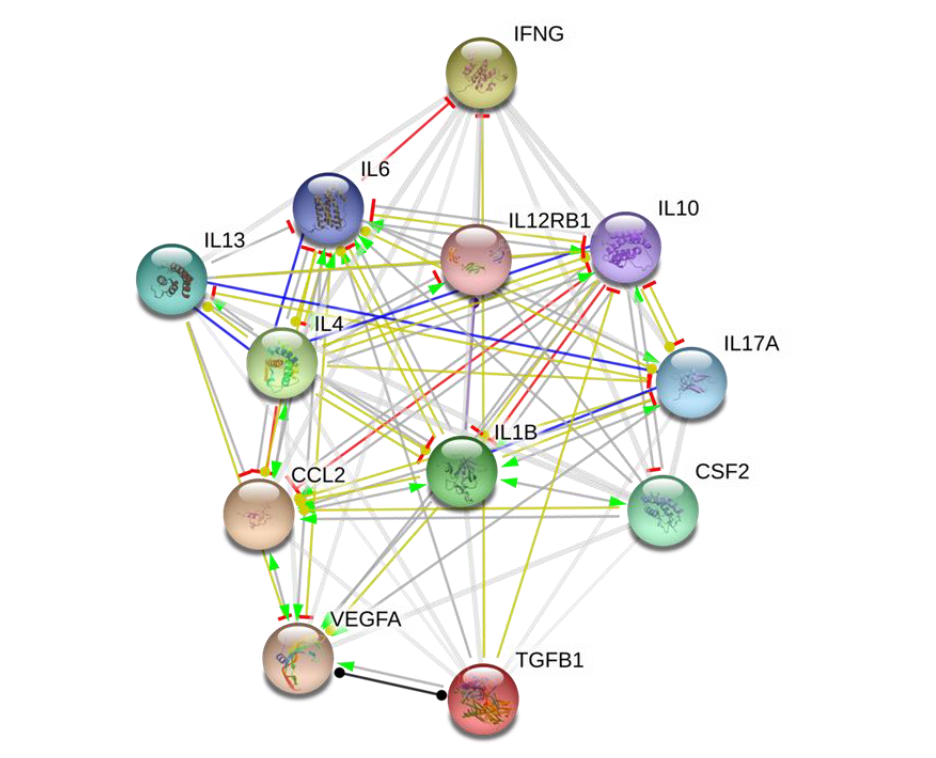
Background: Improving patients’ quality of life (QoL) is a principal objective of all treatment in any clinical setting, including oncology practice. Cancer-associated inflammation is implicated in disease progression and worsening of patients’ QoL. Conventional anticancer therapeutics while selectively eliminating cancerous cells, are evaded by stem cell-like cells, and associated with varying degrees of adverse effects, thus reducing patients’ QoL. This necessitates novel therapeutic approaches with enhanced efficacy, minimal or no treatment-related adverse effects, and improved QoL in patients with cancer, especially those with metastatic/advance stage disease. Methods: Sequel to our team’s previous publication, the present study explores probable effects of Astragalus polysaccharides (PG2) on cancer-related inflammatory landscape and known determinants of QoL, as well as the probable link between the two to provide mechanistic insight. In exploratory double blind randomized controlled trial using patients with metastatic disease (n=23), we comparatively evaluated the therapeutic efficacy of high (500mg) or low (250mg) dose PG2 administered intravenously (i.v.), with particular focus on its suggested anti-inflammatory function and the probable effect of same on QoL indices at baseline, then at weeks 4 and 8 post-PG2 treatment. Results: All 23 patients with metastatic disease treated with either low or high PG2 experienced reduced pain, nausea, vomiting and fatigue, as well as better appetite and sleep, culminating in improved global QoL. This was most apparent in the high dose group, with significant co-suppression of pro-inflammatory interleukin (IL)-1β, IL-4, IL-6, IL-13, IL-17, monocytes chemotactic protein (MCP)1, granulocyte-macrophage colony-stimulating factor (GM-CSF), vascular endothelial growth factor (VEGF), tumor growth factor (TGF)-β1, interferon (IFN)-γ, and immune suppressors IL-10, and IL-12. Univariate and multivariate analyses revealed that IL-1β, IL-13 and GM-CSF are independent prognosticators of improved QoL. Conclusion: This proof-of-concept study provides premier evidence of functional association between PG2 anti-inflammatory effects and improved QoL in patients with advanced stage cancers, laying the groundwork for future larger cohort blinded controlled trials to establish the efficacy of PG2 as adjuvant anticancer therapy in metastatic or advanced stage clinical settings.