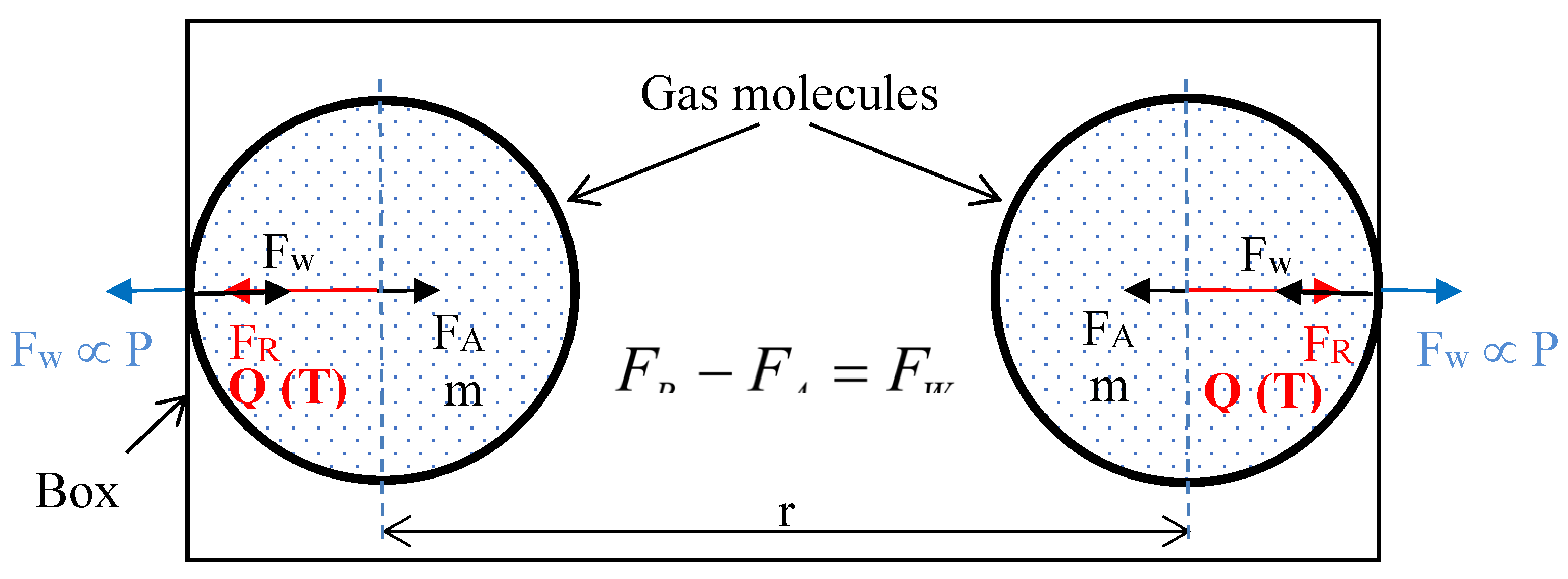
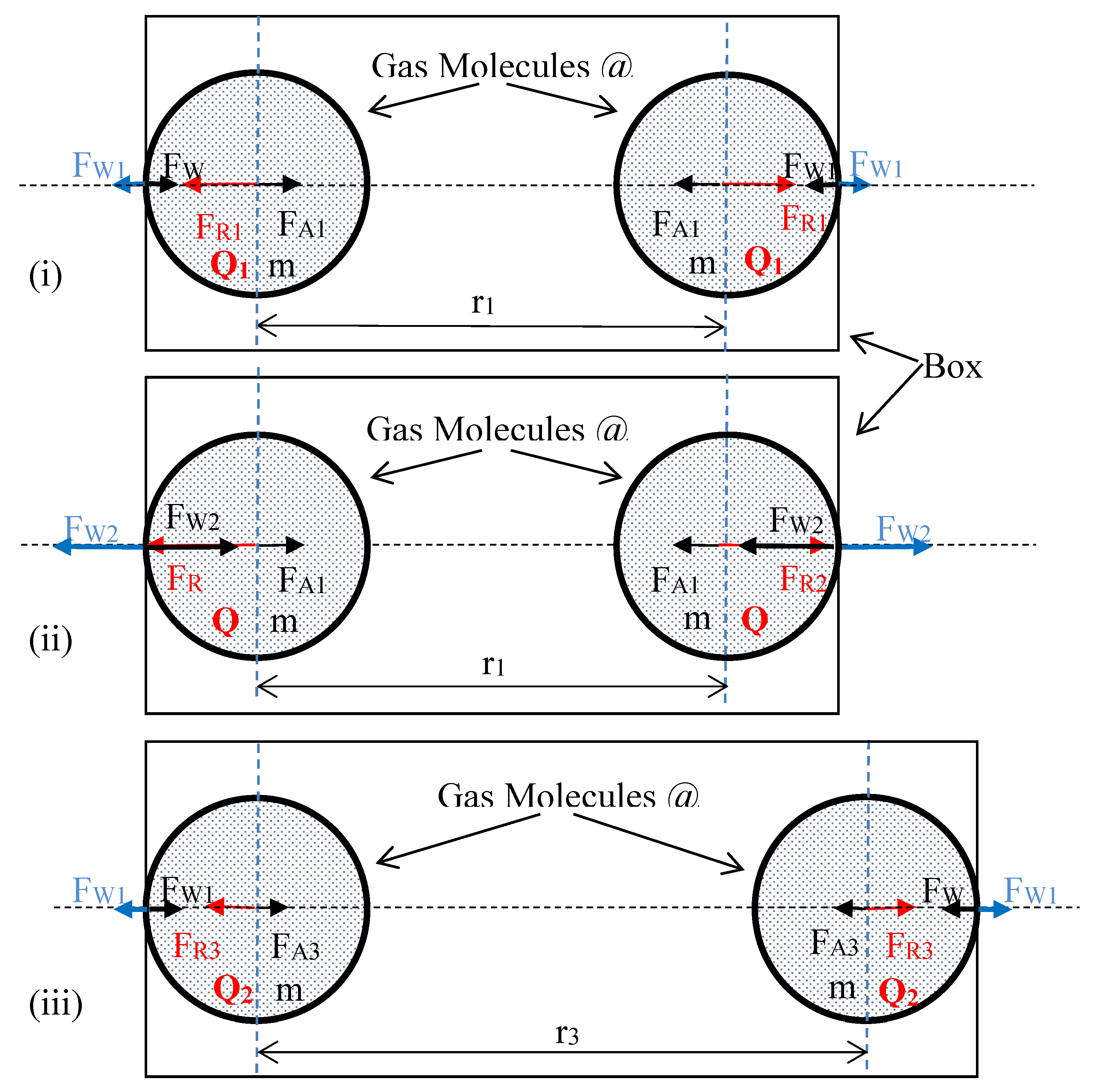
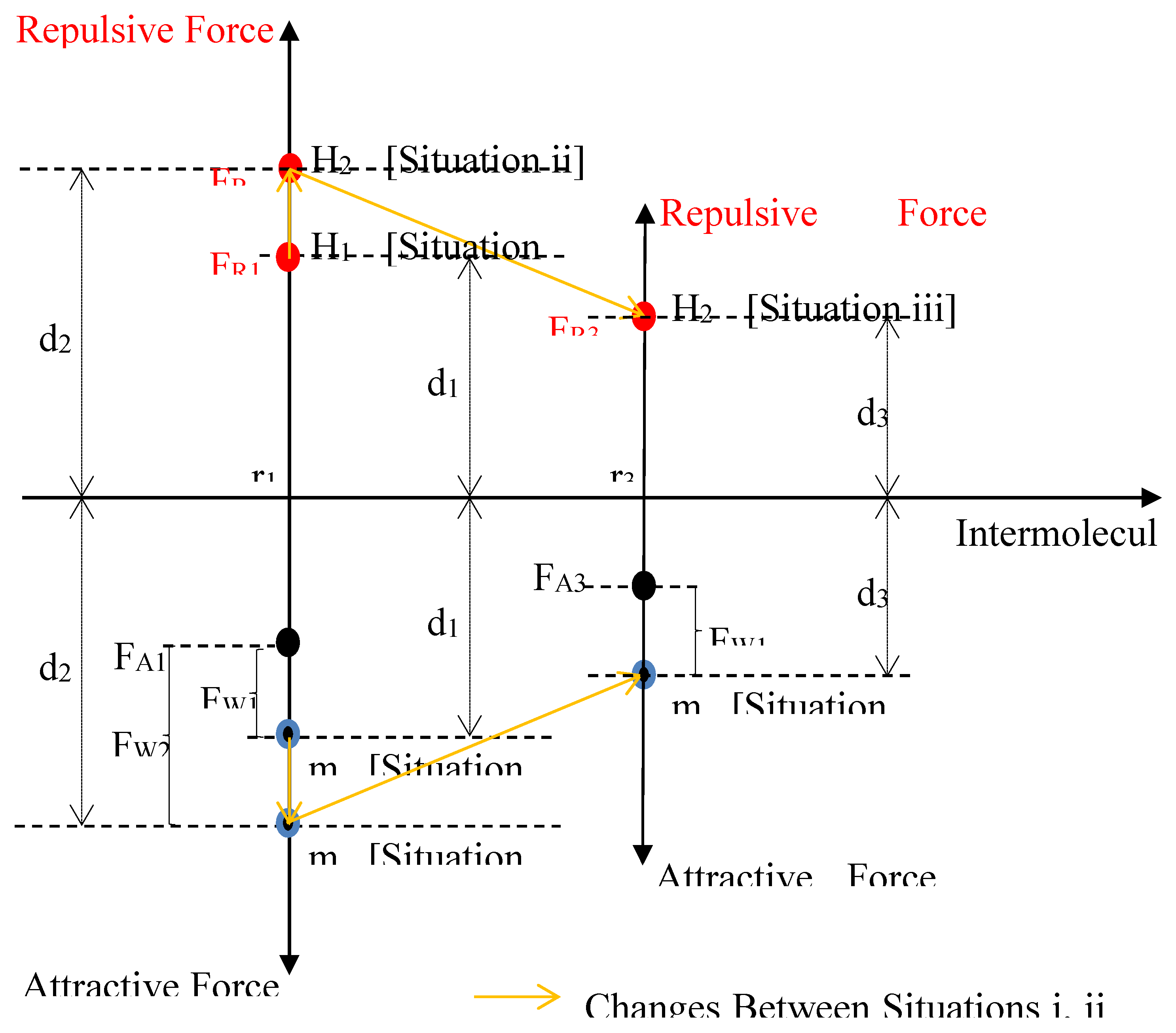
1. Introduction
An alternative mathematical model of the gravitational repulsion force and the gravitational attraction force has been developed herein, considering them as two distinct forces acting on gaseous matter. The model has been validated using experimentally determined and established data utilized in practical thermodynamic applications of mechanical engineering industries. It is a self-standing model, which requires no fitting into existing models. The presented alternative model could more effectively describe the nature of the universe at both micro and macro levels. The gravitational repulsion concept presented in the series of publications [
1,
2,
4,
5] emanating from this research program, are based on experimental observations and natural phenomena; making neither abstruse assumptions nor explanations.
The present understanding of the universe is that there are four fundamental interactions (also known as fundamental forces in classical theory): weak (subatomic), strong (subatomic), electromagnetic and gravitational. None of these fundamental forces are so far defined to be temperature (hence energy) dependent. There are, nevertheless, phenomena observed in the nature/universe (e.g.: pressure, expansion, and so on) that are dependent on temperature which is a manifestation of the thermal energy content. Both gravitational repulsion and attraction forces analytically and experimentally proved, in this research, to be dependent on the thermal energy content, thus fills the critical gap between energy and fundamental forces!
In some literature [
6,
7,
8,
9], the gravitational repulsion force is referred to as the antigravitational force. Therefore, we use both these words in our text to mean the same concept.
Presenting a new scientific revelation that fundamentally challenges our understanding of the universe, requires examining the foundations of our present understanding, viz., Newtonian and Einsteinian gravity concepts, and the kinetic theory of ideal gas. The Author would, hence briefly note:
Newtonian and Einsteinian gravity concepts, highlighting foundations of our present understanding
Early notions of the gravitational repulsion force and its recent revelations
The challenges of a major assumption in the kinetic theory of ideal gas, used in the derivation of the ideal gas equation
1.1. Brief note on Newtonian and Einsteinian Gravity Concepts:
The concept of gravity, the ‘Law of Universal Gravity’, was first proposed and mathematically derived as a relationship based on inductive reasoning of empirical observations by Newton in 1687 [
10]. This empirical solution for the gravitational force between two masses m
1 and m
2, which are distance r apart, is given in the following mathematical form (Equation (1)):
where,
G is the universal gravitational constant.
Secondly, another major interpretation of gravity was given by Einstein in 1907 as General Relativity [
11], which is the theory of gravitation where the observed gravitational effect between masses results from their warping of space-time. Therein, gravity is not considered as a force, it is a consequence of the curvature of spacetime caused by the presence of mass energy. See ‘Einstein Field Equations’ (EFE) in theory of general relativity.
