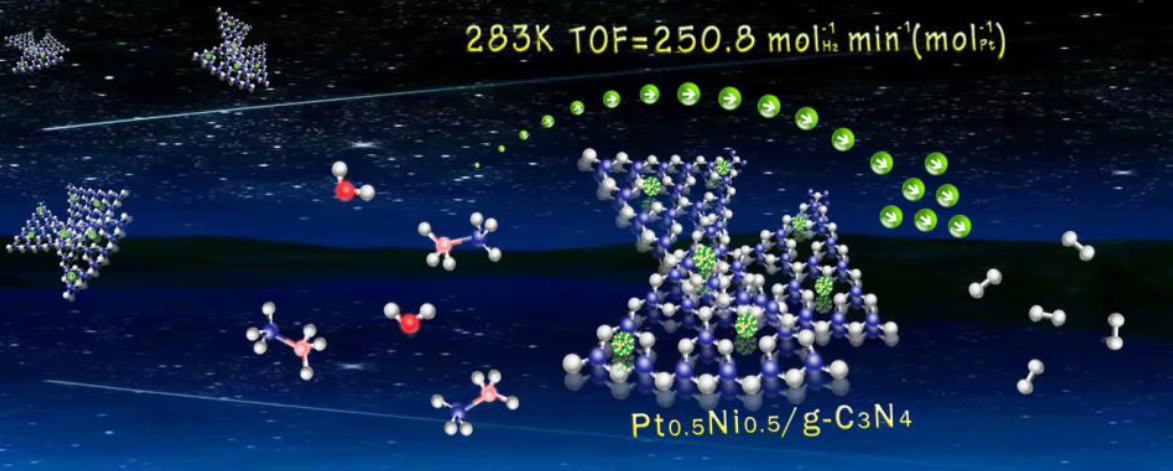
Graphite carbon nitride (g-C3N4) supported PtNi alloy nanoparticles (NPs) were fabricated via a facile and simple impregnation and chemical reduction method and explored their catalytic performance towards hydrogen evolution from ammonia borane (AB). Interestingly, the resultant Pt0.5Ni0.5/g-C3N4 catalyst affords superior performance, including 100% conversion, 100% H2 selectivity, yielding the extraordinary initial total turnover frequency (TOF) of 250.8 molH2 min-1 (molPt)-1 for hydrogen evolution from AB at 10 °C, a relatively low activation energy of 38.09 kJ mol−1, and a remarkable reusability (at least 10 times), which surpass most of the noble metal heterogeneous catalysts. This notably improved activity is attributed to the charge interaction between PtNi NPs and g-C3N4 support. Especially, the nitrogen-containing functional groups on g-C3N4, serving as the anchoring sites for PtNi NPs, may be beneficial for becoming a uniform distribution and decreasing the particle size for the NPs. Our work not only provides a cost-effective route for constructing high-performance catalysts towards the hydrogen evolution of AB but also prompts the utilization of g-C3N4 in energy fields.