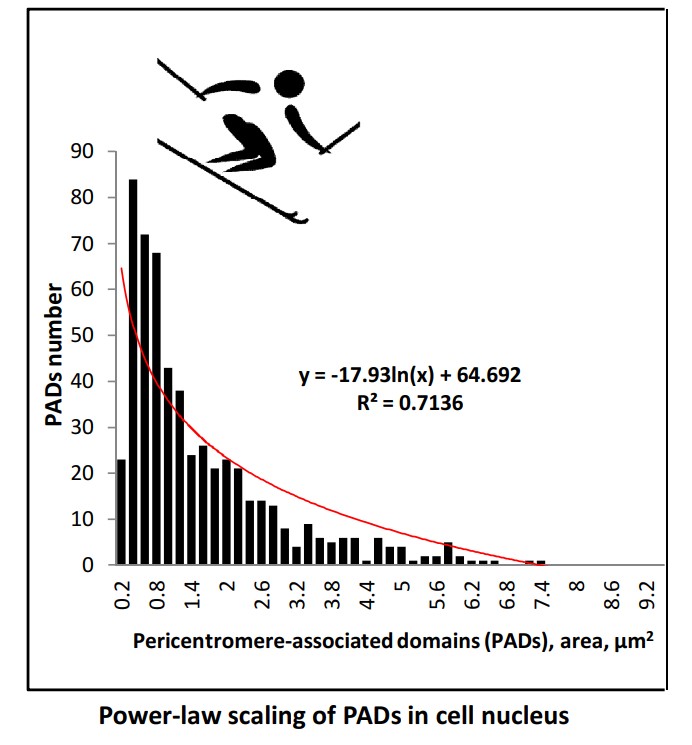
Finding out how cells with the same genome change fates in differentiation commitment is a challenge of biology. We used MCF-7 breast cancer cells treated with the ErbB2 ligand heregulin (HRG), which induces differentiation, to address if and how the constitutive pericentromere-associated domains (PADs) may be involved in this process. PAD-specific repressive heterochromatin (H3K9me3) and active euchromatin (H3K4me3) marking, centromere (CENPA) labelling, qPCR, acridine-orange-DNA structural test, and microscopic image analysis were applied. We found a two-step DNA unfolding, at 15-20 min and 60 min after HRG treatment, coinciding with bi-phasic activation of the early response genes (c-FOS family) and two steps of critical phase transition which were revealed in transcriptome studies. In control, the distribution of PAD number and size displays a power-law scaling with a boundary at the nucleolus. PADs’ clustering correlates with centromere numbers. 15 min after HRG treatment, the unravelling of PADs occurs, coinciding with the first step of euchromatin unfolding. The second step is associated with transcription of long-non-coding-RNA from satellite III DNA. We hypothesize that splitting of the PAD clusters under the critical size threshold of the silencing domain abrupts position effect variegation. It allows the first genome transcription avalanche to occur, starting differentiation commitment.