The general form of EFE is given as (Equation (2)):
where,
is the Ricci curvature tensor,
is the scalar curvature,
is the metric tensor,
is the cosmological constant,
is Newton's gravitational constant,
is the speed of light in vacuum, and
is the stress–energy tensor.
Experiments and observations show that Einstein's description of gravitation accounts for several effects that are unexplained by Newton's laws, such as minute anomalies in the orbits of Mercury and other planets [
12]. General relativity also predicts novel effects of gravity, such as gravitational waves [
13], gravitational lensing [
14] and an effect of gravity on time known as gravitational time dilation [
15]. While some of the astronomical anomalies have been explained by the Einstein’s theory of general relativity, almost all the practical applications (for example all engineering applications) to date are still based precisely on the Newtonian model of gravitation.
The cosmological constant
, the energy density of space, that arises in the Albert Einstein's field equations could be closely associated with the concepts of dark energy and quintessence (hypothetical form of dark energy, postulated in explaining the accelerating expansion of the universe) [
16,
17]. However, some doubts have been raised lately on the accuracy of the cosmological constant [
18,
19,
20].
It has also been shown that by obeying local energy–momentum conservation, the EFE converges to the Newton’s law of gravitation where the gravitational field is considered weak and velocities are much less than the speed of light [
21]. It is worth noting that the empirically derived gravitational constant
in EFE is borrowed from the Newton’s universal gravitation relation; not a constant derived independently by Einstein. It could, hence, be observed that the EFE is, to some extent, related to the Newton’s law of gravity. This aspect, however, is out of the scope of this paper, thus to be discussed separately.
It also has to be noted that, under existing (Newtonian and Einsteinian) models, the gravitational force has been reckoned as a “weak force”. According to
Table 1, the gravitational force is 10
–36 weak compared to the electromagnetic force; accordingly, a negligible force.
1.2. Early Notions of Gravitational Repulsion Force:
The above discussion compares Newtonian and Einsteinian gravity concepts, highlighting foundations of our present understanding. Newton’s model describes gravitation as an attractive force, and the repulsive nature of gravity has never been entertained. It has, however, been shown that a repulsive gravitational force could manifest itself within the theory of general relativity [
23,
24,
25,
26,
27,
28].
Albert Einstein, subsequently, attempted to form a generalized theory of gravitation that would unify the gravitational and electromagnetic forces (and perhaps others), guided by a belief in a single origin for the entire set of physical laws. Models of electromagnetism and gravity were pursued by Einstein in his attempts at a unified field theory (UFT) [
29]. There was an attempt at harnessing electromagnetic interactions with gravitational interactions to induce torsion, which in turn could null out gravity. Thus, a sort of counteraction to gravity or an “antigravity” field would be the result [
30]. UFT could be a point in the history of science that the idea of antigravity surfaced.
