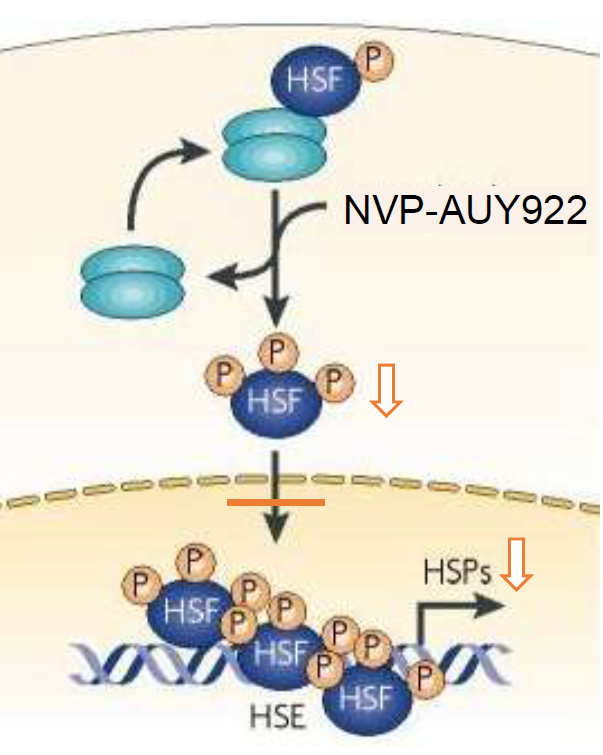
The inhibition of heat shock protein 90 (Hsp90) a molecular chaperone for multiple oncogenic client proteins is considered as a promising approach to overcome radioresistance. Since most Hsp90 inhibitors activate HSF-1 that induces the transcription of cytoprotective and tumor-promoting stress proteins such as Hsp70 and Hsp27, a combined approach consisting of HSF-1 knockdown (k.d.) and Hsp90 inhibition was investigated. A specific HSF-1 k.d. was achieved in H1339 lung cancer cells using RNAi-Ready pSIRENRetroQ vectors with puromycin resistance. The Hsp90 inhibitor NVP-AUY922 was evaluated at low concentrations - ranging from 1-10nM - in control and HSF-1 k.d. cells. Protein expression (i.e., Hsp27/Hsp70, HSF-1, pHSF-1) and transcriptional activity was assessed by Western blot analysis and luciferase assays and radiosensitivity was measured by proliferation, apoptosis (Annexin V, active caspase 3), clonogenic cell survival, alkaline comet, γH2AX, 53BP1 and Rad51 foci assays. The k.d. of HSF-1 resulted in a significant reduction of basal and NVP-AUY922-induced Hsp70/Hsp27 expression levels. A combined approach consisting of HSF-1 k.d. and low concentrations of the Hsp90 inhibitor NVP-AUY922 potentiates radiosensitization which involves an impaired homologous recombination mediated by Rad51. Our findings are key for clinical applications of Hsp90 inhibitors with respect to adverse hepatotoxic effects.