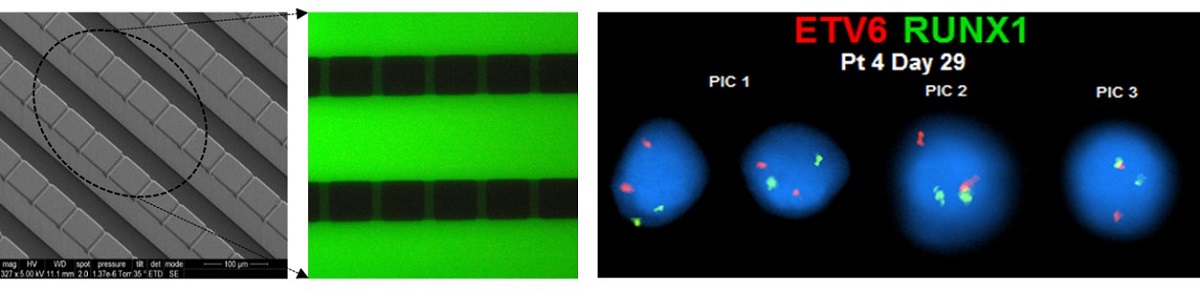
The role of circulating plasma cells (CPCs) and circulating leukemic cells (CLCs) as biomarkers for several blood cancers, such as multiple myeloma and leukemia, respectively, have recently been reported. These markers can be attractive due to the minimally invasive nature of their acquisition through a blood draw (i.e., liquid biopsy) negating the need for painful bone marrow biopsies. CPCs or CLCs can be used for cellular/molecular analyses, such as immunophenotyping or fluorescence in situ hybridization (FISH). FISH, which is typically carried out on slides involving complex workflows, becomes problematic when operating on CLCs or CPCs due to their relatively modest numbers. Here, we present a microfluidic device for characterizing CPCs and CLCs enriched from peripheral blood using immunofluorescence or FISH. The microfluidic possessed an array of cross-channels (2-4 µm in depth and width) that interconnected a series of input and output fluidic channels. Placing a cover plate over the device formed microtraps, the size of which was defined by the width and depth of the cross-channels. This microfluidic chip allowed for automating immunofluorescence and FISH requiring the use of small volumes of reagents, such as antibodies and probes, as compared to slide-based immunophenotyping and FISH. In addition, the device could secure FISH results in <4 h compared to 2-3 d for conventional FISH.