In Newtonian and Einsteinian mechanics, what has been observed as a gravitational effect between two bodies is nothing but the
resultant effect of both gravitational repulsion and attraction forces acting simultaneously [
2]. If gravitation attraction is the only force, the universe must see a contraction instead of expansion with an acceleration [
31,
32], and would eventually collapse. To explain the accelerating expansion of the Universe there have developed numerous modified Einsteinian models, modified Poissonian models [
33] and cosmological models [
34] assuming hypothetical ‘dark energy’ [
35], and a repulsive force of the cosmological scale [
28] relating to ideas of dark matter and dark energy [
6,
7,
36]. Although cosmologists have brought in such ideas as possible reasons for expansion of the universe, they are still speculative and incomplete in explaining the observations in processes occurring/happening in the universe.
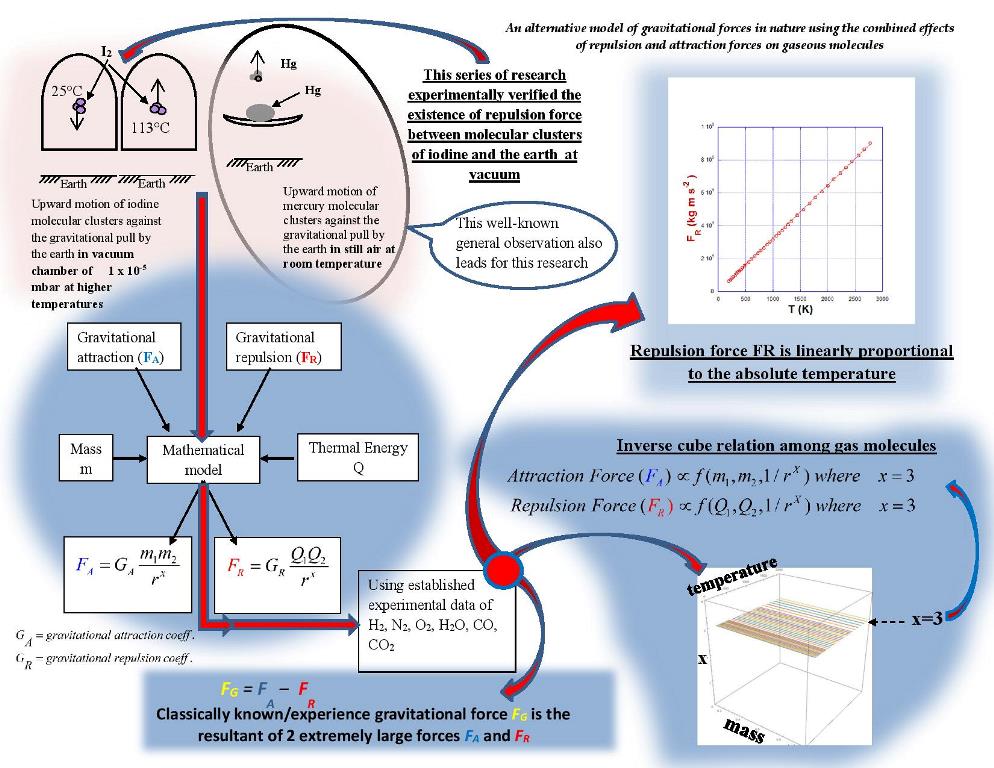
The present research makes an independent approach using of an alternative mathematical model of the gravitational repulsion force and the gravitational attraction force. No used hypothetical assumptions made. The model is validated using experimentally determined and established data utilized in practical thermodynamic applications of mechanical engineering industries.
1.3. Recent Revelation of the Gravitational Repulsion Force:
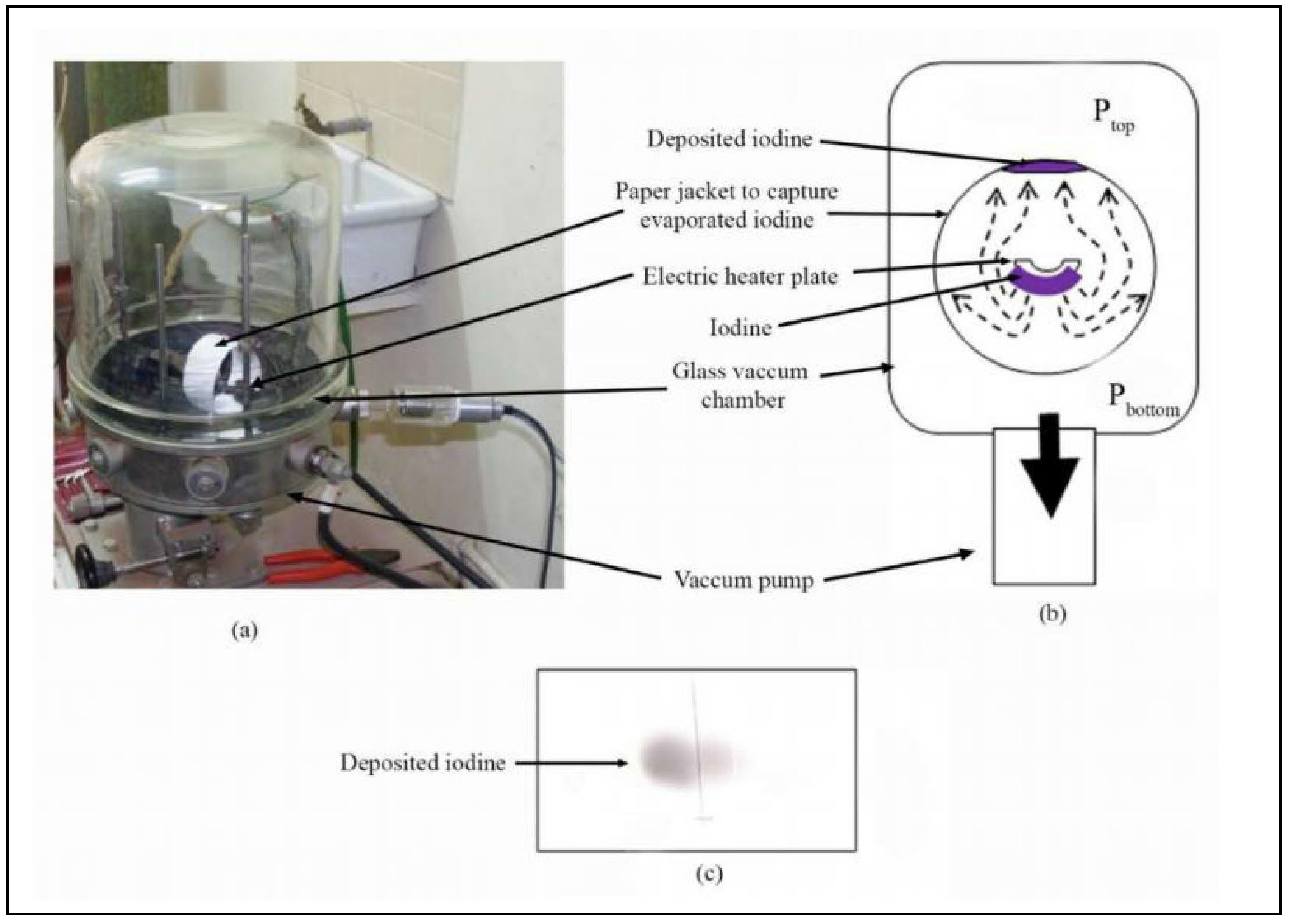
A series of experiments involving mechanistic laboratory studies [
1,
2,
4,
5] by the author, have shown that upward motion of matter against the gravitational attraction of the Earth happens due to a “cryptic” upward force; e.g., the upward movement of iodine in a vacuum[
4] and upward movement water droplets in the air [
2,
5]. A series of studies [
2,
5] on water droplets confirmed that this cryptic force is proportional to the internal thermal energy of the droplets. The said tangible experiments, lead to the understanding of
gravitational repulsion or
antigravity force which persists against the gravitational attraction force. The iodine experiment is discussed in brief, later in this paper. It has further been extended that both gravitational repulsion and attraction forces pervade our surroundings both microscopically and macroscopically [
1].
