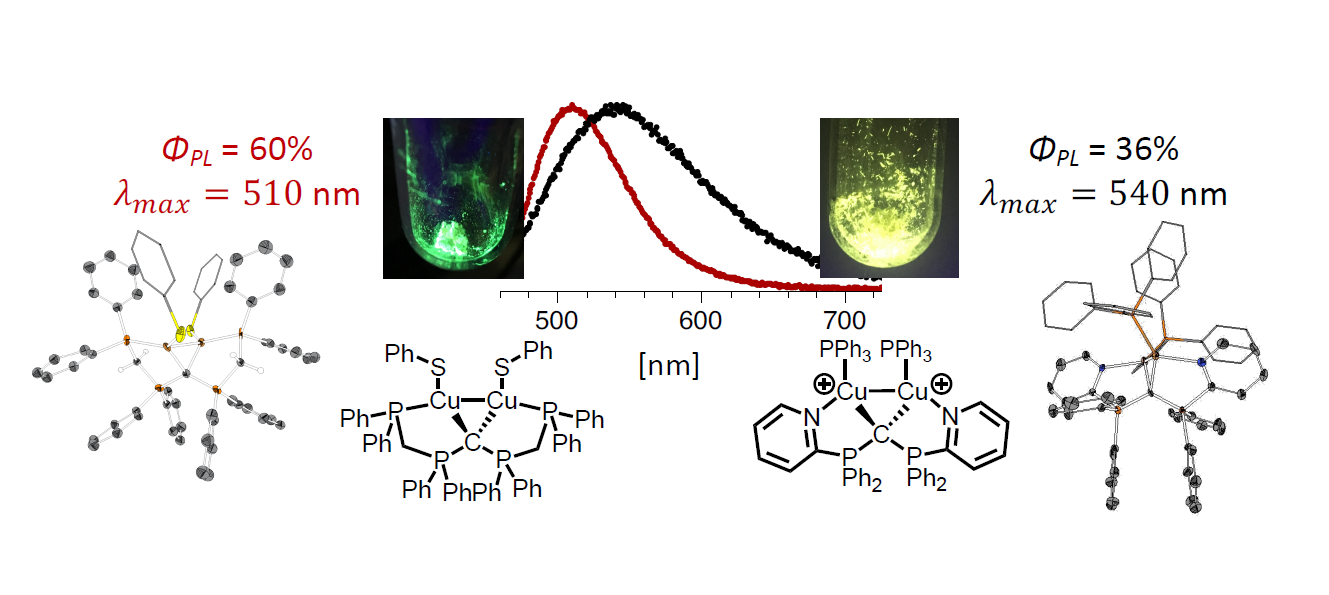
A series of dinuclear copper(I) N,C,N- and P,C,P-carbodiphosphorane (CDP) complexes using multidentate ligands CDP(Py)2 (1) and (CDP(CH2PPh2)2 (13) have been isolated and characterized. Detailed structural information was gained by single crystal XRD analyses of nine representative examples. The common structural motive is the central double ylidic carbon atom with its characteristic two lone-pairs involved into binding of two geminal L-Cu(I) fragments at Cu-Cu distances in the range 2.55 – 2.67 Å. In order to enhance conformational rigidity within the characteristic Cu-C-Cu triangle, two types of chelating side arms were symmetrically attached to each phosphorus atom: two 2-pyridyl functions in ligand CDP(Py)2 (1) and its dinuclear copper complexes 2-9 and 11, as well as two diphenylphosphinomethylene functions in ligand CDP(CH2PPh2)2 (13) and its di- and mononuclear complexes 14-18. Neutral complexes were typically obtained via reaction of 1 with Cu(I) species CuCl, CuI, and CuSPh or via salt elimination reaction of [(CuCl)2(CDP(Py)2] (2) with sodium carbazolate. Cationic Cu(I) complexes were prepared upon treating 1 with two equivalents of [Cu(NCMe)4]PF6, followed by the addition of either two equivalent of an aryl phosphine (PPh3, P(C6H4OMe)3) or one equivalent of a bisphosphine ligands DPEPhos, XantPhos or dppf. For the first time carbodiphosphorane CDP(CH2PPh2)2 (13) could be isolated upon treating its precursor [CH(dppm)2]Cl (12) with NaNH2 in liquid NH3. A protonated and a deprotonated derivative of ligand 13 were prepared and their coordination was compared to neutral CDP ligand 13. NMR analysis and DFT calculations reveal, that the most stable tautomer of 13 does not show a CDP (or carbone) structure in its uncoordinated base form. For most of the prepared complexes, photoluminescence upon irradiation with UV light at room temperature was observed. Quantum yields (PL) were determined to 36% for dicationic [(CuPPh3)2(CDP(Py)2)](PF6)2 (4) and to 60% for neutral [(CuSPh)2(CDP(CH2PPh2)2] (16).