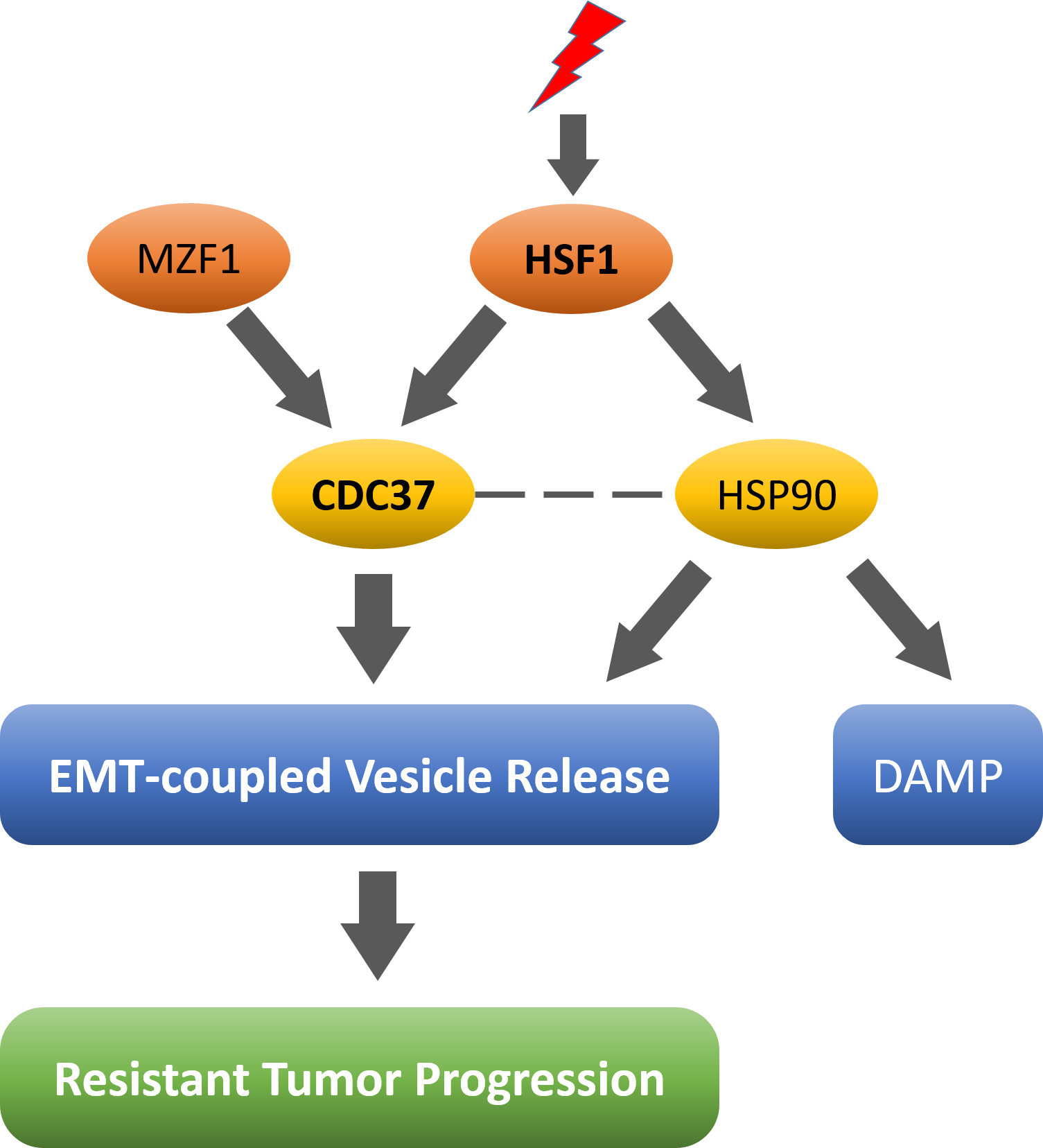
Tumor cells exhibit a resistance-associated secretory phenotype involving extracellular vesicles (EVs) and heat shock proteins (HSPs). This response occurs in response to cell stress and cancer therapeutics. HSPs are stress-responsive molecular chaperones promoting proper protein folding, while also being released from cells with EVs as well as in free form as alarmins. We have here investigated the secretory phenotype of castration-resistant prostate cancer (CRPC) cells using proteome analysis. We have also examined the roles of the key co-chaperone CDC37 in stressome release, epithelial-to-mesenchymal transition (EMT), and tumor progression. A number of HSP family members and their common receptor CD91/LRP1 were enriched at high levels in CRPC cell-derived EVs among over 700 other protein species. The small EVs (30 to 200 nm in size, potentially exosomes) were released even in a non-heated condition from the prostate cancer cells, whereas EMT-coupled release of EVs (200 to 500 nm, likely ectosomes) with associated HSP90α was increased after heat shock stress (HSS). Lactate dehydrogenase, a marker of membrane leakage/damage of cells, was also released upon HSS from the prostate cancer cells. During this stress response, intracellular CDC37 was also transcriptionally inducible by heat shock factor 1, and knockdown of CDC37 decreased EMT-coupled release of EVs. Triple knockdown of CDC37, HSP90α, and HSP90β was required for efficient reduction of the chaperone trio and to reduce tumorigenicity of the CRPC cells in vivo. Taken together, the data indicated that CDC37 and HSP90 are essential for stressome release and for tumorigenesis in resistant cancer.