The discussion so far has briefly indicated why we need a deviation from the existing gravitational models; Newtonian ‘Law of Universal Gravity’ and Einsteinian ‘EFE’. Also, we need to deviate from a major idealistic assumption made in the kinetic theory of ideal gas. Now, our attempt is to develop a relationship to better understand the behavior of gas molecules with the force of repulsive nature on matter; antigravity.
1.4. Challenges of a Major Assumption in the Derivation of the Kinetic Theory of Ideal Gas:
As reasoned above, in application of the kinetic theory of ideal gas in derivation of the ideal gas equation, we are skeptical mainly about one major idealistic assumption [
37]:
The intermolecular force in gaseous state is zero and as such molecules exhibit no force among themselves
In deriving the ideal gas equation, one of the most fundamental forces, the gravitational attraction among matter has been overlooked, both among air molecules themselves and with the Earth. Such steep assumption does not do justice to principles of fundamental science. Even though a gas molecule has minute quantity of matter, it is susceptible to gravitational forces with all entities of matter around. Therefore, it may not be prudent to make an assumption to neglect the gravitational forces on gas molecules with entities small or large. It is accepted that the atmosphere around the Earth exists because of the gravitational force of the Earth and this is a major force between the Earth and air molecules. Even Mars with a lower gravitational attraction accommodates an atmosphere of pressure ~ 0.6% (610 Pa) of the Earth atmosphere [
38]. Also, denser atmospheres are found in planets with higher gravity such as Jupiter and Saturn, which hold even light gases such as hydrogen and helium in their atmospheres [
39].
Further, the postulates of the kinetic molecular theory of ideal gases ignore the volume occupied by the molecules of a gas. Real gases, however, show significant deviations from the behavior expected for an ideal gas [
40], e.g., Van Der Waals Equation.
1.5. Behavior of Real gas – the Van Der Waals Equation
Van der Waals modified [
41] the ideal gas equation to describe the behavior of real gases by explicitly including the effects of molecular size and intermolecular attractive forces. The modified ideal gas equation, the so-called van der Waals equation:
where
a and
b are empirical constants that are dependent on the type of gas.
an2/V2 is added to compensate intermolecular attractive forces and
nb corrects the volume term in the ideal gas equation by subtracting volume taken by the molecules in the space.
The anomalies addressed in the real gas behavior by the Van der Waals equation are only the attraction force and the volume taken by the gas molecules. The attraction force discussed by Van der Waals is considered as a week electrostatic force, and not a gravitational force.
The concept of gravitational repulsion or antigravity or matter moving against the gravitational field, is not a novel concept but one which has been under speculation over the years [
6,
7,
8,
9]. The repulsion between atoms at short distance (within the eigenvolume) has been modeled in Lennard-Jones potential [
42,
43]. This repulsion force, however, acts at very short range (~3-6 A°) usually between diatomic molecules. Van der Waals forces, nevertheless, are attractive interactions between atoms resulting from induced dipoles, and repulsive interactions resulting from overlap of the electron clouds of two atoms (electrostatic repulsion) when they get too close to each other [
44]. The total energy of Van der Waals interactions could be approximated by the Lennard-Jones expression. They, however, did not consider gravitational repulsion and gravitational attraction forces among gaseous atoms/molecules.
Recently published experiments by the author [
1,
2,
4,
5] show that there exists not only gravitational attraction forces acting on matter but also gravitational repulsion forces between them. The following section briefly recalls a groundbreaking laboratory experiment [
4] as it makes a
landmark revelation of the effect of gravitational repulsion.
4. Calculation of x, y, GR, GA, FR and FA Based on Thermodynamic Properties of Gas:
The focus of this section is to engender a reasonable
qualitative and
quantitative picture of the combined effect of gravitational repulsion and attraction forces on matter in the
gaseous state. Results would be considered and compared with conditions and observations encountered in the nature. Thermodynamic properties of gaseous nitrogen are used in the following sample calculation to elucidate the effect. (Calculations using Thermodynamic properties of hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide are also available on request). Established experimental data [
3] (published in 1948) on gas properties of nitrogen (see
Supplementary File 1) were applied to Equations 18–24, to calculate values for
x,
GR,
GA,
FR and
FA. From the said table of properties of nitrogen with average molecular mass
kg/molecule,
cv and
cp at varying temperatures (from 146.65 K to 2888.9 K), were extracted. Molecular distance and pressure were calculated as given below.
4.1. Determining Values of P and r as T Varies:
Pressure P: Guillaume Amontons was the first to empirically establish the relationship between the pressure and the temperature of a gas (~1700 CE), and Joseph Louis Gay-Lussac determined the relationship more precisely (~1800 CE). Because of this, the
P–
T relationship of gases is known as either
Amontons’ Law or
Gay-Lussac’s Law. It states that:
the pressure of a given amount of gas is directly proportional to its temperature on the absolute scale when the volume is held constant [
53]. This empirically established relationship is used to obtain the pressure of a given mass of gas held within a unit volume, as temperature is varied; with reference to the temperature 305.54 K and pressure 101.325 kPa (see
Supplementary File 1).
From the list of values in data [
3], the temperature 305.54 K was chosen, because that is the value closest to the naturally existing livable temperatures. At that temperature, it was assumed that a unit volume of the gas exists at the pressure of 1 atm (standard atmosphere), i.e., 101.325 kPa. Amontons’ Law/Gay-Lussac’s Law was used to obtain the pressure of the given mass of gas held within a unit volume, at other temperatures listed in data tables.
Mean intermolecular distance r: Distances
r1 and
r3 between adjacent nitrogen gas molecules in a unit volume having total number of molecules
n, at varying pressures relative to temperatures were calculated using the Avogadro number, provided that that the molecules are evenly distributed in the space. So, the
r is given by
[
54].
