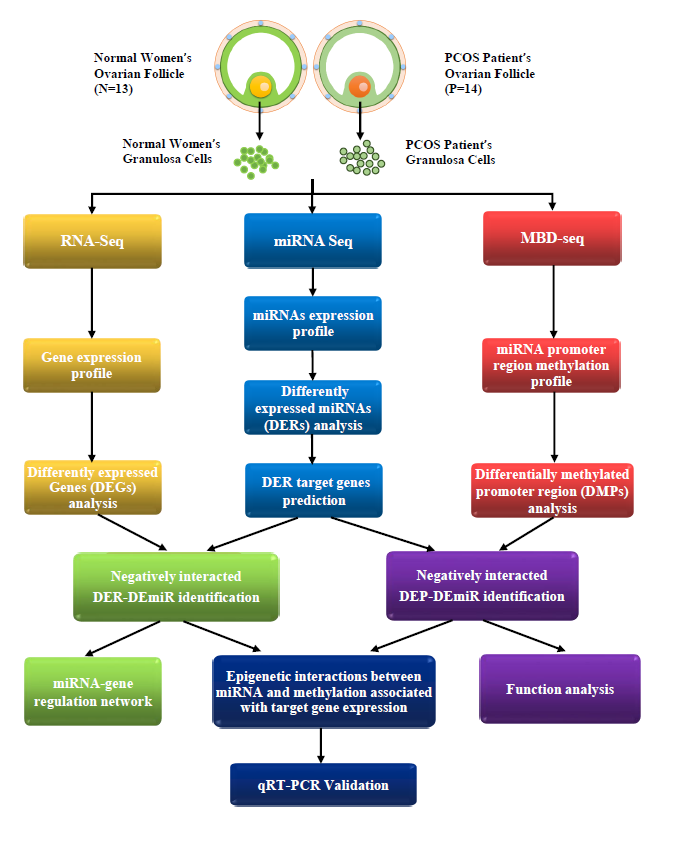
Aberration in microRNA (miRNA) expression or DNA methylation is a causal factor for polycystic ovarian syndrome (PCOS), a common endocrine disorder and leading cause of infertility. However, the epigenetic interactions between miRNA and DNA methylation remain unexplored in PCOS. In this study, we conducted an integrated analysis of RNA-seq, miRNA-seq and MBD-seq on ovarian granulosa cells of PCOS and control groups to reveal the epigenetic interactions involved in the pathogenesis of PCOS. Firstly, we identified 830 genes and 30 miRNAs that were expressed differently in PCOS, and seven miRNAs were found to negatively regulate targeted mRNA expression. Next, in total, 130 miRNAs were found to be significantly differently methylated in promoter regions, while 13 were found to be associated with miRNA expression. Furthermore, the promoter hypermethylation of miR-429, miR-141-3p, and miR-126-3p was proven to suppress miRNA expression and therefore upregulate their corresponding genes, including XIAP, BRD3, MAPK14 and SLC7A5. Our results demonstrate that DNA methylation regulates miRNA expression and therefore controls its corresponding gene expression. The reactivation of the transcription of epigenetically silenced genes may be one of the key elements in PCOS pathogenesis. Meanwhile, the epigenetic mechanisms underlying the regulation of miRNA expression can provide a potential therapeutic target for PCOS in the future.