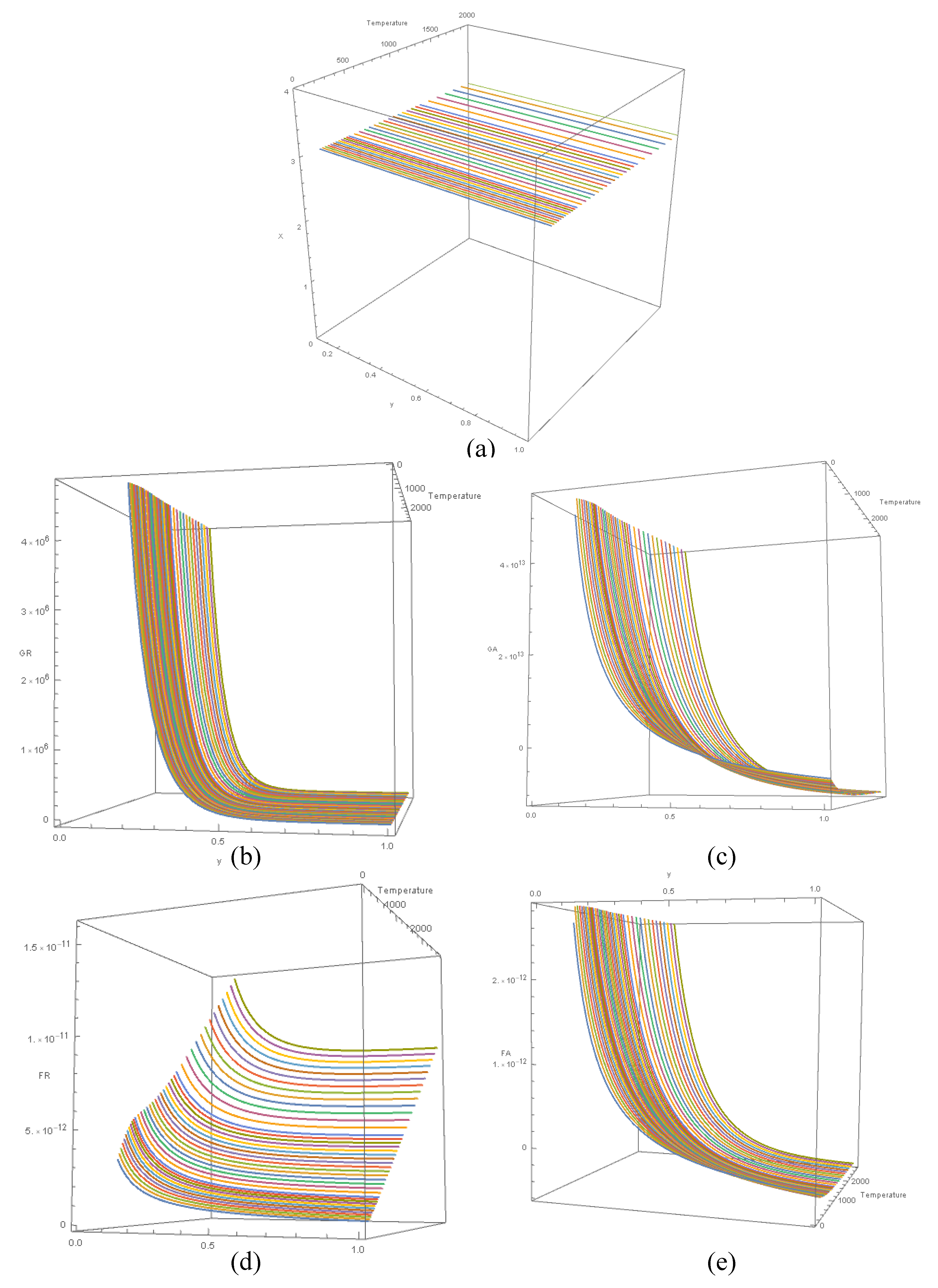
4.2. Observations of Characteristic Behaviors of x, GR, GA, FR and FA:
Characteristic behavior of
x,
GR,
GA,
FR and
FA for temperatures ranging from 194.43 K to 2888.9 K (data availability) relative to
y ranging from 0.1 to 1
(suitable range of
y was determined as 0.1 to 1 by repeated calculations) was determined based on Equations 18–22. Calculations were conducted applying established experimental data [
3], using Wolfram Mathematica 11.2, and the complete set of results is presented in
Supplementary Files 2 and 3. For readers’ convenience, 3D graphs on behavior of
x,
GR,
GA,
FR and
FA with respect to
T and
y are presented in
Figure 7 Graphs (a), (b), (c), (d) and (e).
- (a)
Variation of x relative to T and y. Value of x remains constant at 3 regardless of T and y.
- (b)
-
Variation of GR relative to T and y. GR remains positive over the entire range of T and y. For any given value T:
- i.
When y < 0.5, as y decreases, the rate of change of GR is very high.
- ii.
When y > 0.5, as y increases, GR is much stable, compared when y values are low.
For any given value of y, variation of GR vs. T is comparatively low.
- (c)
-
Variation of GA relative to T and y. For any given value T:
- i.
When y < 0.5, GA is positive, and the rate of change of GA vs. y is high.
- ii.
When y > 0.5, GA is negative, and the rate of change of GA vs. y is comparatively low.
For any given value y: variation of GA vs. T appears significant; comparatively more than GR.
- (d)
-
Variation of FR relative to T and y. FR is linearly proportionate to T at any given value of y. Gradient of the linear relationship FR vs. T is greater when y < 0.5 compared to when y > 0.5. Further, in the linear relationship FR vs. T:
- i.
When y < 0.5, intercept > 0
- ii.
When y ≈ 0.5, intercept ≈ 0
- iii.
When y > 0.5, intercept < 0
- (e)
-
Variation of FA relative to T and y.
- i.
When y < 0.5, FA is positive, and the rate of change of FA vs. y is high.
- ii.
When y > 0.5, FA is negative, and the rate of change of FA vs. y is comparatively low.
- iii.
Hence, when y ≈ 0.5, FA changes polarity.
The above analysis of
Figure 7 shows that,
for the gases investigated:
x is a constant = 3, regardless of
T and
y (
Figure 7(a)). The significance of this finding will be discussed below.
Behaviors of
GR and
GA (
Figure 7(b) and (c)) show that
y = 0.5 is a significant point in this analysis.
At
y = 0.5,
GA changes polarity, hence,
FA (
Figure 7(e)) also changes polarity at
y = 0.5.
GR remains positive over the entire range of
T and
y.
- -
When y < 0.5, the rate of change of both GR and GA vs. y is high as y decreases.
- -
When y > 0.5, both GR and GA are comparatively stable as y increases.
- -
For any given value y, variation of GA vs. T appears significant; comparatively more than GR. In classical models of gravitational forces on matter, the dependency of GA on temperature has not been known; Equation 1 is not dependent on temperature. However, the above analysis for gaseous form of matter shows otherwise. Therefore, FA also depends on T (Equation 24).
FR is linearly proportionate to
T at any given value of
y (
Figure 7(d)); thus, vindicating the mathematical model Equation 5. Significantly, this result confirms the findings presented in a previous paper [
2] of the series of publications emanating from this research program (see
Supplementary Figure S1). It states therein, [
2] p148:
In experiment 2, tf (time-of-fall) of a water-droplet in still air increases with the temperature of the droplet. That is: “The hotter the water-droplet, the slower it falls”.
A significant finding is that, in the linear relationship FR vs. T extrapolated graph intercept ≈ 0 when y ≈ 0.5, while intercept > 0 when y < 0.5, and intercept < 0 when y > 0.5.
4.3. The Significance x = 3.0, Inverse Proportionality to the Cube of the Distance:
Results presented in
Figure 7(a) and
Supplementary Files 1-3, show that, at the length scale of intermolecular distances for gas molecules,
x = 3.0 with a very low variance of 8.4233 x 10
–9 for both
cv and
cp. Calculations show that the
x is constant, independent of the atomic mass
m (analysis was repeated for hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide yielding the same result; information available on request), the Specific Heat Capacity
c and
y, over a wide range of temperature data. It is, hence, concluded that
x = 3.0; a significant finding of this research. This delivers
r3 in the denominators of expressions for both the gravitational repulsion force and the gravitational attraction force. In other words, both gravitational repulsion force and gravitational attraction force, at the length scale of intermolecular distances for gas molecules, are
inversely proportional to the cube of the distance between them. The very low variance of the result
x = 3.0 shows that the inverse cube relationship is dominant.
Until now, under the Newtonian model, it has been considered that the classically known gravitational force between two entities is inversely proportional to the square of the distance between them [
10] (Equation (1)). Such classically known gravitational force is the
resultant of the two distinct forces, viz., gravitational repulsion and attraction forces revealed in this research program. The inverse square concept is also seen in the Coulomb’s Law [
55] for the electromagnetic force between two charged particles.
Inverse proportionality to the cube of the distance is applicable to gravitational repulsion and attraction forces, determined at the length scale of intermolecular distances for gas molecules, is a significant deviation from the classical acceptance.
Value x = 3.0 gives that gravitational repulsion and attraction field/flux flow is closer to the volumetric or solid spherical distribution (4/3 πr3) rather than the area or surface distribution (4πr2) considered in the classical model. With the interpretation of the antigravity effect, we have come up with a volumetric or solid spherical (r3) model which is more relevant to gases in 3-dimensional space. Such volumetric or solid spherical model maybe more relevant to other contexts in the universe in general. Furthermore, this alternative model is a more appropriate application on a force field that fills up free space. This is a significant departure from the Inverse-Square Law. Inverse proportionality to the cube of the distance, revealed in this experiment, is one most important revelation/result in fundamental sciences.
4.4. The Significance of y = 0.5:
As stated, when presenting Equation 10, the parameter
y as the exponent of
T was introduced because the effect of absolute temperature on the thermal energy of an entity is not known in the context of gravitational repulsion. The above analysis (
Figure 7) elaborated that
y = 0.5 is a very noteworthy value when considering behaviors of
GR,
GA,
FR and
FA.
It is very significant that, for both cv and cp, when y ≈ 0.5:
Hence, FW [= FR - FA] may become negative and the gas may condense, when y ≈ 0.5 and T → 0; see Equation 11. The analysis was repeated for hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide, yielding the same result irrespective of atomic mass m; information available on request. This happens only when y ≈ 0.5, although P ∝ T concept of the Amontons’ Law/Gay-Lussac’s Law was applied across all calculations irrespective of the value of y. Therefore, y = 0.5 is observed as the value that satisfies existing empirical observations of the Amontons’ Law/Gay-Lussac’s Law, hence, chosen to conduct subsequent analysis in this paper.
4.5. Units of GR, GA, FR and FA:
When y = 0.5, units of GR and GA would be as follows:
GR: s2 K kg-1
GA: m4 kg-1 s-2
Newtonian Universal Gravitation Constant G has m3 kg-1 s-2 as units; note m3 in the numerator. Units of GA have m4 in the numerator. This difference of one in the power of the unit of length is because the Newtonian model is based on the Inverse Square Law, whereas the model presented above is based on the Inverse Cube Law. The units of the finally calculated forces FR and FA are found to be kg m s-2, i.e., Newton, as expected, thus vindicating the model.
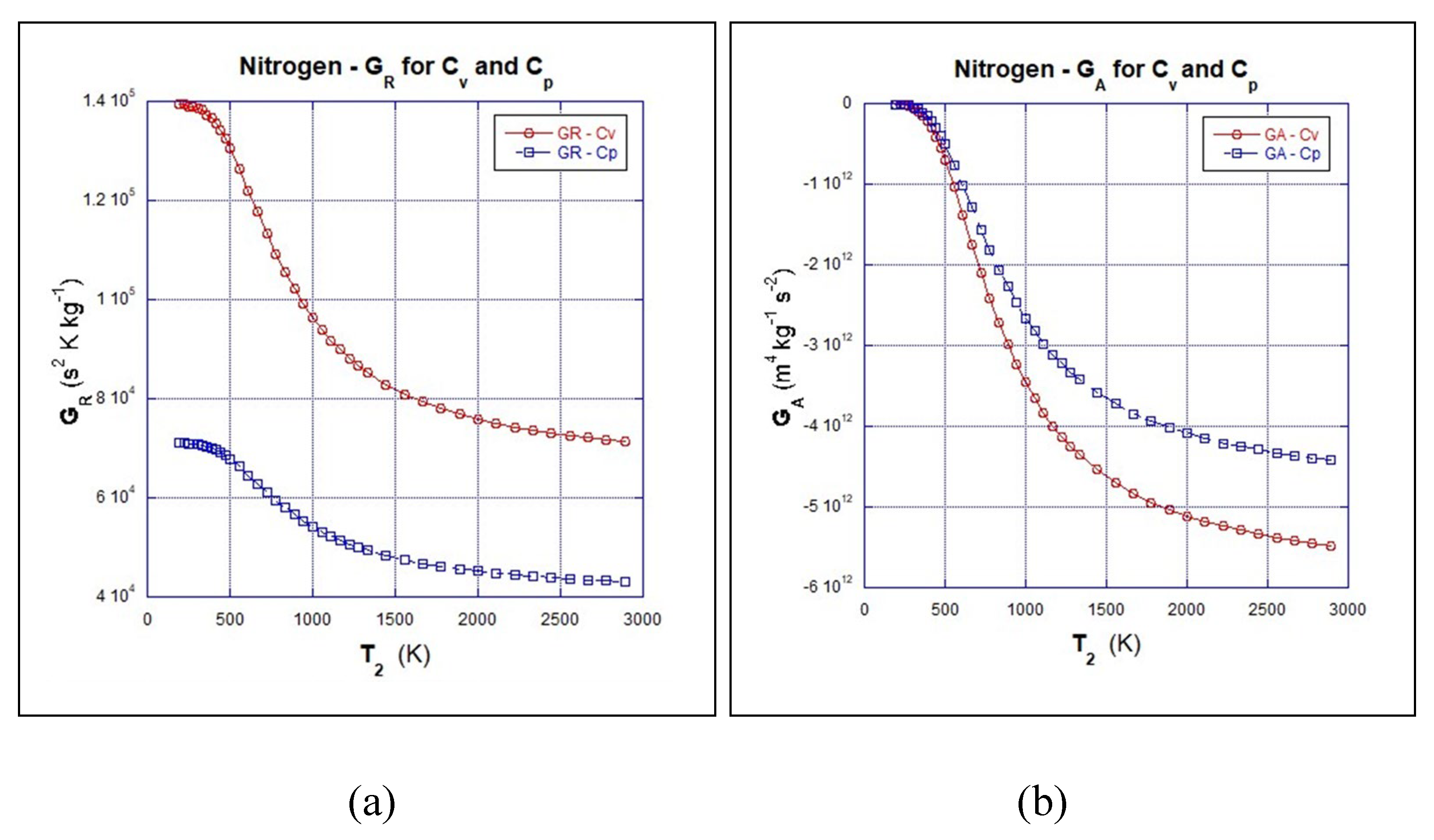
4.6. Orders of the Magnitude of GR and GA:
Figure 8 presents variations of
GR and
GA against
T for gaseous nitrogen; thus, depicts their orders of magnitude. The values were calculated applying the empirical data and the value
y = 0.5 to Equations 21–22 (
Supplementary File 1).
Gravitational attraction was initially, expected to be independent of
T. Results presented in
Figure 8(b), nevertheless, show that
GA is dependent of
T, and this would be discussed later together with
FA.
Results pertaining to both
cp and
cv are presented for comparison in
Figure 8. The variance of
GR and
GA are smaller for
cp compared to
cv. It could be noted that, the model developed herein is where starting (Situation i) and ending (Situation iii) pressures are equal (over all under constant pressure). Hence, data pertaining to
cp is more appropriate to the analysis. Substituting
cp values to Equations 21–22, therefore, shows lesser variations of both
GR and
GA.
- (a)
Results of GR from calculations using cv and cp
- (b)
Results of GA from calculations using cv and cp
The variance of GR and GA are smaller for cp compared to cv.
The orders of the magnitude of
GR and
GA yielded by the gravitational repulsion and attraction model, as presented above, are very large compared to the Newtonian Universal Gravitation Constant
G, which is 6.674 x 10
-11 m
3 kg
-1 s
-2. Considering equilibria in the infinite universe with infinite distances, such large values of
GR and
GA are not impractical. The very small value Newtonian constant has been deduced to represent a very small gravity force observed by the scientists at that time [
56]. The present program of research has shown that the so called ‘weak’ gravitational force is actually the resultant of two extremely large forces, i.e., gravitational repulsion and gravitational attraction, which distinctly act on matter. This would be discussed in more details in the subsequent section that deals with the orders of the magnitude of the forces.
The analysis was repeated for hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide as well, yielding the similar result; information available on request. Magnitudes of GR and GA for the above gases were also in the similar order.
4.7. Orders of the Magnitude of FR and FA:
Values of
FR and
FA for nitrogen gas are calculated applying the empirical data and the value
y = 0.5 to Equations 23–24 (
Supplementary File 1). The analysis was repeated for hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide as well, yielding similar results; information available on request.
To give an example for the convenience of readers, the values of
FR and
FA between two nitrogen gas molecules, at the temperature
T2 333.32 K (≈ 60 °C) and pertaining to
cp are, respectively, 1.324 × 10
-12 N and -3.587 × 10
-15 N. (Note:
FR >>> FA). The resultant of
FR and
FA between two nitrogen gas molecules
= FW = FR - FA = 1.328 × 10
-12 N. [Note: As defined in the mathematical model presented herein,
FW acts in the direction of repulsion].
FW exerted by nitrogen gas molecules, was translated to Pressure
P, in the unit Pa by applying Equations 18 and 19. The result is: 110.537 N on an area of 1 m
2 (110.537 kPa = 1.091 standard atmosphere), which is equal to the value obtained by applying
Amontons’ Law/
Gay-Lussac’s Law. See
Supplementary File 1 for the full set of results.
Further in the
Supplementary File 1, when
FW values obtained based on
cp and
cv (corresponding to any given temperature) are compared, the difference is in the order of a millionth of the
FW values. It shows that difference in
FW caused by the difference of applying either
cp or
cv is very small.
It is useful to compare FR and FA with the gravitational force between gas molecules calculated according to the classical theory as defined in Equation 1. When two nitrogen molecules are 4.557 x 10-9 m apart [which does occur at around 333 K (≈ 60 °C) and 1 atm], the classical theory calculated gravitational force between the two molecules is equal to 1.202 x 10-44 N. [Note: As defined in the classical theory, this gravitational force acts in the direction of attraction]. It is, nevertheless, interesting to note that: FR and FA in nitrogen [at around 333 K (≈ 60 °C) and 1 atm] are respectively 1.101 x 1032 and 2.984 x 1029 times greater than the gravitational force between gas molecules calculated according to the classical theory as defined in Equation 1.
Above pressure calculations were conducted using the mere two molecular system introduced in this paper. It could thus be justified that any two neighboring molecules of gas produces the same force at any given point throughout a gas; provided that the temperature and the molecular distance (volume) are the same. In reality, any small quantity of gas molecules exhibits the same pressure regardless of the number of molecules enclosed. Significance of this analysis is the absence of idealistic assumptions such as: large number of molecules making perfectly elastic collisions in deriving the ideal gas equation.
Gravitational attraction was, as pointed out before, initially expected to be independent of T. Results, nevertheless, show that GR, GA, FR and FA are dependent of T. Four fundamental interactions were not so far defined to be temperature dependent. Nevertheless, phenomena are observed in the nature/universe (e.g.: pressure, expansion, and so on) that are dependent on temperature/thermal energy content. With this research revealing that both FR and FA are temperature dependent, it appears that the critical gap between energy and fundamental forces has been filled.
4.8. Negative Gravitational Attraction Forces Among Gas Molecules:
Applying empirical data to Equation 24 shows that,
FA (the attraction force among gas molecules) is negative at elevated temperatures (
Figure 7(e) and
Supplementary Figure S3), implying that it acts as a repulsive force together with
FR. This negative
FA tends to become positive at lower temperatures where gases are expected to condense. For example, the value calculated for
FA between gaseous nitrogen molecules is -7.118 10
-16 N at 194.43 K and 64.478 kPa, and -2.299 10
-13 N at 2,888.9 K and 958.029 kPa (
Supplementary File 1).
Negative values of FA at elevated temperatures, together with FR, cause the gas to have only repulsive forces among molecules. This gives rise to the property that real gases expand infinitely as the available space increases. Such circumstances of all repulsive forces were observed in other gases studied (hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide) as well. Further for all these gases, tendencies of negative FA becoming positive at lower temperatures could also be observed from the results; suggesting that the gases are expected to condense. All these information on other gases studied are available on request.
The exact
fundamental mechanism of condensation has so far not been explained by existing theories. It is presently believed that condensation is initiated by the formation of atomic/molecular clusters [
57]. How such formation of clusters initiate has not been explained
fundamentally. Both the kinetic theory and the ideal gas law predict that gases compressed to very high pressures and cooled to very low temperatures should still behave like gases. Concepts of thermodynamics, thus far, indicate only that decrease in temperature reduces kinetic energies of particles [
41], but not how atoms/molecules form clusters or how/why aggregation occurs. Molecular vibrational distances are far smaller than intermolecular distances in gases. Reduction of molecular kinetic energy, hence, does not explain how intermolecular distances get smaller with the reduction of the temperature.
In real gases (obeying Van der Waals equation), it is conceptualized that [
42] condensation occurs when the molecules of a gases are cooled to the point where they no longer possess sufficient kinetic energy to overcome intermolecular attractive forces [
58]. Even in this explanation, it is not explained how kinetic energy and intermolecular force relates each other fundamentally.
Values of FR and FA calculated in the above analysis show that as temperature decreases, repulsion forces decrease and attraction forces increase (from negative at elevated temperatures to close to positive at lower temperatures) between gaseous molecules, thus causing aggregation of atoms/molecules together, i.e., causing condensation of the gas.
In other states of matter, different mechanisms pertaining to
FA could be observed. For example, as highlighted in the Author’s previous publication [
1]: collections of water droplets suspended in midair are observed to flocculate as clouds. In such circumstances, gravitational attraction and repulsion forces among water droplets are equal thus in equilibrium (see ‘5.0 Discussion’).
4.9. The Gist of the Model and the Outcomes:
A perceptible alternative model of the gravitational repulsion force and the gravitational attraction force has been developed above, considering them as two distinct forces acting on gaseous matter. The development of this model has been presented in Section 3.0. When established experimental data on nitrogen, hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide were applied, the model performed, yielding significant outcomes. These outcomes have been presented in Section 4.0.
In view of the numerous details, equations, outcomes, etc., of the model presented above, the gist of them all is diagrammatically presented in
Figure 9 for the convenience of the reader.
5. Discussion
It is observed that Newtonian and Einsteinian mechanics experience challenges in explaining certain phenomena seen in laboratory experiments (e.g.: upward motion of heated iodine particles in vacuum) and natural phenomena (e.g.: condensation of gases, accumulative (flocking together) nature of clouds and accelerating expansion of the universe) [
1,
2,
4,
5]. Under these existing models, the gravitational force has been reckoned as a “weak force”. Thus, in deriving the ideal gas equation, one of the most fundamental forces, the gravitational attraction among matter has been overlooked, both among air molecules themselves and with the Earth. Such lapses tempt deviation from the existing models, viz., Newtonian ‘Law of Universal Gravity’, Einsteinian ‘EFE’ and kinetic theory of ideal gases.
The alternative model considering both gravitational repulsion and attraction presented in this paper is self-standing, independent of existing idealistic models. This model has been built without idealistic assumptions such as: “intermolecular force in gaseous state is zero”, “perfectly elastic collisions”, “consists of a large number of molecules”, “volume of the molecules is negligibly small” and so on. In fact, there are no idealistic assumptions involved in this model; hence it is closer to reality.
The entire mathematical model presented in this paper was derived considering the forces of gravitational repulsion and attraction between just two gaseous molecules; the basic building blocks of the gas. Force (net repulsive) between individual gas molecules represents the pressure in the system; a significant deviation from the kinetic theory’s concept of momentum transfer. Hence, pressure does not depend on the number of molecules in a certain mass or volume of the gas, as assumed in the kinetic theory in the derivation of the ideal gas equation. The two gaseous molecule model justifies that any small quantity of gas molecules would exhibit same pressure as a large quantity at the same temperature and molecular number density; even a small quantity of gas could produce the same pressure as a large quantity.
It should be noted that the above relationships (Equations 18–24) are developed for the matter in the gaseous state, hence may not be valid for other states of matter (e.g., liquid, solid, plasma). It is, hence, recommended that future research should focus on analysis of matter in such other states.
The model has been validated using experimentally determined and established data [
3]utilized in practical thermodynamic applications of mechanical engineering industries. The data has been published in 1948, by Joseph Henry Keenan and Joseph Kaye. Applying these data to Equations 18 24, behavior of x,
GR,
GA,
FR and
FA with respect to
T and
y are derived and presented in 3D graphs (a), (b), (c), (d) and (e) in
Figure 7.
The result x = 3.0 as presented in the 3D graph
Figure 7(a) contributes new scientific information on distribution of force fields that fill up the volume in free space, at the length scale of intermolecular distances for gas molecules. The relationship of gravitational repulsion and attraction forces being inversely proportional to the cube of the distance, interprets the gravitational force distributions as volumetric or solid spherical distributions (
4/3 πr3) in free space, rather than the area or surface distributions (
4πr2) considered in the classical model. This is a significant departure from the Inverse-Square Law. Inverse-Square Law describes wave front propagation of energy. In contrast, force fields fill up volume in the free space. The volumetric distribution or fill-up the free space by the force is more appropriate in understanding; as a force field always exists in a 3D space rather than on a 2D surface.
In forces among gravitational fields, the inverse proportionality to cube (Inverse-Cube Law) with the distance is not new. An extra force besides gravity, that is obeying the Inverse-Cube Law, has been mentioned in ‘Principia Mathematica’ [
10] published by Isaac Newton in 1687; see Prepositions XLIII-XLV of Book 1, pp171-182. It has also been demonstrated [
59] experimentally that, in magnetostatic fields where both poles geometrically coincide, attraction and repulsion forces obey the Inverse-Cube Law with the distance. Future research should focus on discerning where inverse proportionality to the cube of the distance is more appropriate in applications of fundamental physics.
The analysis presented in this paper signifies that y = 0.5 is a very special value when considering behaviors of GR, GA, FR and FA. Most noteworthy points were that, for both cv and cp, when y ≈ 0.5:
extrapolation of graphs FR vs. T crosses (0,0)
negative FA tends to become positive as T approaches 0 K
This was found to be true for all gases considered: nitrogen, hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide; yielding similar results irrespective of atomic mass m. How these results resonate with other empirically established models such as Boyle’s Law, Charles’ Law and Amontons’ Law/Gay-Lussac’s Law will be discussed in a future publication.
In the 3D graphs
Figure 7(b) and (c), both Gravitational Repulsion Coefficient and Gravitational Attraction Coefficient appeared dependent on the temperature
T. This result is unexpected, as temperature dependency of
GA is not previously known. Further to that, as presented in 3D graphs
Figure 7(d) and (e), both
FR and
FA are temperature dependent; being gravitational forces, they are fundamental interactions in nature. Significant departure from the existing knowledge is that, the four fundamental interactions (fundamental forces) in classical theory are not defined to be temperature dependent. Existing theories, nevertheless, state that increase of thermal energy increases the potential energy of the gas molecules. Results showed that, increase of thermal energy increased repulsion (Eqs. 5 and 23) between gas molecules. See graphs of
FR vs.
T and
FA vs.
T, where, as
T increases:
That implies that thermal energy is directly proportional to the resultant of FR and FA, confirming the relationship between energy and fundamental forces. With this revelation, the critical gap between energy and fundamental forces has been filled. Fundamental forces could be more readily linked with observable temperature dependent phenomena (e.g.: pressure, expansion, and so on) in the nature/universe; thus, enabling better explanations.
In the 3D graph
Figure 7(d), gravitational repulsion force appeared linearly proportionate to the temperature. This vindicated the experiment conducted in this research program by the Author, presented in paper [
2]. The said experiment demonstrated that the time-of-fall of water droplets is linearly proportionate to the temperature (
Supplementary Figure S1). [Note:
FA also acts in this context. It was presented that
FR >>>
FA; see section ‘4.7 Orders of the Magnitude of
FR and
FA’].
Negative values of
FA at elevated temperatures [
Figure 7(e)], together with
FR, cause the gas to have only repulsive forces among molecules. This gives rise to the property that real gases expand infinitely as the available space increases. Such circumstances of all repulsive forces were observed in other gases studied (hydrogen, oxygen, water vapor, carbon monoxide and carbon dioxide) as well (information available on request).
Analysis presented in this paper further shows that as temperature decreases, repulsion forces decrease and attraction forces increase (from negative at elevated temperatures to close to positive at lower temperatures) between gaseous molecules, thus causing aggregation of atoms/molecules together, i.e., causing condensation of the gas. This finding is significant in a context where exact fundamental mechanism of condensation has so far not been explained by existing theories.
The present program of research has shown that, the so called ‘weak’ gravitational force (
Table 1), is actually the resultant of two extremely large forces, i.e., gravitational repulsion and gravitational attraction, which distinctly act on matter. Newly determined gravitational repulsion and attraction forces between two nitrogen molecules at 305 K are in the order of 10
30 times greater than the classically calculated gravitational attraction force. It thus reveals that gravitational repulsion and attraction forces in fact are of similar orders of magnitude as the other three forces in the nature.
Earlier in this paper, an experiment was described, where heated iodine particles moved upwards against the Earth’s gravitational pull. This is a groundbreaking experiment where the said phenomenon occurred in a situation where all factors which are believed to be causing the upward movement of particles against the gravitational pull in air, viz., buoyancy and convective forces, are eliminated by experimental design. Initially, at the room temperature (~25°C), the iodine particles detached from the iodine sample moved downward under gravitational attraction force with the Earth, and deposited in the bottom part of the paper jacket. When the iodine sample was heated, the experiment revealed that iodine particles move against gravitational pull in the vacuum and deposited in the top part of the paper jacket. In electronic vacuum tubes (also called electronic valves) also, evaporated tungsten and thorium particles from the filament moves upwards despite the gravitational pull and the strong radial electric fields, and deposit at the top of the glass tube.
The above was a laboratory experiment at a micro scale. The antigravity concept could also be extended to macro level phenomena in the nature such as clouds and the accelerating expansion of the universe. Review paper by the Author [
1] on the previous papers in this research program states:
In addition to attractive and repulsive forces of water-droplets of a cloud with earth, there exist attractive and repulsive forces among water droplets within the cloud. These forces acting inside the cloud explain the accumulative (flocking together) nature of the cloud which has not been explained by the classical theories. The equilibrium of these two forces will confine the droplets to a certain area as a floccule. The repulsiveness does not allow shrinking and finally collapsing the cloud while the attractive force keeps the droplets together without dispersion. [
1]
p4
The above is an ideal example where there is no net outward force (no net pressure exerted outward) among water droplets. Water droplets behave as a flock under the equilibrium of gravitational attraction and gravitational repulsion forces. The paper [
2] dispelled the classical belief that clouds float due to convection currents, and showed that the force that holds the water droplets up in the air is a cryptic force, known as “antigravity”.
Considering both gravitational attraction and gravitational repulsion on matter and filling the critical gap between energy and fundamental forces, opens the doors for more research enabling stronger scientific explanations of observable temperature dependent phenomena, e.g.:
Heavy gas molecules (such as CFC) in the upper atmosphere
Brownian Motion
Condensation/evaporation/sublimation
Expansion/contraction of gas/liquid/solid
and more
The concept of gravitational repulsion and gravitational attraction forces could be further applied at macro level to explain the accelerating expansion of the universe. Even in our solar system:
… the distance of the Earth’s [sic] from the sun. Various measurements indicate that this distance (or at least the length of the Earth’s semimajor axis) is increasing at the rate of 15 cm per year (plus or minus 4 cm). [
60,
61]
Galaxies and other interstellar objects are not in a state of equilibrium as a result of increasing thermal energy content due to various reasons including atomic fission and fusion causing mass-energy conversion (E = mc
2). Such mass-energy conversion has dual effects on equilibria in the universe: (1) increasing thermal energy increases gravitational repulsion forces, while (2) decreasing mass decreases gravitational attraction forces. Gravitational repulsion forces, hence, supersede gravitational attraction forces, thus causing outward expansion of the universe with acceleration [
62].
This research has shown that, gravitational repulsion and gravitational attraction forces are colossal (larger in the order of 1032 and 1029 times respectively at around 333 K and 1 atm) than the traditionally known gravitational force between gas molecules. What has been calculated as gravity force so far according to the classical theory is, the resultant of the two large forces gravitational repulsion and attraction, which are nearly equal thus nearly in equilibrium. These forces pervade our environments at both micro and macro levels. Once fully understood, such nearly in balance colossal forces offer the prospect of learning to control and manipulate to achieve hitherto unknown results, outcomes and developments. This is another direction for future research.